Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6989
Revised: February 7, 2014
Accepted: March 12, 2014
Published online: June 14, 2014
Processing time: 193 Days and 4.4 Hours
AIM: To develop a safe, simple, noninvasive and affordable system to predict esophageal variceal bleeding (EVB) in decompensated cirrhosis patients.
METHODS: Four hundred and eighty-six patients with decompensated cirrhosis (238 males and 248 females), with a mean age of 63.1 ± 11.2 years, were admitted to Changshu Affiliated Hospital of Suzhou University between May 2008 and March 2011. Patients enrolled in this study underwent ultrasound-Doppler (US-Doppler) to assess left gastric vein (LGV) blood flow velocity (LGVV) and blood flow direction (LGVBFD), and were evaluated by the Model For End-Stage Liver Disease (MELD) scoring system. All patients received follow-up evaluations every three months. The resulting data were entered into a database after each time point collection.
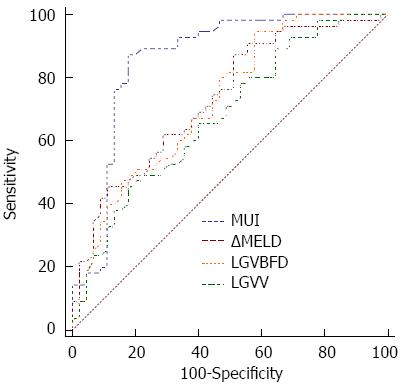
RESULTS: Four hundred and sixteen patients completed follow-up evaluations for an average of 31.6 mo (range: 12 to 47 mo). Fifty-one (12.3%) patients experienced EVB. The change in the MELD score over three months (ΔMELD), LGVV and LGVBFD were independently associated with EVB occurrence. MELD-US-Doppler Index (MUI), a new index, was developed and calculated using the following logistic regression equation: MUI = Logit (P) = 1.667 (ΔMELD) + 2.096 (LGVV) - 3.245 (LGVBFD) - 1.697. The area under the receiver operating characteristic curve for prediction of EVB occurrence was significantly higher for the MUI [0.858 (95%CI: 0.774-0.920)] than for ΔMELD [0.734 (95%CI: 0.636-0.817); P < 0.05], LGVV [0.679 (95%CI: 0.578-0.769); P < 0.05] or LGVBFD [0.726 (95%CI: 0.627-0.810); P < 0.05] alone. When the MUI was set at 46, the index had high diagnostic accuracy (85.8%), with high specificity (80%) and sensitivity (87.27%).
CONCLUSION: The MUI, a noninvasive and affordable index, can predict EVB occurrence in decompensated cirrhotic patients and serve as an alternative when conventional endoscopic screening is declined.
Core tip: Bleeding is a common occurrence in cirrhotic patients with portal hypertension and establishing indicators of risk is critical. Although screening by endoscopy is the recommended approach for patients with decompensated cirrhosis, it is an invasive, uncomfortable and costly procedure. In the present study, a new index that combines ultrasound-Doppler and the Model For End-Stage Liver Disease was evaluated as a suitable alternative. This MUI represents a noninvasive, low-cost and convenient method for assessing the risk of esophageal variceal bleeding in decompensated cirrhotic patients.
- Citation: Xu XD, Dai JJ, Qian JQ, Pin X, Wang WJ. New index to predict esophageal variceal bleeding in cirrhotic patients. World J Gastroenterol 2014; 20(22): 6989-6994
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6989
In patients with cirrhosis, esophageal varices are commonly observed, with an estimated prevalence of 50%[1]. After esophageal varices have formed, the annual risk for bleeding can range from 10% to 30%[2,3]. In patients with decompensated cirrhosis, acute esophageal variceal bleeding (EVB) represents a predominant cause for morbidity and mortality. Due to the increased risk of fatality in cirrhotic patients with EVB, the risk status in patients must be routinely evaluated such that the appropriate prophylactic therapy is administered to prevent variceal bleeding events. Typically, the EVB risk is assessed through endoscopic screening to assign a grade to the varices and determine if red signs are present, which is currently recommended for all patients with diagnosed cirrhosis[4-6]. Endoscopy, however, is invasive, painful and costly, especially to those in developing countries, which ultimately limits the frequency of examinations.
One way to overcome the obstacle of frequent endoscopies is to develop a safe, noninvasive and affordable system to better predict the risk of variceal hemorrhage in cirrhotic patients. An ultrasound approach that includes duplex ultrasonography and color Doppler is a simple, precise, noninvasive and affordable imaging method that is predominantly used to diagnose and stage cirrhotic portal hypertension. The Model for End-stage Liver Disease (MELD) prognostic scoring system, which has an equal or better predictive ability than the traditional Child-Pugh system, is another method to evaluate portal hypertension for short- or intermediate-term outcome[7-9]. Monitoring changes in the MELD score over time (ΔMELD) has also been proposed as another way to estimate the risk for complications[10]. Patients with a rapidly worsening ΔMELD score are at risk for more severe liver disease and complications such as EVB, which ultimately increase the risk of mortality.
The objective, noninvasive and affordable natures of the MELD score and color ultrasound-Doppler (US-Doppler) allow data to be obtained quickly. The capability of a combined approach using the MELD scoring system and US-Doppler to predict EVB has not yet been described. In the present study, the predictive capability of a new index, the MELD-US-Doppler Index (MUI), was evaluated in a cohort of cirrhotic patients.
This study was approved by the ethical committee of Changshu Hospital, a Suzhou University affiliate. All patients provided informed consent prior to study enrollment. The following exclusion criteria were applied: (1) patients who declined the evaluation schedule; (2) patients with hepatocellular carcinoma; (3) patients who frequently took medicines such as proton pump inhibitors, propranolol or antivirals; and (4) patients who underwent a splenectomy, received transjugular intrahepatic portosystemic shunts (TIPS), sclerotherapy, or band ligation endoscopic treatments before or after enrollment in this study. A total of 486 decompensated cirrhotic patients (238 males and 248 females), with an average age of 63.1 ± 11.2 years, were enrolled in the study between May 2008 and March 2011 at the Changshu Affiliated Hospital of Suzhou. A diagnosis of decompensated cirrhosis was made after analyzing a combination of physical, laboratory and radiological examination results. A diagnosis of EVB was confirmed when esophageal varices with stigmata from recent bleeding (e.g., fibrin plug on the varix, adhered blood clot, etc.) or active bleeding (e.g., oozing or spurting blood) were observed by endoscopy.
Patients enrolled in this study were subjected to US-Doppler and MELD evaluations every three months. After each patient evaluation, the database was updated with the newly obtained data. The occurrence of EVB served as the end point for patient follow-up. Patients who died for reasons unrelated to cirrhosis or were unable to be reached for follow-up were excluded from the trial.
MELD scores were calculated as specified by the United Network of Organ Sharing (UNOS) database using the following equation: MELD = 9.57 [Ln creatinine (mg/dL)] + 3.78 [Ln bilirubin (mg/dL)] + 11.2 (Ln international normalized ratio) + 6.43 (constant for liver disease etiology). For calculations, the minimum values were set to 1. The maximum serum creatinine level applied to the MELD score equation was 4.0 mg/dL. The change in the MELD score over a three month period was represented as ΔMELD.
One of two sonographers (WMF and YYG, each with over 20 years of experience), who were blinded to the clinical data for the study duration, performed all examinations using an Aplio color US-Doppler unit (Toshiba, Tokyo, Japan) and a 3.75 MHz phased array curved electronic probe. All patients were imaged while resting (> 15 min) in a supine position the morning following an overnight fast. Quantitative measurements were obtained during a brief inspiratory apnea. To obtain images, the transducer was oriented along the longitudinal axis of the portal vein (PV) and splenic vein (SV), and Doppler traces were obtained at an insonation angle < 60°. The Doppler signal for the PV was obtained at the halfway point between the convergence of the splenic and superior mesenteric veins, and the PV hilar split. For the SV, the Doppler signal was obtained halfway between the spleen and the PV, prior to the left gastric vein (LGV). The LGV appears as a tubular structure within the gastrohepatic ligament near the esophagogastric junction, emanating from the SV or PV with blood flowing towards the head. The maximum diameter of the LGV was measured from long-axis sonograms taken in the sagittal or transverse oblique plane, depending on the orientation of the vein. The direction of the LGV blood flow was assessed with respect to the portal system (hepatopetal vs hepatofugal). Software associated with the US unit was used to calculate velocity and flow for each vessel.
All statistical analyses were performed with SPSS 13.0 (IBM, Chicago, IL, United States) and MedCalc 12.7.0.0 (MedCalc Software, Ostend, Belgium). For analysis of categorical data, a χ2 test or Fisher’s exact test (two-tailed) was used, while an independent samples t-test was used to analyze continuous data. A normality test was applied to continuous data to ensure that a t-test was the most appropriate to use. All patient characteristics are expressed as mean ± SD or as percentages when appropriate. A multivariate logistic regression analysis with the Wald test was used to analyze MELD scores. The ΔMELD and US parameters were analyzed to identify the independent factors that were predictive of EVB risk. Variables from the univariate analysis with a P value < 0.20 were analyzed by multivariate logistic regression analysis to identify independent predictive factors. Receiver operating characteristic (ROC) curves were constructed for the independent predictive factors, and the area under the curve (AUC) was then determined. To define the best discriminating probability threshold, sensitivity, specificity and predictive values were calculated and compared for diagnostic accuracy. All statistical tests were two-tailed and the threshold for statistical significance was set at P < 0.05.
Seventy of the original 486 patients were excluded from the trial. Of these, 43 patients declined to undergo US-Doppler or MELD evaluation during the first three-month follow-up, 12 patients had been medicated with proton pump inhibitors, propranolol or antivirals, eight patients underwent a splenectomy and seven patients received other endoscopic procedures after study enrollment. A total of 416 patients were followed until March 2012, with a mean follow-up period of 31.6 mo (range: 12-47 mo). Fifty-one patients had an EVB episode (EVB+ group), while the remaining 365 patients did not (EVB- group), resulting in an incidence of 12.3%. The characteristics and results for the EVB+ and EVB- groups are displayed in Table 1.
| Variable | EVB+ (n = 51) | EVB- (n = 365) | P value |
| Age (yr) | 67.5 ± 13.4 | 62.3 ± 11.8 | 0.0085 |
| Sex (male/female) | 27/24 | 201/164 | 0.7749 |
| Etiology of liver disease | |||
| Hepatitis B virus | 36 | 255 | 0.9157 |
| Primary biliary cirrhosis or primary sclerosing cholangitis | 0 | 2 | 0.5962 |
| Alcohol-related liver disease | 1 | 4 | 0.5955 |
| Schistosomiasis-associated liver disease | 14 | 104 | 0.8771 |
| MELD score | 26.5 ± 9.8 | 18.9 ± 10.3 | 0.0000 |
| ΔMELD score | 1.89 ± 1.23 | 0.66 ± 0.47 | 0.0001 |
| US parameters | |||
| PV diameter (cm) | 1.37 ± 0.25 | 1.31 ± 0.18 | 0.0979 |
| PV velocity (cm/s) | 16.9 ± 10.1 | 19.9 ± 8.4 | 0.0428 |
| PV flow (mL/min) | 809 ± 396 | 776 ± 412 | 0.5907 |
| SV diameter (cm) | 1.23 ± 0.45 | 1.01 ± 0.23 | 0.0006 |
| SV velocity (cm/s) | 27.1 ± 11.3 | 29.1 ± 12.0 | 0.2623 |
| SV flow (mL/min) | 1124 ± 412 | 896 ± 331 | 0.0002 |
| LGV diameter (cm) | 0.73 ± 0.33 | 0.61 ± 0.21 | 0.0115 |
| LGV velocity (hepatopetal) (cm/s) | -21.1 ± 8.1 | 14.1 ± 6.9 | 0.0002 |
| LGVBFD (hepatofugal) | 49 | 73 | 0.0000 |
| Spleen longitudinal diameter (cm) | 20.7 ± 4.1 | 18.6 ± 3.6 | 0.3356 |
Patients in the EVB+ group were significantly older than in the EVB- group (67.5 ± 13.4 years vs 62.3 ± 11.8 years; P < 0.05) and had significantly higher MELD (26.5 ± 9.8 vs 18.9 ± 10.3; P < 0.05) and ΔMELD (1.89 ± 1.23 vs 0.66 ± 0.47; P < 0.05) scores. EVB+ patients also had significantly larger diameters of the SV (1.23 ± 0.45 cm vs 1.01 ± 0.23 cm; P < 0.05) and LGV (0.73 ± 0.33 cm vs 0.61 ± 0.21 cm; P < 0.05), and significantly higher SV blood flow (1124 ± 412 mL/min vs 896 ± 331 mL/min; P < 0.05). Moreover, the EVB+ group demonstrated a significantly higher proportion of patients with hepatofugal LGV blood flow (49/51 patients vs 73/365 patients; P < 0.05) and with a higher velocity (21.1 ± 8.1 cm/s vs 14.1 ± 6.9 cm/s; P < 0.05), while the velocity of the PV was significantly lower (16.9 ± 10.1 cm/s vs 19.9 ± 8.4 cm/s; P < 0.05). Furthermore, multivariate analyses identified ΔMELD, LGVV and LGV blood flow direction (LGVBFD) as being independently associated with EVB occurrence (Table 2). Hepatopetal LGVBFD results in an LGVBFD of 1 and a negative value for the LGVV. Hepatofugal blood flow results in an LGVBFD of -1 and a positive value for the LGVV.
| Variable | Coefficient | SE | Wald | P value | OR (95%CI) |
| ΔMELD | 1.667 | 0.781 | 4.719 | 0.023 | 6.195 (1.193-22.624) |
| LGVV | 2.096 | 0.658 | 13.116 | 0.001 | 7.129 (2.725-24.256) |
| LGVBFD | -3.245 | 1.271 | 7.001 | 0.006 | 23.306 (2.376-337.186) |
| Constant | -1.697 | 0.659 | 6.635 | 0.010 | 0.183 |
As indicated by the logistic regression model, the predicted probability of EVB occurrence in cirrhotic patients was due to an increase in ΔMELD and LGVV and a decrease in the hepatopetal LGV blood flow. Thus a new index was proposed, the MELD-US-Doppler index (MUI), which includes ΔMELD, LGVV and LGVBFD in order to strengthen the diagnostic accuracy: MUI = Logit (P) = 1.667 (ΔMELD) + 2.096 (LGVV) - 3.245 (LGVBFD) - 1.697. The AUCs for prediction of EVB occurrence were substantially higher for the MUI compared to the ΔMELD, LGVV or LGVBFD alone (Figure 1). The highest diagnostic accuracy (85.8%) was demonstrated with a MUI of 46, with a specificity of 80% (95%CI: 65.4-90.4) and a sensitivity of 87.27% (95%CI: 75.5-94.7).
In cirrhotic patients, esophageal varices are a common complication, occurring in 35% to 80% of individuals. Approximately one-third of patients with esophageal varices will go on to develop EVB, a potentially fatal complication[11-14], with 70% of these at risk for additional bleeding episodes[15]. Prophylactic therapies such as endoscopic variceal ligation, sclerotherapy and surgery should be offered to patients at high risk for EVB. The serious nature of EVB and its complications necessitates the development of a reliable system to predict the risk of bleeding in patients. Although screening by endoscopy is currently recommended for all cirrhotic patients[1-5], can identify esophageal varices and provide information on variceal severity, the procedure is difficult to implement in developing countries due to the high cost, inconvenience and potential complications.
The MELD system, initially developed to assess mortality risk in patients receiving TIPS[16], was based on a highly selective subgroup of cirrhotic patients. Despite this caveat, the MELD score provides a reliable short-term survival estimate for a broad spectrum of liver diseases[17]. The MELD scoring system was then adopted by UNOS in February 2002 as a way to prioritize livers for transplantation. A more recent study performed by Merion et al[10] analyzing the utility of MELD in assessing survival of patients awaiting liver transplantation, showed that a change in the MELD over a 30-day period was a better predictor of mortality than a one-time MELD score. The alterations in prothrombin time and serum bilirubin and creatinine levels are factors known to perturb the MELD score. Increased MELD scores are indicative of more severe liver disease and may signify the presence of complications, such as EVB, which ultimately increase the risk for patient mortality.
The results of this prospective study further demonstrate the prognostic value of the MELD score in prediction of EVB risk in cirrhotic patients. The change in MELD score over time provides a more dynamic profile of disease severity that may be indicative of cirrhotic complications. Previously, most studies have calculated a change in the MELD score over a one-month time interval[18,19], which is insufficient for an accurate estimate of the dynamically occurring changes. Moreover, the frequency of MELD score evaluations may incite patient resistance to follow-up visits. To overcome this obstacle, the follow-up interval in this study was lengthened to three months. A multivariate logistic regression analysis demonstrated that the ΔMELD score was an independent predictive factor for EVB risk, indicating that patients with a rapid deterioration in liver function, and thus a higher ΔMELD, are at a higher risk for EVB.
Portal hypertension, characterized by an increase in PV pressure, is caused by a blockage in blood flow through the liver. This increase in PV pressure results in the development of varices in the stomach and esophagus in an effort to bypass the blockage. These enlarged vessels are fragile and bleed more easily. Noninvasive, rapid, highly sensitive and specific ultrasound approaches such as duplex ultrasonography or color US-Doppler are the modalities of choice to examine patients for bleeds. Although attention has been predominantly focused on measurement of PV and SV size, dilation of the LGV can also occur as a result of portal hypertension. LGV blood flow direction and velocity within its trunk and branches may affect blood flow to esophageal varices or contribute to their development. To support this idea, previous studies have demonstrated a strong correlation between LGV hepatofugal blood flow velocity and EVB[20-23]. To this end, Wachsberg et al[22] showed that portal hypertension patients with preserved hepatopetal LGV flow were at a lower risk for bleeding. In the present study, the EVB+ group had a significantly higher proportion of patients with hepatofugal LGV blood flow and significantly larger LGV diameters. However, a multivariate logistic regression analysis showed that hepatofugual LGV blood flow and an enlarged LGV diameter were not independent predictive factors for EVB. Rather, the results indicate that both the LGVV and the LGVBFD were independently associated with incidences of EVB.
An ROC analysis demonstrated that the ΔMELD, LGVV, or LGVBFD alone were insufficient in providing diagnostic accuracy for EVB prediction. When the three independent factors were combined to form the MUI, however, a significant diagnostic accuracy was achieved, with a high AUC. Moreover, a MUI of 46 provides high sensitivity, specificity and positive and negative predictive value for the study population. By establishing the above cutoff value, a correct prediction for EVB would have been obtained in 85.8% of patients, without the need for an invasive endoscopy. Thus, the MUI represents a low-cost, convenient and noninvasive index to predict EVB occurrence during a short time-period (three months). The combination of a rapid increase in the MELD score, indicating liver function deterioration, with an increase in hepatofugal LGV blood flow leads to portal hypertension, ultimately causing varices to become fragile and more prone to bleeding.
Although this study demonstrates that a newly developed MUI results in an improved diagnostic accuracy for EVB prediction, there are still some limitations of this study. First, the results suggest that a small percentage of high-risk patients could be improperly diagnosed with EVB, which can have serious consequences for the patient. For example, seven patients in the EVB+ group would have been misdiagnosed. Second, because a majority of the patients were unwilling to undergo frequent screening endoscopies, a side-by-side comparison of the predicative ability of the MUI versus endoscopy was impossible. Third, the current study was a single-center study, and independent external validation in other patient populations is required to validate the predictive ability of the MUI. Fourth, as MELD score is particularly effective in predicting one-year patient survival[24,25], an investigation of the predictive ability for the MUI in an intermediate-term is needed.
In conclusion, the newly developed MUI presented here is a noninvasive, affordable and convenient index to assess EVB risk in decompensated cirrhosis patients. It is a suitable alternative for patients who have declined regular screening via endoscopy. It is currently unclear whether the MUI can reduce the requirement for screening endoscopies, and future prospective studies are warranted to further validate the clinical strength of this index.
A predominant cause of morbidity and mortality in decompensated cirrhosis patients is acute esophageal variceal bleeding (EVB). To determine whether a cirrhotic patient is at risk for EVB, they must undergo screening endoscopies to grade the varices and look for the presence of red signs. However, screening endoscopies are invasive, uncomfortable and expensive to those in developing nations, thus the frequency of endoscopic examination is often quite limited.
The Model for End-stage Liver Disease (MELD) score is widely used in Western countries to evaluate the status of cirrhotic patients, and has been adopted by the United Network of Organ Sharing to prioritize patients awaiting a liver transplant from a deceased donor. Increased MELD scores are indicative of more severe liver disease and may signify the presence of complications such as EVB, which ultimately increase the risk for patient mortality. Analysis of the patient’s liver through an ultrasound approach using duplex ultrasonography and color Doppler is a noninvasive, affordable and simple method to diagnose and stage cirrhotic portal hypertension.
This study demonstrates the use of a new index combining the MELD score and ultrasound-Doppler, the MUI, to predict EVB in a cohort of cirrhotic patients.
The MELD-US-Doppler Index (MUI) is a noninvasive, affordable and convenient index to assess EVB risk in decompensated cirrhosis patients, and is a suitable alternative for patients who have declined regular endoscopic screening. However, it is currently unclear whether the MUI can reduce the requirement for screening endoscopies, and future prospective studies are warranted to further validate the clinical strength of this index.
MELD score is a widely used index to evaluate the status of cirrhotic patients and is based on the following objective parameters: total bilirubin, prothrombin time, international normalized ratio and serum creatinine. Portal hypertension, when portal pressure rises above 12 mmHg, is a feature associated with several disease conditions, though cirrhosis is the most common cause. Portal hypertension can promote the formation of esophageal varices, or enlarged vessels, which can lead to massive gastrointestinal hemorrhage.
The study presented here is a useful and clinically relevant study on a newly developed index, the MUI, to evaluate the risk of esophageal variceal bleeding in cirrhotic patients. The MUI represents a suitable alternative for patients that have declined screening by endoscopy.
P- Reviewer: Liaskou E S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Pagliaro L, D’Amico G, Sörensen TI, Lebrec D, Burroughs AK, Morabito A, Tiné F, Politi F, Traina M. Prevention of first bleeding in cirrhosis. A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 222] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 733] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 5. | Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091. [PubMed] |
| 6. | Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, Bosch J, Stiegmann GV, Henderson JM, de Franchis R. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28:868-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 259] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1864] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 8. | Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, Testa E, Risso D, Colla G, Testa R. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, Meregaglia D, Nicolini A, Lubatti L, Riggio O. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Brandenburger LA, Regenstein FG. Variceal Hemorrhage. Curr Treat Options Gastroenterol. 2002;5:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Seewald S, Seitz U, Yang AM, Soehendra N. Variceal bleeding and portal hypertension: still a therapeutic challenge? Endoscopy. 2001;33:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bhasin DK, Malhi NJ. Variceal bleeding and portal hypertension: much to learn, much to explore. Endoscopy. 2002;34:119-128. [PubMed] |
| 15. | Tsokos M, Türk EE. Esophageal variceal hemorrhage presenting as sudden death in outpatients. Arch Pathol Lab Med. 2002;126:1197-1200. [PubMed] |
| 16. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2067] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 17. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 18. | Huo TI, Wu JC, Lin HC, Lee FY, Hou MC, Lee PC, Chang FY, Lee SD. Evaluation of the increase in model for end-stage liver disease (DeltaMELD) score over time as a prognostic predictor in patients with advanced cirrhosis: risk factor analysis and comparison with initial MELD and Child-Turcotte-Pugh score. J Hepatol. 2005;42:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Bae WK, Lee JS, Kim NH, Kim KA, Moon YS, Oh MK. [Usefulness of DeltaMELD/month for prediction of the mortality in the first episode of variceal bleeding patients with liver cirrhosis: comparison with CTP, MELD score and DeltaCTP/month]. Korean J Hepatol. 2007;13:51-60. [PubMed] |
| 20. | Li FH, Hao J, Xia JG, Li HL, Fang H. Hemodynamic analysis of esophageal varices in patients with liver cirrhosis using color Doppler ultrasound. World J Gastroenterol. 2005;11:4560-4565. [PubMed] |
| 21. | Matsutani S, Furuse J, Ishii H, Mizumoto H, Kimura K, Ohto M. Hemodynamics of the left gastric vein in portal hypertension. Gastroenterology. 1993;105:513-518. [PubMed] |
| 22. | Wachsberg RH, Simmons MZ. Coronary vein diameter and flow direction in patients with portal hypertension: evaluation with duplex sonography and correlation with variceal bleeding. AJR Am J Roentgenol. 1994;162:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Hino S, Kakutani H, Ikeda K, Uchiyama Y, Sumiyama K, Kuramochi A, Kitamura Y, Matsuda K, Arakawa H, Kawamura M. Hemodynamic assessment of the left gastric vein in patients with esophageal varices with color Doppler EUS: factors affecting development of esophageal varices. Gastrointest Endosc. 2002;55:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, Archimandritis AJ. MELD vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol. 2005;11:3099-3104. [PubMed] |
| 25. | Gheorghe L, Popescu I, Iacob R, Iacob S, Gheorghe C. Predictors of death on the waiting list for liver transplantation characterized by a long waiting time. Transpl Int. 2005;18:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |