Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.187
Revised: September 25, 1996
Accepted: November 10, 1996
Published online: December 15, 1996
AIM: To detect whether hepatitis B virus (HBV) and hepatitis C virus (HCV) can infect the same host cell.
METHODS: We established a method of non-isotope double in situ hybridization by combining a degoxigenin-labeled probe with a biotin-labeled probe, and detected HBV and HCV infections in 10 cases of hepatocellular carcinoma.
RESULTS: It was found that HBV and HCV can infect the same host cell, which is a unique biological phenomenon.
CONCLUSION: Infections with HBV and HCV might not always follow the rule of “viral interference”, which provides a new clue for investigating the synergistic action of HBV and HCV in the pathogenesis of hepatocellular carcinoma.
- Citation: Liu YJ, Cong WM, Xie TP, Wang H, Shen F, Guo YJ, Chen H, Wu MC. Detecting the localization of hepatitis B and C viruses in hepatocellular carcinoma by double in situ hybridization. World J Gastroenterol 1996; 2(4): 187-189
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.187
Hepatocellular carcinoma (HCC) in Mainland China is closely associated with hepatitis B virus (HBV)[1]. The relationship between HCC and hepatitis C virus (HCV) has attracted more and more attention and about 20% of HCC patients have HBV and HCV co-infection in their sera[2]. An investigation has indicated that HBV and HCV co-infection accounted for about 14% of chronic hepatitis patients in Shanghai[3]. However, little is known about whether or not synergistic actions of HBV and HCV exist in the pathogenesis of HCC.
People generally believe that two sorts of viruses could not infect the same host cell, and this is the phenomenon of “viral interference”[4]. Direct methods of detecting HBV and HCV co-infection to identify whether HBV and HCV infect different cells respectively or the same cell simultaneously are still lacking. So in this study, we first established a method of non-isotope double in situ hybridization for detecting the two sorts of target nuclear acids in one cell, and then studied whether HBV and HCV co-infection would obey the rule of “viral interference”. Finally, we discuss the biologic significance of HBV and HCV co-infection.
The PUC18 plasmid carrying full length HCV cDNA was given by professor Qi-Zhong Tian (Department of Microbiology, Second Military Medical University). The PBR322 plasmid carrying full length HBV cDNA was provided by professor Gu-Jian Ren (Institute of Tumor, Shanghai). Specimens of HCC and their surrounding tissues were obtained from Eastern Hospital of Hepatobiliary Surgery, Second Military Medical University.
HBV and HCV plasmids were transformed into E. coli DH5α. Plasmids were prepared from E. coli by alkaline lysis method after bacteria had been amplified. The target gene fragments were cut by specific restriction endonuclear enzymes (HBV cDNA length is 3.4 kb, and the restriction enzyme used is EcoR I; HCV cDNA length is 0.54 kb, and the restriction enzymes used are EcoR I and Pst I). HBV and HCV cDNA probes were labeled by digoxigenin (DIG) using a DIG-Labelling and Detection Kit (Boehringer Mannheim Inc.); HCV cDNA probe was also labeled by biotin with the help of a Nick Translation Kit (Enzo Inc.).
Sections of HCC and their surrounding tissues, 6 μm thick, were cut by a cryostat microtome, fixed in 4% paraformaldehyde (pH 7.4) for 10 min, and washed with Poly Butylenes Succinate (PBS) for 2 × 5 min. They were then incubated in 0.2 mol/L HCl for 20 min, washed with 2 × SSC for 2 × 5 min, treated with 2 μg/mL proteinase K (containing 10 mmol/L Tris-HCl and 2 mmol/L CaCl2, pH 7.4) for 15 min at 37 °C, washed with 2 mg/mL glycine in PBS, fixed in 4% paraformaldehyde (pH 7.4) for 10 min, and washed with PBS for 2 × 5 min. Finally, sections were prehybridized at 42 °C in prehybridization buffer for 2 h (the buffer was composed of 4 × SSC, 50% formamide, 5 × Denhardt′s solution, 5% dextran sulfate and predenatured 0.5 mg/mL ssDNA). After prehybridization buffer was removed, hybridization buffer (5 ng/μL DIG-HBV and BIO-HCV cDNA probes, 4 × SSC, 50% formamide, 5 × Denhardt′s solution, 5% dextran sulfate and 0.5 mg/mL ssDNA) was added to slides which had already been incubated for 15 min at 100 °C. Then these slides were placed into a humid chamber for 10 min at 100 °C, followed by incubation in the humid chamber at 42 °C overnight. Colour detection of biotin system (ABC method with substrates of DAB and H2O2) was done before that of digoxigenin system (based on the procedure of DIG-labelling and detection kit, Boehringer Mannheim Inc., Cat. No. 1093657, with the substrates of NBT and BCIP).
DIG-PBR328 and BIO-PBR328 were adopted as negative controls instead of target probes. The results of single in situ hybridization were taken as parallel controls for the results of double in situ hybridization.
The background information of 10 cases of HCC patients is shown in Table 1.
| Specimen number | Age | Sex | HBV serum marker | AFP (ρB/μg·L-1) | Pathological diagnosis |
| 1 | 48 | Male | HBsAg (+) HBeAb (+) HBcAb (+) | 42 | HCC, chronic hepatitis |
| 2 | 27 | Male | HBsAg (+) | 900 | HCC, hepatitis in porta tract |
| 3 | 61 | Male | HBsAg (+) HBcAb (+) | 260 | HCC, cirrhosis |
| 4 | 38 | Male | (-) | 12000 | HCC, cirrhosis |
| 5 | 44 | Male | HBeAb (+) HBcAb (+) | 40 | HCC, cirrhosis |
| 6 | 46 | Male | (-) | 150 | HCC, non-cirrhosis |
| 7 | 45 | Male | (-) | < 25 | HCC, cirrhosis |
| 8 | 62 | Male | (-) | 52 | HCC, cirrhosis |
| 9 | 40 | Male | HBsAg (+) | - | HCC, cirrhosis |
| 10 | 58 | Male | - | < 25 | HCC, cirrhosis |
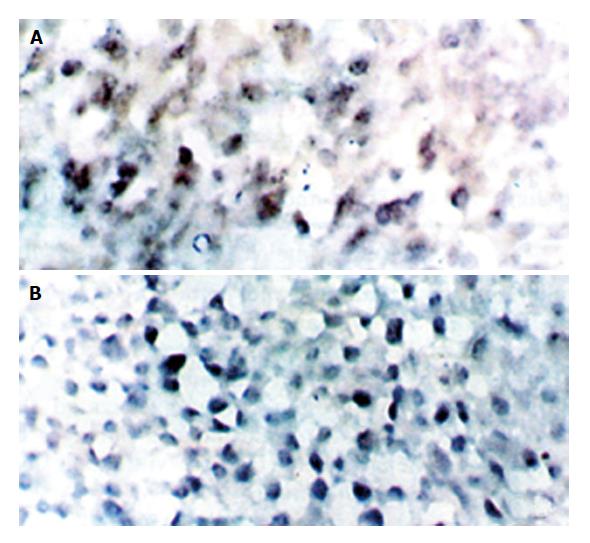
HBV or HCV positive cells showed diffuse distribution in HCC; most HBV or HCV positive cells in tumor surrounding tissues showed local distribution, concentrating to the porta tract (Table 2).
| Specimen number | Dig-HBV | Dig-HCV | ||
| C | S | C | S | |
| 1 | + | + | - | - |
| 2 | + | + | + | + |
| 3 | + | + | - | - |
| 4 | + | + | + | + |
| 5 | + | + | - | - |
| 6 | - | - | - | - |
| 7 | + | + | - | - |
| 8 | + | + | - | - |
| 9 | + | + | - | - |
| 10 | + | + | - | - |
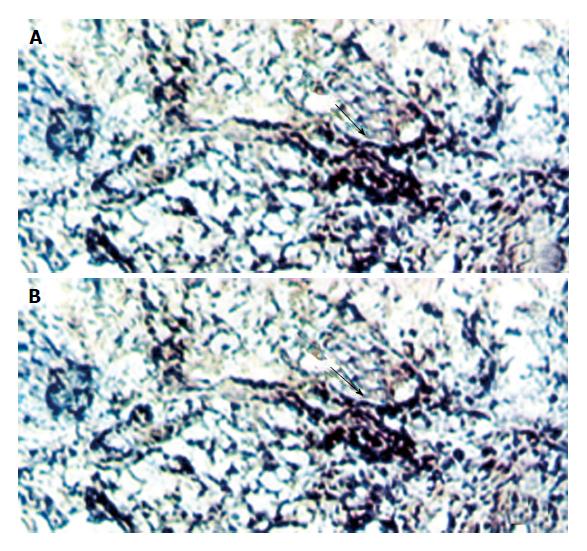
HBV and HCV co-infection was detected only in No. 2 and No. 4 cases. HBV or HCV positive cells were distributed diffusely in HCC and locally in tumor surrounding tissues, some of them were shown at single cell level. The signal of BIO-HCV was weaker than that of DIG-HBV.
HBV infection is the most important factor associated with HCC in Chinese people. With the improvement of sensitivity of detecting methods, the importance of HCV infection and HBV/HCV co-infection in the pathogenesis of HCC has gradually been recognized.
Scientists from Taiwan discovered that both HBsAg and anti-HCV were important risk factors for HCC (relative risk ratio, 13.96 and 27.12, respectively), and the risk ratio was elevated significantly to 40.05 when HBsAg and anti-HCV were considered simultaneously, which suggested that the two viruses might have independent but synergistic effects on the pathogenesis of HCC[5].
The results of double in situ hybridization conform with those of single in situ hybridization. We found that there were two positive cases of HCV infection accompanied by HBV infection with the help of single and double in situ hybridization, respectively. These results directly provide experimental evidence that HBV and HCV may play a synergistic role in the pathogenesis of HCC (Figure 1, Figure 2, Figure 3).
People generally believe that two kinds of viruses could not infect the same host cell simultaneously (the phenomenon of viral interference). There are two sorts of viral interference, homologous and heterologous, and the latter is more common[4]. HBV is a double stranded DNA virus, and HCV is a single stranded RNA virus. There is no homology between them. In 1983, Bradley noted that non-A non-B hepatitis virus (NANBV) infection could resist hepatitis A virus (HAV) infection in two cases of chimpanzees, and symptoms of one HBV infected chimpanzee were relieved and the level of serum HBsAg reduced after infection with NANBV. So he suggested that NANBV could interfere with HBV and HAV infections[6]. Brotman et al[7] indicated that HCV could inhibit the replication of HBV. Dr. Du et al[8] discovered that acute HAV infection could obviously affect the level of HBV markers, and particularly reduce the level of serum HBsAg. But these results which came from serum detection, for example, the reduction or disappearance of serum HBsAg, could not accurately show the actual change of liver cells.
The result that HBV and HCV could co-infect the same host cell indicates that HBV and HCV might not always follow the rule of “viral interference”; the above conclusion conforms with the results that liver functions of HBV patients were severely damaged by HCV infection caused by blood transfusion. So it laid down a theoretical basis that HBV and HCV might play a synergistic role in the pathogenesis of HCC, which indicates that vaccines of HBV and HCV will not interfere with each other in their preventive effects.
We noted that there were 5 cases positive for HBV serum markers, 4 cases negative and one case undetected; but the results of in situ hybridization showed that 9 cases were positive with hybridized signals of HBV both in HCC and their surrounding tissues, and one case was negative. Considering their pathologic diagnoses, we found that 3 cases negative for HBV serum markers but positive for in situ hybridization showed liver cirrhosis, and one case negative for these two methods showed no liver cirrhosis. These results suggest that in situ hybridization is more sensitive than serum immunodetection for HBV infection. It was a pity that we could not compare in situ hybridization with serum detection for HCC because we lack these patients′ background information of HCV serum markers.
Original title:
S- Editor: Cao LB L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Gu GW. Prevention and treatment for liver cancer. Shanghai: Scientific and Technical Press 1991; 148. |
| 2. | Yang JM, Liu WW. Relationship of hepatitis C virus infection with primary hepatic carcinoma. Zhonghua Ganzangbing Zazhi. 1994;2:11-15. |
| 3. | Li JQ, Xiao SD, Jiang ZJ, Zeng MD, Zhao JM, Qiu DK. Investigation on infections of HBV and HCV in Shanghai area. Zhonghua Xiaohua Zazhi. 1992;12:87-89. |
| 4. | Yu H. Microbiology. Beijing: People Health Press 1983; 329-330. |
| 5. | Chuang WL, Chang WY, Lu SN, Su WP, Lin ZY, Chen SC, Hsieh MY, Wang LY, You SL, Chen CJ. The role of hepatitis B and C viruses in hepatocellular carcinoma in a hepatitis B endemic area. A case-control study. Cancer. 1992;69:2052-2054. |
| 6. | Bradley DW, Maynard JE, McCaustland KA, Murphy BL, Cook EH, Ebert JW. Non-A, non-B hepatitis in chimpanzees: interference with acute hepatitis A virus and chronic hepatitis B virus infections. Zhonghua Yixue Zazhi. 1983;11:207-213. |
| 7. | Brotman B, Prince AM, Huima T, Richardson L, van den Ende MC, Pfeifer U. Interference between non-A, non-B and hepatitis B virus infection in chimpanzees. Zhonghua Yixue Zazhi. 1983;11:191-205. |
| 8. | Du ZX, Li Y, Luo CY, Wang XN, Ma WB. Effect of hepatitis A virus infection on hepatitis B virus infection. Zhonghua Chuanranbing Zazhi. 1994;12:57-58. |