Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3180
Revised: December 10, 2013
Accepted: January 19, 2014
Published online: March 28, 2014
Processing time: 188 Days and 21.6 Hours
There is evidence that inflammatory bowel diseases (IBD) combine both inflammation and coagulation in their pathogenesis and clinical manifestations. Although platelets (PLT) are well known for their role in hemostasis, there are a rising number of studies supporting their considerable role as inflammatory amplifiers in chronic inflammatory conditions. IBD are associated with several alterations of PLT, including number, shape, and function, and these abnormalities are mainly attributed to the highly activated state of circulating PLT in IBD patients. When PLT activate, they increase in size, release a great variety of bio-active inflammatory and procoagulant molecules/particles, and express a variety of inflammatory receptors. These inflammatory products may represent a part of the missing link between coagulation and inflammation, and can be considered as possible IBD pathogenesis instigators. In clinical practice, thrombocytosis is associated both with disease activity and iron deficiency anemia. Controlling inflammation and iron replacement in anemic patients usually leads to a normalization of PLT count. The aim of this review is to update the role of PLT in IBD and present recent data revealing the possible therapeutic implications of anti-PLT agents in future IBD remedies.
Core tip: Many platelets (PLT) changes have been described in IBD, including morphological alterations (mean PLT volume, PLT distribution width, plateletcrit, and augmented granular content), count increase, microparticles release, over-excretion of granular content, and increased formation of PLT-PLT and PLT-leukocyte aggregates, which are all linked to PLT activation induced by inflammatory agonists. In this review article, we present the multipotent role of PLT in human biological paths and emphasize on how PLT participate in the chronic intestinal inflammation process in IBD.
- Citation: Voudoukis E, Karmiris K, Koutroubakis IE. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. World J Gastroenterol 2014; 20(12): 3180-3190
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3180.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3180
Inflammatory bowel diseases (IBD), namely Crohn’s disease (CD) and ulcerative colitis (UC), are disorders that primarily affect the gastrointestinal tract. The immune system, with its active components, dominates IBD pathogenesis, but many genetic and environmental factors have been also implicated. A growing number of studies are highlighting the importance of non-immune cells like endothelial, mesenchymal, and nerve cells, as well as platelets (PLT), as key players in the IBD inflammatory cascade[1].
PLT dysfunction is considered as participating in IBD pathogenesis, although the existing evidence is rather weak. On the other hand, there is solid evidence supporting PLT having functions of potent proinflammatory cells in addition to their role in hemostasis. Several studies have shown that PLT constitute a crucial link between inflammation and coagulation in both UC and CD, creating a vicious circle in which participating parameters conserve and propagate each other[2].
Many PLT changes have been described in IBD, including morphological alterations [mean PLT volume (MPV), PLT distribution width (PDW), plateletcrit (PCT), augmented granular content], count increase, microparticles (MPs) release, over excretion of granular content, and increased formation of PLT-PLT and PLT-leukocyte aggregates (PLA), which are all linked to PLT activation induced by inflammatory agonists (Table 1). In the following sections, we will present the multipotent role of PLT in human biological paths and emphasize how PLT participate in the chronic intestinal inflammation process in IBD.
| Number and morphological changes | Loss of discoid shape |
| Acquisition of pseudopodia | |
| Size increase | |
| Count increase (reactive thrombocytosis) | |
| Density increase | |
| Granular content augmentation | |
| MPV value decrease | |
| PDW value increase | |
| PCT value increase | |
| Other abnormalities | |
| Overproduction and excretion of granular content products | P-selectin, β-TG, PF-4, fibrinogen, vWF, fibrinolytic inhibitors, coagulation, angiogenic and mitogenic factors |
| Increased incorporation of receptors in PLT membrane | CD40, P-selectin, GP53, GP IIb/IIIa, receptors for chemokines, cytokines and complement components |
| Overproduction of PLT-derived microparticles | |
| PLT-PLT aggregates formation | |
| Increased PLT-leukocytes formation |
PLT are small anuclear fragments (1-6 μm) derived from bone marrow megakaryocytes, with a 5-9 d lifespan in humans. Their primary role is hemostatic; surveying endothelial barrier consistence and interfering when vessel integrity is threatened[3]. A significant decrease of PLT (< 20000/mm3) in septic models resulted in the disruption of the endothelial barrier in clinical studies[4]. Collagen from the exposed subendothelial layer at the injured vessel site binds to plasma von Willebrand factor and recruits circulating PLT to form a glycoprotein (GP) Ib-IX-V complex. PLT adhesion to the site of injury initiates a cascade of signaling transduction through GP VI and integrin family surface receptors. PLT become activated and transform into high affinity platforms which are suitable for participating in inflammatory reactions, ligand binding, and clot formation promotion[5]. In addition, PLT participate in wound repair and tissue regeneration by interacting with components of extracellular matrix and endothelium[6,7].
It has been demonstrated that PLT present innate immunological properties. They express Toll-like receptors which can bind to lipopolysaccharides on the outer membrane of gram(-) bacteria[8]. In vitro and in vivo studies have also demonstrated that PLT can internalize pathogens resistant to clearance such as Staphylococcus aureus or HIV virus, promoting further PLT activation changes[9]. Moreover, PLT stimulate the formation of extracellular DNA nets by neutrophils that trap and kill gram(-) microbes, via the lipopolysaccharides - Toll-like receptor 4 interaction in septic models[10,11].
PLT can also act as mediators between innate and adaptive immune systems. When activated at inflammatory sites, they excrete large amounts of pro-inflammatory substances located in their intracellular granules[12], by which they crosstalk, recruit, and activate leukocytes, endothelial, and immune-like cells even at distant sites. A typical example of the remote PLT actions is the ability of PLT-derived CD40 ligand (CD40L) to activate dendritic cells in the injured tissue[13] and to stimulate immunoglobulin production by B-cell compartment[14].
PLT ability to interact with a large variety of cells is also implicated in the generation of vascular inflammation. Endothelium dysfunction triggers PLT activation processes and possibly renders PLT as the first in line to initiate atherosclerotic immune responses. Therefore production and release of PLT highly inflammatory cargo at the injured vessel wall induces and propagates the recruitment of leukocytes and the further construction of atherosclerotic lesions.
Elevation in PLT count (> 450000 × 109/L), defined as reactive thrombocytosis (RT), may frequently occur in certain conditions like hypo- or asplenism, blood loss, acute or chronic inflammatory disorders, malignancies, and iron deficiency. The first study reporting IBD RT in 1968 by Morowitz et al[15] noted markedly-elevated concentration of circulating PLT during a period of increased clinical activity in a case series of IBD patients. This effect is the result of aberrant bone marrow thrombopoiesis under the influence of inflammatory mediators and the aftermath of reduced PLT lifespan due to accelerated activation and consumption of thrombocytes at the sites of inflammation.
Thrombopoiesis is mainly regulated by plasma thrombopoietin (TPO). Plasma TPO binds to C-Mpl receptors on the PLT surface, and the remaining fraction promotes thrombopoiesis by binding to the same receptors on progenitor megakaryocytes in bone marrow. Thus, in normal conditions thrombopoiesis is controlled by a negative feedback mechanism based on PLT mass in blood[16,17]. Cytokines and other inflammatory agents, especially interleukin 6 (IL-6), promote hepatic TPO production[18], which is considered an acute phase reactant[19]. Heits et al[20] have shown that IBD patients with thrombocytosis have elevated plasma TPO and IL-6 levels. However, the existing data are vague, as other studies display a lack of correlation between PLT number and TPO concentration, indicating other possible regulating factors in IBD RT[21]. Although PLT count is correlated to IBD disease activity[22], it is not considered an independent risk factor for the increased risk of thromboembolic (TE) events observed in IBD patients as it is for cancer[23]. Properly designed and adequately powered clinical studies evaluating predictive laboratory indices for TE events in IBD are still lacking.
Moreover, some conflicting data have emerged over the last decade about the role of preoperative RT in the occurrence of chronic pouchitis in patients undergoing ileal pouch-anal anastomosis. Two studies from the Surgery Department Division of Colon and Rectal Surgery in California have pointed out that the presence of elevated PLT count before surgery was associated with an increased risk for chronic pouchitis postoperatively[24,25]; a severe complication that can result in the removal or diversion of the pouch. In discontinuity with these studies, Lian et al[26] failed to predict the occurrence of inflammatory pouch disorders based on pre-colectomy laboratory tests, including PLT count. Larger prospectively well-designed series with patients requiring ileal pouch-anal anastomosis are needed in order to verify possible implication of PLT in this subject.
Chronic inflammatory disorders are connected to several morphological changes in PLT indices calculated in whole blood count, such as MPV, PDW, and PCT. The most widely-studied PLT parameter in humans is MPV. PLT volume decreases when an inflammatory process is present, which is mainly attributed to thrombopoiesis abnormalities and increased PLT consumption. Inflammatory mediators stimulate bone marrow precursors to enhance PLT generation at the cost of maturation time, delivering smaller PLT in circulation, while at the same time larger and more active PLT are consumed at inflammatory sites, as is proposed in the intestinal microvasculature of IBD patients[27].
MPV changes are correlated to inflammatory disorders like myocardial infarction, stroke, diabetes mellitus, acute appendicitis, rheumatoid arthritis, chronic hepatitis B, celiac disease[28-31], paroxysmal atrial fibrillation, obesity[32], amyloidosis[33], and retinal vein occlusion[34]. Moreover, MPV could serve as a reliable predictor of high risk patients for portal venous thromboembolism[35], acute coronary syndromes[36], and stroke in patients with atrial fibrillation. MPV has also been proposed as a useful biomarker for early gastric, pancreatic, and hepatocellular carcinoma diagnosis[37], dietary compliance to celiac disease and exacerbation of chronic obstructive pulmonary disease[38].
In IBD patient studies a MPV value decrease has long been observed[39] which has been inversely correlated with endoscopic and disease activity indices, such as C-reactive protein and erythrocyte sedimentation rate[40-44]. This MPV reduction can be attributed to the decreased circulating reticulated PLT number that was found in patients with active UC compared to inactive and healthy control subjects[44]. In line, studies have reported an inverse relationship between extent of intestinal inflammation and MPV in IBD patients[40,41]. Öztürk et al[45] suggested that all PLT parameters (PDW, PCT, MPV) can prove to be useful surrogate markers for IBD follow-up, as they reveal strong relationship with activity indices. We observed that MPV, PCT, and PDW were correlated with certain iron deficiency markers (soluble transferrin receptors, hemoglobin) but not with activity indices such as C-reactive protein, Crohn’s disease activity index score, or simple clinical colitis activity index score in IBD patients. This observation reflects a possible role of iron capacity as a regulator of megakaryopoiesis and PLT morphology[46]. Literature reports about MPV correlations with clinical and laboratory parameters in IBD patients are presented in Table 2.
| Ref. | Disease (n) | HC (n) | MPV correlations |
| Yüksel et al[41] | UC (61) | 27 | Reduced MPV in UC compared to HC |
| Inverse correlation between MPV and disease activity | |||
| Inverse correlation between MPV and disease extent | |||
| Järemo et al[39] | UC (18), | 12 | Reduced MPV in UC patients compared to HC |
| CD (9) | Inverse correlation between MPV and disease activity | ||
| Güçlü et al[42] | UC (41) | (-) | Reduced MPV in active compared to non-active disease |
| Voudoukis et al[46] | UC (91), CD(107) | 102 | Reduced MPV in IBD patients compared to HC Correlation of MPV with Hb and sTfR |
| Öztürk et al[45] | UC (103), CD (72) | 40 | Reduced MPV in IBD compared to HC |
| MPV decreases after remission in UC | |||
| MPV increases after remission in CD | |||
| Kapsoritakis et al[40] | UC (93), CD (66) | 38 | Correlation between MPV and disease activity indices |
| Correlation between MPV and disease extent | |||
| Kayahan et al[44] | UC (37) | 20 | Correlation between MPV and disease activity indices |
| Reduced MPV in UC compared to HC | |||
| Liu et al[43] | CD (61) | 50 | Reduced MPV in CD patients compared to HC |
| MPV value did not correlate to disease activity |
Anemia is the most frequent extra-intestinal manifestation of IBD, affecting approximately one third of patients[47,48]. The most prevailing type of IBD-associated anemia is iron deficiency anemia (IDA)[48,49]. Iron deficiency is related both with up-and downregulation in PLT count, with RT reported more frequently[50].
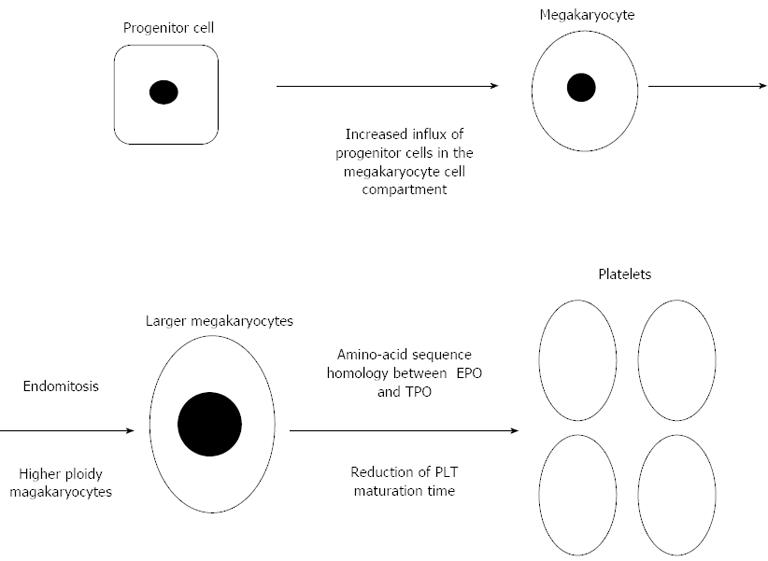
Several mechanisms related to iron deficiency have been implicated in PLT overproduction. Iron scarcity could trigger an increase influx of progenitor cells to the megakaryocyte cell compartment, a diminution of PLT maturation time[51], and the generation of high ploidy megakaryocytes. Megakaryocytes can proliferate through a procedure called endomitosis, and augment DNA ploidy and cytoplasmic volume and further abandon mitosis before cytokinesis take place[52]. Iron deficiency may lead to the production of larger polyploid megakaryocytes capable of generating numerically more PLT, as it is observed in an iron deficient rat model[53]. Moreover, striking amino-acid sequence homology between erythropoietin (key hormone controlling erythropoiesis) and TPO, both being members of the same hematopoietic growth factor subfamily, could be a tempting explanation for the thrombocytosis observed in children with IDA[54] (Figure 1). This assumption, however, is in discordance with the study by Kulnigg-Dabsch et al[55] that didn’t observe any alteration in PLT production with the concomitant use of erythropoietin combined to iron replacement therapy in IBD patients with RT.
A special interest in IDA associated RT has arisen over the last few years in IBD[55,56]. In a Kulnigg-Dabsch et al[55] study, iron replacement was associated with dose-dependent normalization of PLT count which remained within normal range after therapy, highlighting a regulatory rather than a toxic effect of iron on PLT. Patients presented with mildly elevated or within-normal range inflammatory indices at baseline and during treatment, demonstrating that RT could be mainly attributed to IDA rather than systemic inflammatory response[55]. In another study, iron replacement was not only associated with PLT count decrease, but also to a significant decrease in PLT activation markers, such as P-selectin and PLT-aggregation, suggesting that iron management may express anti-thromboembolic properties in IBD patients with increased risk for TE events. However, the small number of participants and the need for study protocol modification during the active phase does not allow us to make safe conclusions[56].
Studies have also identified a correlation between PLT count, red blood cell parameters, and anemic indices in otherwise healthy IDA patients[57,58]. In a recent study we observed a mutual relationship between PLT count and iron deficiency parameters. Inflammatory indices (C-reactive protein, Crohn’s disease activity index score, and simple clinical colitis activity index score) and iron deficiency markers (ferritin, soluble transferrin receptors, and index) were correlated to PLT count in 198 consecutive IBD patients, indicating that RT is probably a multifactorial event in which iron deficiency and inflammation hold a major role. Moreover, taking into account the low inflammatory indices in our patients’ cohort, we assumed that iron deficiency could be the main factor affecting PLT count in IBD[46].
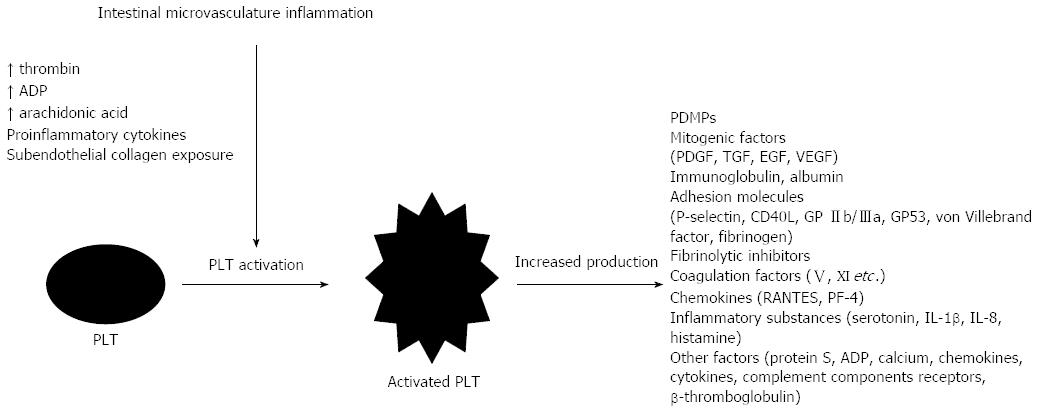
PLT circulate at a highly-activated state in IBD, as it is demonstrated by an increased concentration of circulating PLT activation markers in the systemic circulation of patients[59]. This activation possibly takes place in the mesenteric microcirculation, where PLT are exposed to several inflammatory mediators[60]. Molecules in the site of injury like subendothelial collagen, cytokines from activated leukocytes, and endothelial cells, increased local adenosine diphosphate (ADP) concentration due to reduced capillary blood flow, substances released from neighboring cells, arachidonic acid, PLT activating factor (PAF), and thrombin generation augment PLT accumulation and activation in the intestinal microvasculature in IBD[2]. During activation, PLT lose their normal discoid shape, obtain projecting forms called pseudopodia, release an increased amount of microparticles (PDMPs), and grow in size and density. Numerous metabolic reactions happen within their cytoplasm, where various inflammatory mediators are being produced[1,39]. Proteomic studies have identified more than 300 proteins accumulated in granules of activated PLT[61]. PLT granules are rich in PLT factor-4, β-thromboglobulin, fibrinogen, von Willebrand factor, fibrinolytic inhibitors, coagulation V and XI factors, protein S, angiogenic and mitogenic factors (PLT-derived growth factor, transforming growth factor, endothelial growth factor, and vascular endothelial growth factor), immunoglobulins, membrane ligand proteins (P-selectin), ADP, serotonin, IL-1β, chemokines, RANTES, IL-8, and various other substances[12]. Certain PLT granular products, such as P-selectin, GP IIb/IIIa, CD40L, and GP53, are incorporated into the cytoplasmic membrane, giving them a more adhesive and interacting phenotype. Moreover, PLT during activation develop receptors for chemokines, cytokines, and complement components, enabling them to participate in various inflammatory cascades in IBD[1] (Figure 2). Molecules released from the activated PLT induce an inflammatory phenotype in endothelial cells and leukocytes. Polymorphonuclear cells enhance their superoxide, PAF, and leukotriene production, and endothelial cells stimulated by certain PLT factors (PAF, histamine, and RANTES) increase vascular permeability[2]. CD40L(+) PLT of IBD patients induce I- and V-cell adhesion molecules (CAM) and IL-8 overexpression when co-cultured with human intestinal microvascular endothelial cells in an experimental colitis model[62].
P selectin is a member of the CAMs family mainly produced in PLT. A soluble fraction of P-selectin is also detected in patients with inflammatory disorders, including IBD, and possibly serves as selectin binding inhibitor[63]. The lectin containing N-terminal domain of P-selectin binds to P-selectin glycoprotein ligand (PSGL-1) found in leukocytes (mainly polymorphonuclears) mediating recruitment and rolling of infiltrating leukocytes in the gut mucosa, and initiating activation processes like chemokines production by monocytes and CD4(+) T-cells, as well as superoxide overexcretion by neutrophils[1]. P-selectin ligation to PSGL-1 also serves in PLT-PLT aggregation and PLA formation[2], induces tissue factor (TF) generation, and stimulates the release of PDMPs bearing TF by leukocytes[64]. The above mentioned findings highlight the significant role of P-selectin in IBD pathogenesis.
CD40L (CD 154) is a protein, strongly related to tumor necrosis factor (TNF) and expressed on the surface of activated PLT and immune system cells. CD40L has the ability to bind CD40 located on the surface of most immune, endothelial, and other mesenchymal cells[65]. There are three CD40 family members encountered in humans: CD40, CD40L, and the soluble form of CD40L (sCD40L) derived by enzymatic fragmentation of CD40L in serum[66]. The latter is believed to be produced and released only by activated PLT in IBD patients[67]. Increased levels of CD40L(+) PLT and sCD40L are demonstrated in disorders combining inflammation and thrombosis, such as unstable angina, myocardial infarction[68], and IBD[67,69].
CD40L interactions have a significant role in immune mediated activation of inflammation and thrombosis. They induce TF expression by endothelial cells and monocytes[65]. SCD40L is able to bind onto GPIIb/IIIa to promote arterial thrombosis stabilization, as was demonstrated in CD40L deficient mice[70]. Pro-inflammatory responses of CD40L/CD40 result in chemokines, ILs, and CAMs (V-CAMs, I-CAMs, P/E-Selectin) upregulation in PLT and other immune cells[65]. CD40L can stimulate PAF production, thus inducing PLT activation, propagating immune mediated angiogenesis in IBD in both human and murine models[71], and provoking cytokine overexcretion by human intestinal microvascular endothelial cells such as IL-8, which constitutes a major neutrophil chemoattractant[67]. Finally, PLT CD40L (+)-derived vesicles seem to display an immunoregulatory role by activating peripheral blood B-cells in producing immunoglobulins when co-cultured with them in vitro[72] and stimulating antigen specific IgG production by germinal center modulation in the B-cell compartment[14].
CD40L is essential in activating components of the immune system in IBD. The infiltration of neutrophils in the colonic mucosa of UC patients and macrophage chemoattraction in granulomatous lesions in CD has been found to be mediated mainly by CD40/CD40L interactions[1]. CD40(+) immune fluorescence staining was observed only at inflamed intestinal sites and not at intact mucosal segments in intestinal endoscopic biopsies from IBD patients[73]. A positive correlation between sCD40L and the extent of anatomical involvement in IBD was also found[67]. Finally, CD40 deficient mice experienced significantly milder dextran sodium sulfate (DSS) colitis than wild type littermates[74].
PLT spontaneous aggregation is a unique feature found in the blood of IBD patients that is not encountered in other inflammatory conditions[59]. Aggregation is believed to be primarily accomplished in the mesenteric microcirculation where PLT come into close contact with increased inflammatory mediators[60]. PLT aggregates are independent of disease activity, as their existence has been noted in colonic biopsies of IBD patients in remission, but not in healthy controls[75].
PLT aggregation in IBD seems to represent the initial response of PLT leading to an increased risk for TE. The reported prevalence of TE events (arterial or venous thrombosis) in IBD is between 1.3% and 6.0%, with a 1.5-3.6 fold increased risk compared to the general population and other inflammatory disorders[76,77]. The development of TE in IBD seems to be multifactorial, with interaction of genetic and acquired factors (e.g., inflammation, hospitalization, and operations). TE events in IBD indicate a higher predilection towards younger age compared to non-IBD subjects[2]. Thromboembolism is considered a negative prognostic outcome and represents one of the four leading causes of death in these patients. Thrombosis may correlate with disease activity, but it is interesting to note that one third of the events happen during clinical remission, indicating a continuous activate state of PLT and coagulation systems in IBD[78,79].
Moreover, the increased concentration of PLA in circulation[80] is also considered as an aftermath of leukocyte sequestration in mesenteric circulation, where they bind to activated PLT[81]. This interaction is mainly guided by PLT(+) P-selectin ligation to leukocytes PSGL-1. After this initial step, further ligation of PLT GPIIb/IIIa to MAC-1 leukocyte membrane receptors, with fibrinogen serving as the bridging connector, intensifies binding and promotes PLA formation. PLA are major inflammatory agent carriers, more active than circulating leukocytes or activated PLT alone[82,83] and exhibiting an enhanced ability to adhere to mucosal endothelium[82]. Increased PLA formation is noted in many chronic inflammatory disorders like diabetes mellitus, cardiovascular and collagenous tissue diseases, asthma, systemic lupus, and rheumatoid arthritis[84]. Therefore, PLA is indexed as a sensitive marker of inflammation and PLT activation though not in consistency with IBD activity in recent studies[82].
Eukaryotic cells are capable of budding small vesicles like exosomes (endosomal products), apoptotic bodies (byproducts of cell death), and MPs. Circulating MPs are a heterogeneous mixture of cellular membrane fragments that are derived from a great variety of cells, and recapitulate the functions of their cellular origin. They influence a diverse series of physiological and pathological functions, as they can transfer genetic material (m-RNA, micro-RNA, DNA), membrane receptors, and a series of parental molecules to target cells[85]. MP formation is a well regulated process consisting of local concentration changes in specific intracellular molecules, cytoskeleton disruption, and phosphatidylserine inversion in the outer membrane layer of ancestral cells[86].
Although MPs are detected in low concentrations in health, a great variety of cardiovascular diseases, inflammatory disorders, cancer, and diabetes are associated with increased MP production. They are considered major procoagulant factors, due to TF and phosphatidylserine exposure on their membrane[87]. PDMPs represent the most abundant MP population in humans, approximately 70%-90% of cell-derived MPs[88]. Among them a large amount of PDMPs originate from megakaryocytes[89]. PDMP production is enhanced in vitro by PLT agonists like Ca2+, thrombin, ADP, collagen, fibrinogen, and high shear stress, confirming the statement that PDMPs are mainly derived by activated PLT[86].
PDMPs are increased in autoimmune disorders such as mixed connective tissue disease, systemic sclerosis, primary Sjögren’s syndrome, systematic lupus erythematosus, rheumatoid arthritis, Raynaud’s phenomenon, and psoriasis[87,90-92], as well as in cardiovascular diseases such as atherosclerosis, acute coronary syndrome, pulmonary embolism, and pulmonary arterial hypertension[93-96]. Moreover, they can be used as antithrombotic indicators and side-effect markers following blood transfusion[97,98].
Few studies have been conducted in IBD patients. Andoh et al[99] showed increased PDMPs in active IBD patients compared to inactive ones and healthy controls. PDMPs correlated with clinical disease activity indices and PLT activity markers, and significantly reduced after remission achievement. However, this study included a small sample size for exporting safe conclusions and PDMPs were measured using ELISA and not flow cytometry, the latter being considered a more reliable method. Chamouard et al[100] demonstrated that infliximab therapy induced a significant decrease in circulating MPs, mainly of PLT origin, in CD but not in UC, implicating that PDMPs shedding is important in the IBD inflammatory response. Finally, Palkovits et al[101] noted that TF(+) MP and especially TF(+) PDMPs were significantly increased in IBD patients compared to healthy controls, although they didn’t correlate with markers of coagulation activity and inflammation. These results indicate that PDMPs may have an important role in IBD. Taking into account the high procoagulant and proinflammatory predisposition of PDMPs, they can be useful targets, or even vectors, of future IBD therapies.
Anti-PLT therapy is unanimously certified as evidence-based primary and secondary prevention therapy in high risk cardiovascular patients resulting in reduced mortality rates[102]. Based on existing evidence, one can assume that PLT could be an ambitious target cell for IBD therapies, as it represents the critical crossroad between inflammation and coagulation.
Clopidogrel is a potent suppressor of PLT activation, PLA formation and production of PLT activation markers such as P-selectin[103-105]. Clopidogrel significantly inhibited PLT inflammatory markers and resolved IBD symptoms in rats after a single intra-colonic administration of trinitrobenzenesulfonic acid and oxazolone[106]. Moreover, salicylic compounds like 5-aminosalicylic acid regimens, which are broadly used in IBD, significantly reduced PLT activation markers in IBD patients[107]. However, the use of aspirin even in a low dose in IBD is still uncertain, as it is associated with exacerbation symptoms and should be offered in patients with a strong indication for it[108]. Larger randomized controlled studies evaluating its systematic anti-inflammatory effect in IBD are needed in order to verify possible benefits.
Azathioprine and 6-mercaptopurine are reported to inhibit collagen, ADP, and arachidonic acid-dependent PLT aggregation, as well as PLA aggregate formation[109]. GPIIb/IIIa antagonists (eptifibatide, abciximab, and tirofiban) have been shown to be more competent in sCD40L down regulation compared to aspirin in high risk cardiovascular patients, an observation that might be proved useful in IBD[110]. Moreover, infliximab therapy induced significant disruption of CD40/CD40L dependent cognate interactions[111] and reduced circulated MPs[100] in CD patients, suggesting a potent drug effect on TNF, CD40L, and MPs production in IBD.
Other studies evaluating anti-PLT activation marker products in experimental colitis have also been conducted. CD40/CD40L pathway inhibitor (Trapidil) administration resulted in a significant reduction of colonic inflammation in wild type murine DSS induced colitis[74]. Moreover, CD40L deficient mice exhibited a reduced thrombotic response that was restored after sCD40L administration, highlighting the possible anticoagulant effect of anti-CD40L drugs in IBD where the risk for TE events is increased[112]. Finally, P-selectin deficient mice or P-selectin, PSGL-1 blocking antibody utilization induced significantly decreased PLT recruitment in a DSS colitis mouse model[113].
In conclusion, there an increasing data suggesting that PLT are important key regulators in inflammatory disorders beyond hemostasis and thrombosis. Inflammation, wound repair, angiogenesis, atherosclerosis, and tumor metastasis are only some examples that reveal PLT multifactorial role. In IBD pathogenesis, PLT activation could be the missing link between inflammation and coagulation, two “independent” processes linked in such a way that each one activates and propagates the other.
Thrombocytosis has been associated with IBD manifestations such as disease activity, iron deficiency anemia, and development of pouchitis, whereas PLT parameters (PDW, PCT, and MPV) have been suggested as surrogate markers for IBD. PLT count increase cannot be attributed only to inflammation, as we believe that iron deficiency should be considered a major governor of thrombopoiesis. Until now, no study was designed in such a way as to discriminate to what extent inflammation and iron deficiencies are responsible for PLT increase. However, particular interest should be given to iron replacement in IBD patients, and especially those with thrombocytosis and low inflammatory indices or/and low hematocrit. The possible association between iron replacement therapy and reduction of PLT activation markers raises new questions regarding the involvement of iron scarcity in the increased incidence of TE events in IBD patients, although the data are as yet inconclusive. Additionally, PLT parameters seem to display good predictive value regarding disease activity, and can be cautiously used as cost-effective follow-up biomarkers in IBD.
Despite the increasing number of studies revealing the dominant role of PLT in IBD, little has been clarified regarding the efficacy of anti-PLT drugs in IBD. Perhaps different existing pathways between PLT hemostasis and coagulation could explain the lack of potent anti-PLT drugs approved in IBD. Breaking this vicious cycle by encountering PLT inflammation properties appears to be a challenging ordeal for future investigators and clinical physicians, who will need to come up against resisting IBD flares with a reduced selection of effective drugs.
P- Reviewers: Bai AP, Cheng JF, Kupcinskas L, Miheller P S- Editor: Zhai HH L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 2. | Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1485] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 4. | Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993;104:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Huang HS, Chang HH. Platelets in inflammation and immune modulations: functions beyond hemostasis. Arch Immunol Ther Exp (Warsz). 2012;60:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Danese S. Nonimmune cells in inflammatory bowel disease: from victim to villain. Trends Immunol. 2008;29:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Ripoche J. Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol. 2011;35:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40L release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. 2007;33:382-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Youssefian T, Drouin A, Massé JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1809] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 11. | Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10725] [Article Influence: 397.2] [Reference Citation Analysis (0)] |
| 14. | Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028-5036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Morowitz DA, Allen LW, Kirsner JB. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med. 1968;68:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 108] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Skoda RC. Thrombocytosis. Hematology Am Soc Hematol Educ Program. 2009;159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Schafer AI. Thrombocytosis. N Engl J Med. 2004;350:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009;147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Cerutti A, Custodi P, Mduranti M, Balduini CL. Circulating thrombopoietin in reactive conditions behaves like an acute phase reactant. Clin Lab Haematol. 1999;21:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Papa A, Danese S, Piccirillo N, Toriani-Terenzi C, Bartolozzi F, Piscaglia AC, Grillo A, Leone G, Gentiloni-Silveri N, Gasbarrini G. Thrombopoietin serum levels in patients with inflammatory bowel disease with and without previous thromboembolic events. Hepatogastroenterology. 2003;50:132-135. [PubMed] |
| 22. | Harries AD, Fitzsimons E, Fifield R, Dew MJ, Rhoades J. Platelet count: a simple measure of activity in Crohn’s disease. Br Med J (Clin Res Ed). 1983;286:1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Fleshner P, Ippoliti A, Dubinsky M, Ognibene S, Vasiliauskas E, Chelly M, Mei L, Papadakis KA, Landers C, Targan S. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5:952-98; quiz 887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Okon A, Dubinsky M, Vasiliauskas EA, Papadakis KA, Ippoliti A, Targan SR, Fleshner PR. Elevated platelet count before ileal pouch-anal anastomosis for ulcerative colitis is associated with the development of chronic pouchitis. Am Surg. 2005;71:821-826. [PubMed] |
| 26. | Lian L, Fazio VW, Lavery IC, Hammel J, Remzi FH, Shen B. Evaluation of association between precolectomy thrombocytosis and the occurrence of inflammatory pouch disorders. Dis Colon Rectum. 2009;52:1912-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Collins CE, Rampton DS. Platelet dysfunction: a new dimension in inflammatory bowel disease. Gut. 1995;36:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:CR202-CR205. [PubMed] |
| 29. | Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, Kiraz S, Ertenli I, Calguneri M. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Balcik ÖS, Bilen S, Ulusoy EK, Akdeniz D, Uysal S, Ikizek M, Ak F, Kosar A. Thrombopoietin and mean platelet volume in patients with ischemic stroke. Clin Appl Thromb Hemost. 2013;19:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Albayrak Y, Albayrak A, Albayrak F, Yildirim R, Aylu B, Uyanik A, Kabalar E, Güzel IC. Mean platelet volume: a new predictor in confirming acute appendicitis diagnosis. Clin Appl Thromb Hemost. 2011;17:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Varol E, Ozaydin M. Decreased mean platelet volume in patients with amylodiosis. Clin Appl Thromb Hemost. 2013;19:578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Sahin A, Sahin M, Yüksel H, Türkcü FM, Cınar Y, Cingü AK, Arı S, Caça I. The mean platelet volume in patients with retinal vein occlusion. J Ophthalmol. 2013;2013:236371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Aliosmanoglu I, Gul M, Oguz A, Basol O, Uslukaya O, Keles C. Can mean platelet volume be a new risk factor in portal venous thrombosis? Clin Appl Thromb Hemost. 2013;19:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Khode V, Sindhur J, Kanbur D, Ruikar K, Nallulwar S. Mean platelet volume and other platelet volume indices in patients with stable coronary artery disease and acute myocardial infarction: A case control study. J Cardiovasc Dis Res. 2012;3:272-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Kılınçalp S, Ekiz F, Başar O, Ayte MR, Coban S, Yılmaz B, Altınbaş A, Başar N, Aktaş B, Tuna Y. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Wang RT, Li JY, Cao ZG, Li Y. Mean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18:1244-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Järemo P, Sandberg-Gertzen H. Platelet density and size in inflammatory bowel disease. Thromb Haemost. 1996;75:560-561. [PubMed] |
| 40. | Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, Kouroumalis EA. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Yüksel O, Helvaci K, Başar O, Köklü S, Caner S, Helvaci N, Abayli E, Altiparmak E. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | Mustafa Güçlü, Hakan Sakallı, Tolga Yakar. Mean Platelet Volume may be Reflects the Disease Activity of Ulcerative Colitis. Eur J Gen Med. 2010;7:259-263. |
| 43. | Liu S, Ren J, Han G, Wang G, Gu G, Xia Q, Li J. Mean platelet volume: a controversial marker of disease activity in Crohn’s disease. Eur J Med Res. 2012;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Kayahan H, Akarsu M, Ozcan MA, Demir S, Ates H, Unsal B, Akpinar H. Reticulated platelet levels in patients with ulcerative colitis. Int J Colorectal Dis. 2007;22:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Öztürk ZA, Dag MS, Kuyumcu ME, Cam H, Yesil Y, Yilmaz N, Aydinli M, Kadayifci A, Kepekci Y. Could platelet indices be new biomarkers for inflammatory bowel diseases? Eur Rev Med Pharmacol Sci. 2013;17:334-341. [PubMed] |
| 46. | Voudoukis E, Karmiris K, Oustamanolakis P, Theodoropoulou A, Sfiridaki A, Paspatis GA, Koutroubakis IE. Association between thrombocytosis and iron deficiency anemia in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2013;25:1212-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Gasche C. Anemia in IBD: the overlooked villain. Inflamm Bowel Dis. 2000;6:142-150; discussion 151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Gomollón F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 126] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 49. | Oustamanolakis P, Koutroubakis IE, Messaritakis I, Kefalogiannis G, Niniraki M, Kouroumalis EA. Measurement of reticulocyte and red blood cell indices in the evaluation of anemia in inflammatory bowel disease. J Crohns Colitis. 2011;5:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Dan K. Thrombocytosis in iron deficiency anemia. Intern Med. 2005;44:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Choi SI, Simone JV, Jackson CW. Megakaryocytopoiesis in experimental iron deficiency anemia. Blood. 1974;43:111-120. [PubMed] |
| 52. | Lordier L, Jalil A, Aurade F, Larbret F, Larghero J, Debili N, Vainchenker W, Chang Y. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Choi SI, Simone JV. Platelet production in experimental iron deficiency anemia. Blood. 1973;42:219-228. [PubMed] |
| 54. | Bilic E, Bilic E. Amino acid sequence homology of thrombopoietin and erythropoietin may explain thrombocytosis in children with iron deficiency anemia. J Pediatr Hematol Oncol. 2003;25:675-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Kulnigg-Dabsch S, Evstatiev R, Dejaco C, Gasche C. Effect of iron therapy on platelet counts in patients with inflammatory bowel disease-associated anemia. PLoS One. 2012;7:e34520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Kulnigg-Dabsch S, Schmid W, Howaldt S, Stein J, Mickisch O, Waldhör T, Evstatiev R, Kamali H, Volf I, Gasche C. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflamm Bowel Dis. 2013;19:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Kadikoylu G, Yavasoglu I, Bolaman Z, Senturk T. Platelet parameters in women with iron deficiency anemia. J Natl Med Assoc. 2006;98:398-402. [PubMed] |
| 58. | Kuku I, Kaya E, Yologlu S, Gokdeniz R, Baydin A. Platelet counts in adults with iron deficiency anemia. Platelets. 2009;20:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840-845. [PubMed] |
| 60. | Collins CE, Rampton DS, Rogers J, Williams NS. Platelet aggregation and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:1213-1217. [PubMed] |
| 61. | Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16:329-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Danese S, de la Motte C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Magro F, Araujo F, Pereira P, Meireles E, Diniz-Ribeiro M, Velosom FT. Soluble selectins, sICAM, sVCAM, and angiogenic proteins in different activity groups of patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA. 1994;91:8767-8771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 425] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 65. | Scaldaferri F, Lancellotti S, Pizzoferrato M, De Cristofaro R. Haemostatic system in inflammatory bowel diseases: new players in gut inflammation. World J Gastroenterol. 2011;17:594-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Russo S, Bussolati B, Deambrosis I, Mariano F, Camussi G. Platelet-activating factor mediates CD40-dependent angiogenesis and endothelial-smooth muscle cell interaction. J Immunol. 2003;171:5489-5497. [PubMed] |
| 67. | Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 68. | Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost. 2003;90:377-384. [PubMed] |
| 69. | Koutroubakis IE, Theodoropoulou A, Xidakis C, Sfiridaki A, Notas G, Kolios G, Kouroumalis EA. Association between enhanced soluble CD40 ligand and prothrombotic state in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Danese S, Scaldaferri F, Vetrano S, Stefanelli T, Graziani C, Repici A, Ricci R, Straface G, Sgambato A, Malesci A. Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease. Gut. 2007;56:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogné M, Richard Y, Garraud O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Borcherding F, Nitschke M, Hundorfean G, Rupp J, von Smolinski D, Bieber K, van Kooten C, Lehnert H, Fellermann K, Büning J. The CD40-CD40L pathway contributes to the proinflammatory function of intestinal epithelial cells in inflammatory bowel disease. Am J Pathol. 2010;176:1816-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Vowinkel T, Anthoni C, Wood KC, Stokes KY, Russell J, Gray L, Bharwani S, Senninger N, Alexander JS, Krieglstein CF. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007;132:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Dhillon AP, Anthony A, Sim R, Wakefield AJ, Sankey EA, Hudson M, Allison MC, Pounder RE. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992;21:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 77. | Koutroumpakis EI, Tsiolakidou G, Koutroubakis IE. Risk of venous thromboembolism in patients with inflammatory bowel disease. Semin Thromb Hemost. 2013;39:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Talbot RW, Heppell J, Dozois RR, Beart RW. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 413] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 79. | Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM. 1997;90:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Irving PM, Macey MG, Shah U, Webb L, Langmead L, Rampton DS. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Irving PM, Macey MG, Feakins RM, Knowles CH, Frye JN, Liyanage SH, Dorudi S, Williams NS, Rampton DS. Platelet-leucocyte aggregates form in the mesenteric vasculature in patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2008;20:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Irving PM, Pasi KJ, Rampton DS. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2005;3:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 517] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 85. | Nieuwland R, Sturk A. Why do cells release vesicles? Thromb Res. 2010;125 Suppl 1:S49-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Montoro-García S, Shantsila E, Marín F, Blann A, Lip GY. Circulating microparticles: new insights into the biochemical basis of microparticle release and activity. Basic Res Cardiol. 2011;106:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 840] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 88. | Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 89. | Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 90. | Oyabu C, Morinobu A, Sugiyama D, Saegusa J, Tanaka S, Morinobu S, Tsuji G, Kasagi S, Kawano S, Kumagai S. Plasma platelet-derived microparticles in patients with connective tissue diseases. J Rheumatol. 2011;38:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. 2010;62:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 93. | Nomura S, Uehata S, Saito S, Osumi K, Ozeki Y, Kimura Y. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb Haemost. 2003;89:506-512. [PubMed] |
| 94. | Inami N, Nomura S, Kikuchi H, Kajiura T, Yamada K, Nakamori H, Takahashi N, Tsuda N, Hikosaka M, Masaki M. P-selectin and platelet-derived microparticles associated with monocyte activation markers in patients with pulmonary embolism. Clin Appl Thromb Hemost. 2003;9:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Nomura S, Imamura A, Okuno M, Kamiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. Platelet-derived microparticles in patients with arteriosclerosis obliterans: enhancement of high shear-induced microparticle generation by cytokines. Thromb Res. 2000;98:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Nadaud S, Poirier O, Girerd B, Blanc C, Montani D, Eyries M, Imbert-Bismut F, Pacheco A, Vigne J, Tregouet DA. Small platelet microparticle levels are increased in pulmonary arterial hypertension. Eur J Clin Invest. 2013;43:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Taube J, McWilliam N, Luddington R, Byrne CD, Baglin T. Activated protein C resistance: effect of platelet activation, platelet-derived microparticles, and atherogenic lipoproteins. Blood. 1999;93:3792-3797. [PubMed] |
| 98. | Nomura S, Okamae F, Abe M, Hosokawa M, Yamaoka M, Ohtani T, Onishi S, Matsuzaki T, Teraoka A, Ishida T. Platelets expressing P-selectin and platelet-derived microparticles in stored platelet concentrates bind to PSGL-1 on filtrated leukocytes. Clin Appl Thromb Hemost. 2000;6:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Andoh A, Tsujikawa T, Hata K, Araki Y, Kitoh K, Sasaki M, Yoshida T, Fujiyama Y. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am J Gastroenterol. 2005;100:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Chamouard P, Desprez D, Hugel B, Kunzelmann C, Gidon-Jeangirard C, Lessard M, Baumann R, Freyssinet JM, Grunebaum L. Circulating cell-derived microparticles in Crohn’s disease. Dig Dis Sci. 2005;50:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Palkovits J, Novacek G, Kollars M, Hron G, Osterode W, Quehenberger P, Kyrle PA, Vogelsang H, Reinisch W, Papay P. Tissue factor exposing microparticles in inflammatory bowel disease. J Crohns Colitis. 2013;7:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1570] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 103. | Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 104. | Storey RF, Judge HM, Wilcox RG, Heptinstall S. Inhibition of ADP-induced P-selectin expression and platelet-leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb Haemost. 2002;88:488-494. [PubMed] |
| 105. | Storey RF, Wilcox RG, Heptinstall S. Comparison of the pharmacodynamic effects of the platelet ADP receptor antagonists clopidogrel and AR-C69931MX in patients with ischaemic heart disease. Platelets. 2002;13:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 106. | Patel SH, Rachchh MA, Jadav PD. Evaluation of anti-inflammatory effect of anti-platelet agent-clopidogrel in experimentally induced inflammatory bowel disease. Indian J Pharmacol. 2012;44:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 107. | Carty E, MacEy M, Rampton DS. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1095-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Thomas G, Skrinska VA, Lucas FV. The influence of glutathione and other thiols on human platelet aggregation. Thromb Res. 1986;44:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 111. | Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J Immunol. 2006;176:2617-2624. [PubMed] |
| 112. | André P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 113. | Mori M, Salter JW, Vowinkel T, Krieglstein CF, Stokes KY, Granger DN. Molecular determinants of the prothrombogenic phenotype assumed by inflamed colonic venules. Am J Physiol Gastrointest Liver Physiol. 2005;288:G920-G926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |