Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4446
Revised: December 22, 2013
Accepted: January 20, 2014
Published online: April 21, 2014
Processing time: 216 Days and 14.4 Hours
AIM: To characterize the clinical, radiological, endoscopic and pathological features of intestinal tuberculosis (ITB) and primary small intestinal lymphoma (PSIL).
METHODS: This was a retrospective study from February 2005 to October 2012 of patients with a diagnosis of ITB (n = 41) or PSIL (n = 37). All patients with ITB or PSIL underwent computed tomography (CT) and pathological examination. Thirty-five patients with ITB and 32 patients with PSIL underwent endoscopy. These patients were followed for a further 18 mo to ascertain that the diagnosis had not changed. Clinical, endoscopic, CT and pathological features were compared between ITB and PSIL patients.
RESULTS: Night sweating, fever, pulmonary TB and ascites were discovered significantly more often in ITB than in PSIL patients (P < 0.05), however, abdominal mass, hematochezia and intestinal perforation were found significantly more frequently in PSIL than in ITB patients (P < 0.05). Ring-like and rodent-like ulcers occurred significantly more often in ITB than in PSIL patients (P < 0.05), however, enterorrhagia and raised lesions were significantly more frequent in PSIL than in ITB patients (P < 0.05). The rate of granuloma was significantly higher in ITB than in PSIL patients (87.8% vs 13.5%, χ2 = 43.050, P < 0.05), and the incidence of confluent granulomas with caseous necrosis was significantly higher in ITB than in PSIL patients (47.2% vs 0.0%, χ2 = 4.034, P < 0.05). Multi-segmental lesions, mural stratification, mural gas sign, and intestinal stricture were more frequent in ITB than in PSIL patients (P < 0.05), however, a single-layer thickening of bowel wall, single segmental lesions, and intussusception were more common in PSIL than in ITB patients (P < 0.05). Necrotic lymph nodes, comb sign and inflammatory mass were more frequent in ITB than in PSIL patients (P < 0.05). The bowel wall enhancement in ITB patients was greater than that in PSIL patients (P < 0.05), while the thickening and lymph node enlargement in PSIL patients were higher than those in ITB patients (P < 0.05).
CONCLUSION: Combined evaluation of clinical, radiological, endoscopic and pathological features is the key to differentiation between ITB and PSIL.
Core tip: Treatment for intestinal tuberculosis (ITB) differs completely from that for primary small intestinal lymphoma (PSIL). Differentiating ITB from PSIL continues to be a challenge. Combined evaluation of clinical, radiological, endoscopic and pathological features is the key to differentiation between ITB and PSIL. For example, night sweating, ascites, ring-like and rodent-like ulcers, granuloma, multi-segmental lesions, mural stratification, necrotic lymph nodes, comb sign, and inflammatory mass are more suggestive of ITB. However, abdominal mass, hematochezia, enterorrhagia, raised lesions, single-layer thickening of bowel wall, single segmental lesions, and intussusception are more suggestive of PSIL.
- Citation: Zhu QQ, Zhu WR, Wu JT, Chen WX, Wang SA. Comparative study of intestinal tuberculosis and primary small intestinal lymphoma. World J Gastroenterol 2014; 20(15): 4446-4452
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4446.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4446
Intestinal tuberculosis (ITB) is a specific chronic intestinal disease caused by Mycobacterium tuberculosis (M. tuberculosis) infection[1]. In recent decades, with improvement of economics, quality of life, and sanitary conditions, the incidence of TB has declined and the prevalence of ITB has gradually decreased[2]. However, there is still no sensitive, accurate, convenient and specific marker to diagnose ITB. Therefore, clinicians still need to pay much attention to ITB.
The clinical manifestations of primary small intestinal lymphoma (PSIL) are nonspecific, such as abdominal pain, vomiting, weight loss and intestinal perforation[3]. Although the incidence is not high, it is similar to ITB in clinical manifestations and still needs to be distinguished[4].
Many studies have reported that ITB is similar to PSIL with regard to clinical, endoscopic, pathological and computed tomography (CT) features[5,6]. Treatment for ITB is completely different from that for PSIL. The first-line therapy for ITB is the combined anti-TB medication, while the major therapies for PSIL patients include surgery and radiotherapy[7]. It is clear that misdiagnosis between ITB and PSIL leads to severe clinical events, such as M. tuberculosis diffusion and delaying the medical management of PSIL[8]. An accurate diagnosis is important for appropriate treatment. Therefore, the aim of this study was to investigate the clinical, CT, endoscopic and pathological features in 41 cases of ITB and 37 of PSIL.
Upon searching our hospital pathology and image archiving and communications system, we found 41 patients with ITB and 37 with PSIL who were admitted to our hospital from February 2005 to October 2012. All patients with ITB or PSIL underwent CT and pathological examination. Thirty-five patients with ITB and 32 with PSIL underwent upper GI endoscopy.
The diagnosis of ITB complied with the established clinical, CT, histological and microbiological criteria. The diagnosis of PSIL conformed with the 1961 Dawson standards. All patients with ITB or PSIL were followed for a further 18 mo to ascertain that the diagnosis had not changed.
Two gastrointestinal radiologists analyzed the images together, which resulted in a consensus interpretation. Statistical analysis was undertaken using SPSS version 17.0 (SPSS, Chicago, IL, United States). Numerical data are expressed as mean and standard deviation, and categorical data are expressed as percentages. Evaluated characteristics were compared using the χ2 test or independent-samples t test. P < 0.05 was considered statistically significant.
Night sweating, fever, pulmonary TB, and ascites were discovered significantly more often in ITB than in PSIL patients (P < 0.05). However, abdominal mass, hematochezia and intestinal perforation were significantly more frequent in PSIL than in ITB patients (P < 0.05) (Table 1).
| Diarrhea | Ascites | Febrility | Night sweating | Hematochezia | Pulmonary TB | |
| ITB | 12 (29.2) | 22 (53.6) | 23 (56.1) | 25 (60.9) | 3 (7.3) | 26 (63.4) |
| PSIL | 10 (27.0) | 8 (21.6) | 2 (5.4) | 5 (13.5) | 19 (51.3) | 2 (5.4) |
| χ2 value | 0.048 | 8.434 | 22.948 | 18.510 | 18.623 | 28.441 |
| P value | 0.826 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 |
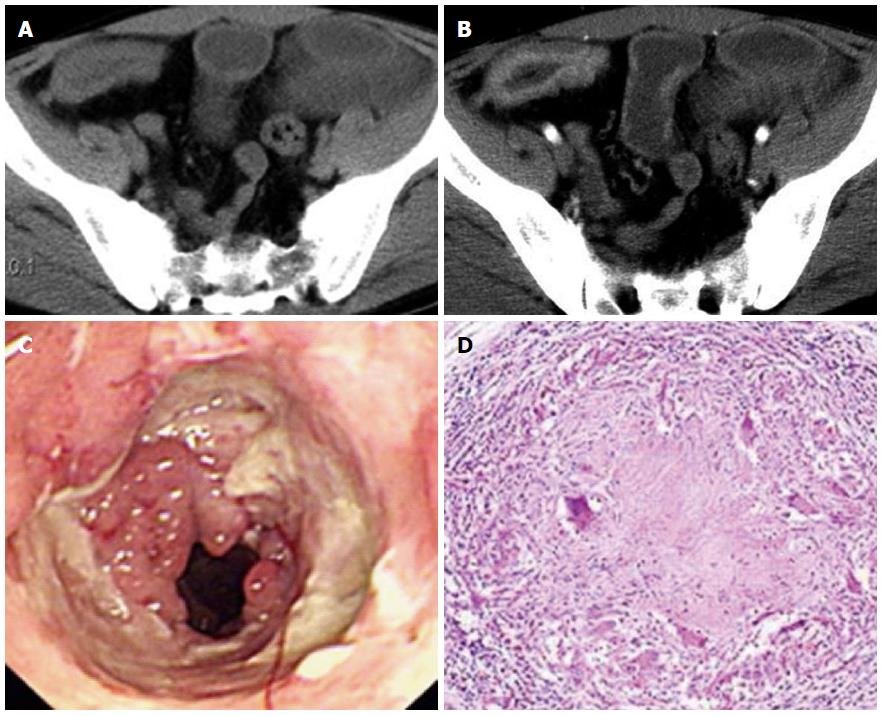
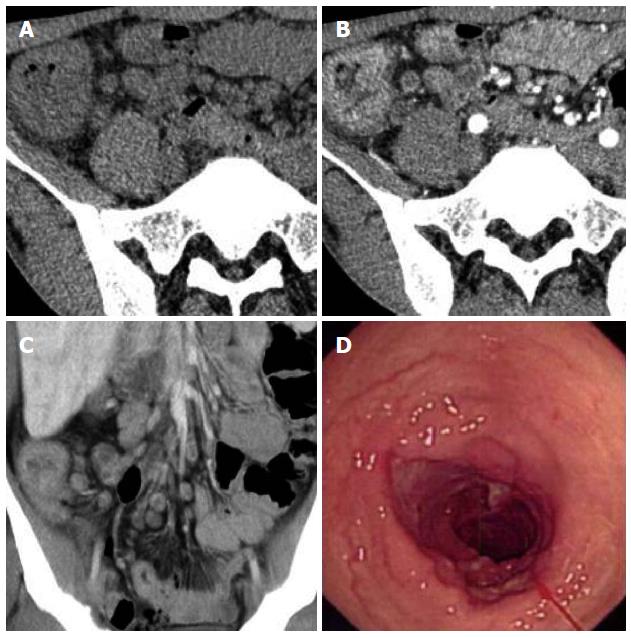
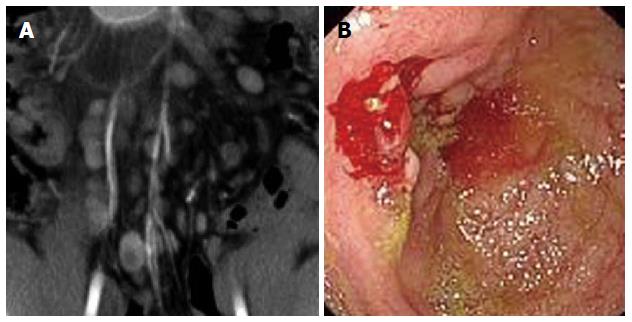
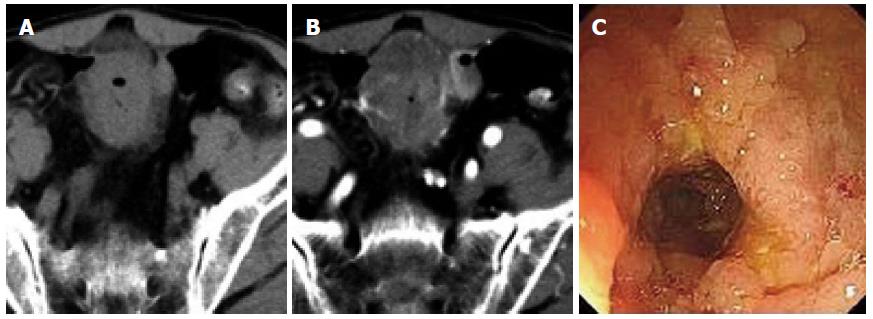
Rodent-like (Figure 1) and ring-like (Figure 2) ulcers were found significantly more often in ITB than in PSIL patients (P < 0.05). However, enterorrhagia (Figure 3) and raised lesions (Figure 4) were found significantly more frequently in PSIL than in ITB patients (P < 0.05). The rate of granuloma was significantly higher in ITB than in PSIL patients (87.8% vs 13.5%, χ2 = 43.050, P < 0.05), and the incidence of confluent granulomas with caseous necrosis was significantly more frequent in ITB than in PSIL patients (47.2% vs 0.0%, χ2 = 4.034, P < 0.05) (Table 2).
| Ring-like ulcer | Rodent-like ulcer | Enterorrhagia | Raised lesions | Stricture | |
| ITB | 13 (37.1) | 12 (34.3) | 3 (8.6) | 0 (0) | 22 (62.8) |
| PSIL | 0 (0) | 0 (0) | 17 (53.1) | 21 (65.6) | 6 (18.7) |
| χ2 value | 14.747 | 13.365 | 15.846 | 33.454 | 13.369 |
| P value | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 |
Multisegmental lesions, mural stratification (Figure 1), mural gas sign, and intestinal stricture (Figure 1) were seen significantly more often in ITB than in PSIL patients (P < 0.05). Single-layer thickening of the bowel wall, single segmental lesions, and intussusception were significantly more frequent in PSIL than in ITB patients (P < 0.05). Necrotic lymph nodes (Figure 2) and comb sign were discovered significantly more often in ITB than in PSIL patients (P < 0.05). Bowel wall enhancement in ITB patients was significantly greater than that in PSIL patients (83.3 ± 7.6 HU vs 55.9 ± 4.2 HU, P < 0.05), while lymph node enlargement (Figure 3) (19.6 ± 3.2 mm vs 9.8 ± 2.7 mm) and bowel thickening (Figure 4) (18.6 ± 3.3 mm vs 11.1 ± 3.7 mm) were more common in PSIL than in ITB patients (P < 0.05) (Tables 3 and 4).
| Mural stratification | Mural single layer | Bowel gas sign | Multi segmental lesions | |
| ITB | 24 (58.5) | 6 (14.6) | 13 (31.7) | 35 (85.4) |
| PSIL | 4 (10.8) | 27 (73.0) | 0 (0) | 8 (21.6) |
| χ2 value | 19.251 | 27.119 | 14.078 | 31.947 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
| Inflammatory mass | Comb sign | Peritoneal abscess | Necrotic lymph nodes | Intussusception | |
| ITB | 5 (12.2) | 26 (63.4) | 3 (7.3) | 19 (46.3) | 0 (0) |
| PSIL | 0 (0) | 4 (10.8) | 1 (2.7) | 2 (5.4) | 21 (56.7) |
| χ2 value | 4.821 | 22.738 | 0.851 | 16.565 | 31.844 |
| P value | 0.0285 | 0.000 | 0.356 | 0.000 | 0.000 |
The differential diagnosis between ITB and PSIL is still a challenge because of the lack of an economic, simple and reliable diagnostic method. Current clinical research demonstrates that ITB and PSIL have marked overlap in clinical, CT and endoscopic features, thus, differentiating between ITB and PSIL can be a major diagnostic challenge, particularly in developing countries where ITB remains common[9]. The misdiagnosis of ITB and PSIL can lead to serious problems in the subsequent treatment of these two conditions[10]. Therefore, it is particularly important to distinguish ITB from PSIL.
In our study, we found that the first symptom of ITB was abdominal discomfort or pain, while that of PSIL tended to be hematochezia or intestinal perforation. This differed from diarrhea as the first symptom of Crohn’s disease (CD). We speculated that ITB lesions were not only inflammatory ulcers[11], but also proliferative lesions, whereas in PSIL, inflammation and ulcers were both involved in intestinal wall thickening and damage[12]. Therefore, when symptoms in patients are complex and lack specificity, the first symptom plays a role in differentiating ITB and PSIL.
Both ITB and PSIL are chronic granulomatous conditions and show an overlap in their histological features. PSIL lesions are located in the ileocecum and more limited than those of ITB[13]. PSIL endoscopic mucosal biopsies are mainly taken from a single lesion, whereas for ITB, there are multiple biopsy sites due to the wide range of lesions. This may have an impact on the efficiency of endoscopic biopsy[14].
Mucosal hallmarks of CD, such as ulcer shape, also contribute to the differential diagnosis between CD and ITB[15]. For example, ring-like and rodent-like ulcers suggest a diagnosis of ITB, while enterorrhagia and raised lesions suggest PSIL. However, longitudinal and grid-like ulcers and cobblestone pattern suggest a diagnosis of CD.
In our study, granuloma detection rate in the ITB and PSIL groups was 87.8% (n = 36) and 13.5% (n = 5), respectively. Among these lesions, the incidence of confluent granulomas with caseous necrosis in the ITB group was 47.2% (n = 17), while that in the CD group was zero. Caseous granuloma remains a specific diagnostic marker for ITB. Therefore, if pathological examination only finds noncaseating granuloma, it is not immediate evidence of PSIL, which requires a combination of other pathological changes[16]. If pathological examination finds both noncaseating granuloma and submucosal lymphocyte aggregation, the patient is more likely to have a diagnosis of PSIL[17].
Abdominal CT has a certain value for the differential diagnosis between ITB and PSIL[18]. These two diseases have their own characteristic distribution of lesions, so it is important to master lesions by perfecting checks for the differential diagnosis of the diseases.
Bowel wall thickness normally measures 1-3 mm in distended small bowel, and generally ranges from 5 to 10 mm in bowel affected by ITB. Wall thickening is the most consistent imaging finding of ITB and has been shown to correlate with the presence and severity of disease[19]. However, wall thickness generally ranges from 15 to 20 mm in bowel affected by PSIL. We noted significant differences in bowel wall thickness in patients with ITB and PSIL (P < 0.05).
Bowel wall enhancement plays an important role in determining disease severity and may be one of the earliest signs of disease[20]. Enhancement can be assessed during several phases based on the timing of the scan relative to contrast injection. The optimal scan time has still not been determined. Peak wall enhancement in normal volunteers was 60-70 s (portal venous phase). However, Zappa et al[21] have found that differentiation is best achieved by the level of enhancement in delayed phase images. In our study, the enhancement was lower in PSIL than in ITB patients in the portal venous phase (P < 0.05).
Increased mesenteric blood flow resulting in vascular engorgement, known as the comb sign, has mostly been reported in active ITB disease[22,23]. There was a significant difference in the comb sign in patients with ITB and PSIL. Mesenteric necrotic lymph nodes on CT scanning are suggestive of ITB, while lymph node enlargement in PSIL was more frequent than in ITB. Lymph node enlargement and the percentage of necrotic mesenteric lymph nodes were greater in ITB than in CD.
In our study, PSIL patients with hematochezia and intestinal perforation were common, however, these manifestations are rare in ITB patients[24,25]. These differences may be due to mild progression of ITB in China, but the exact cause needs to be further studied. Besides, emergency surgery is more common in PSIL patients because of the serious complications[26], whereas medicinal treatment is more common in ITB because complications of ITB are less severe and the disease course is chronic[27]. This phenomenon indicates that complications are more frequent in PSIL than in ITB patients and their progression is faster. This indicates that patients with serious complications and surgical procedures are more likely to have a diagnosis of PSIL[28].
In conclusion, differentiating ITB from PSIL continues to be a challenge. At present, combination of clinical, endoscopic, radiological and pathological features continues to be the key to differentiation between the two conditions. We need to continue to develop new differential diagnostic tests. Our study was limited by the relatively small number of patients with these two diseases. Further research is needed to verify these findings in larger patient populations.
Many studies have reported that intestinal tuberculosis (ITB) is similar to primary small intestinal lymphoma (PSIL) with regard to clinical, endoscopic, pathological and computed tomography (CT) features. Treatment for ITB is completely different from that for PSIL. Misdiagnosis between ITB and PSIL leads to severe clinical events, such as Mycobacterium tuberculosis diffusion and delaying the medical management of PSIL. Accurate diagnosis is important for appropriate treatment. Previously published reports on ITB and PSIL have documented the pathological and clinical features. However, there are only relatively small-sample reports focusing on a comparative study of CT imaging findings.
Combined evaluation of clinical, radiological, endoscopic and pathological features is the key to differentiation between ITB and PSIL.
The authors used a multimodal method to characterize the differences between ITB and PSIL. The results showed that combined evaluation of clinical, radiological, endoscopic and pathological features was the key to differentiation between ITB and PSIL.
The results showed that radiological and endoscopic features were the key to differentiation between ITB and PSIL.
Intestinal tuberculosis (ITB) is a specific chronic intestinal disease caused by Mycobacterium tuberculosis infection. The clinical manifestations of primary small intestinal lymphoma (PSIL) are nonspecific, such as abdominal pain, vomiting, weight loss and intestinal perforation.
The paper has novel information on comparison between ITB and PSIL. This article presents useful information about differential diagnosis of intestinal diseases. The study was well designed and the experimental and statistical methods used are described in detail.
P- Reviewers: Bugaj AM, Benjamin P, Ray G, Subhada PP S- Editor: Song XX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Pulimood AB, Peter S, Ramakrishna B, Chacko A, Jeyamani R, Jeyaseelan L, Kurian G. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn’s disease. J Gastroenterol Hepatol. 2005;20:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Oostenbrug LE, van Dullemen HM, te Meerman GJ, Jansen PL, Kleibeuker JH. Clinical outcome of Crohn’s disease according to the Vienna classification: disease location is a useful predictor of disease course. Eur J Gastroenterol Hepatol. 2006;18:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 4. | Epstein D, Watermeyer G, Kirsch R. Review article: the diagnosis and management of Crohn’s disease in populations with high-risk rates for tuberculosis. Aliment Pharmacol Ther. 2007;25:1373-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Jayanthi V, Robinson RJ, Malathi S, Rani B, Balambal R, Chari S, Taghuram K, Madanagopalan N, Mayberry JF. Does Crohn’s disease need differentiation from tuberculosis? J Gastroenterol Hepatol. 1996;11:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 7. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1506] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 8. | Marra F, Cox VC, FitzGerald JM, Moadebi S, Elwood RK. Successful treatment of multidrug-resistant tuberculosis following drug-induced hepatic necrosis requiring liver transplant. Int J Tuberc Lung Dis. 2004;8:905-909. [PubMed] |
| 9. | Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Bruining DH, Loftus EV, Ehman EC, Siddiki HA, Nguyen DL, Fidler JL, Huprich JE, Mandrekar JN, Harmsen WS, Sandborn WJ. Computed tomography enterography detects intestinal wall changes and effects of treatment in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:679-683.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Choi D, Jin Lee S, Ah Cho Y, Lim HK, Hoon Kim S, Jae Lee W, Hoon Lim J, Park H, Rae Lee Y. Bowel wall thickening in patients with Crohn’s disease: CT patterns and correlation with inflammatory activity. Clin Radiol. 2003;58:68-74. [PubMed] |
| 12. | Baker ME, Walter J, Obuchowski NA, Achkar JP, Einstein D, Veniero JC, Vogel J, Stocchi L. Mural attenuation in normal small bowel and active inflammatory Crohn’s disease on CT enterography: location, absolute attenuation, relative attenuation, and the effect of wall thickness. AJR Am J Roentgenol. 2009;192:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, Fries W, Balzarini L, Montorsi M, Malesci A. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Knollmann FD, Dietrich T, Bleckmann T, Böck J, Mäurer J, Radtke C, Felix R. Magnetic resonance imaging of inflammatory bowel disease: evaluation in a rabbit model. J Magn Reson Imaging. 2002;15:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Allen BC, Baker ME, Einstein DM, Remer EM, Herts BR, Achkar JP, Davros WJ, Novak E, Obuchowski NA. Effect of altering automatic exposure control settings and quality reference mAs on radiation dose, image quality, and diagnostic efficacy in MDCT enterography of active inflammatory Crohn’s disease. AJR Am J Roentgenol. 2010;195:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | De Backer AI, Mortelé KJ, Deeren D, Vanschoubroeck IJ, De Keulenaer BL. Abdominal tuberculous lymphadenopathy: MRI features. Eur Radiol. 2005;15:2104-2109. [PubMed] |
| 18. | Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227-7236. [PubMed] |
| 19. | Mackalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733-741. [PubMed] |
| 20. | Kam KM, Yip CW. Surveillance of Mycobacterium tuberculosis susceptibility to second-line drugs in Hong Kong, 1995-2002, after the implementation of DOTS-plus. Int J Tuberc Lung Dis. 2004;8:760-766. [PubMed] |
| 21. | Zappa M, Stefanescu C, Cazals-Hatem D, Bretagnol F, Deschamps L, Attar A, Larroque B, Tréton X, Panis Y, Vilgrain V. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17:984-993. [PubMed] |
| 22. | Wong WM, Lai KC, Yiu WC, Wong BC, Chan FL, Lai CL. Intestinal tuberculosis mimicking fistulizing Crohn’s disease. J Gastroenterol Hepatol. 2007;22:137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Kataoka H, Mizuno K, Hayashi N, Tanaka M, Nishiwaki H, Ebi M, Mizoshita T, Mori Y, Kubota E, Tanida S. Diagnostic utility of small-caliber and conventional endoscopes for gastric cancer and analysis of endoscopic false-negative gastric cancers. World J Gastrointest Endosc. 2013;5:440-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Xu W, Liu Y, Lu Z, Jin ZD, Hu YH, Yu JG, Li ZS. A new endoscopic ultrasonography image processing method to evaluate the prognosis for pancreatic cancer treated with interstitial brachytherapy. World J Gastroenterol. 2013;19:6479-6484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Winther KV, Føgh P, Thomsen OØ, Brynskov J. Inflammatory bowel disease (ulcerative colitis and Crohn’s disease): diagnostic criteria and differential diagnosis. Drugs Today (Barc). 1998;34:935-942. [PubMed] |
| 26. | Zhu QQ, Wu JT, Chen WX, Wang SA, Zheng J. [Differential diagnosis of intestinal tuberculosis and primary small intestinal lymphoma using endoscopy and computerized tomography]. Zhonghua Weichang Waike Zazhi. 2012;15:1247-1251. [PubMed] |
| 27. | Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn’s disease: a diagnostic challenge. Am J Gastroenterol. 2009;104:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Simpson P, Papadakis KA. Endoscopic evaluation of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |