Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8678
Revised: September 21, 2013
Accepted: September 29, 2013
Published online: December 14, 2013
Processing time: 126 Days and 10.7 Hours
AIM: To investigate alanine aminotransferase (ALT) and sustained virological response (SVR) in chronic hepatitis C (CHC) during peginterferon-ribavirin treatment.
METHODS: One hundred and fifty-one genotype 1 CHC patients underwent treatment for 48 wk with peginterferon and ribavirin, and were retrospectively divided into two groups as having a rapid virological response (RVR) (Group 1, n = 52) and not having an RVR (Group 2, n = 99). We also subdivided each group into two according to the initial ALT level being high (Group 1h and Group 2h) or normal (Group 1n and Group 2n). HCV RNA and ALT levels were measured at baseline; at 4, 12, 24 and 48 wk during the treatment period; and at 24 wk follow-up. ALT levels were also obtained at 8 wk. According to the results of ALT, patients were enrolled in either the follow-up abnormal or follow-up normalized ALT groups at each interval. Patients with high and normal ALT levels were compared for each interval in terms of SVR.
RESULTS: The SVR rates were 83% vs 40% (P = 0.000), 82% vs 84% (P = 0.830), and 37% vs 44% (P = 0.466) when comparing Group 1 with 2, 1h with 1n, and 2h with 2n, respectively. In Group 2h, the SVR rates were 34% vs 40% (P = 0.701), 11% vs 52% (P = 0.004), 12% vs 50% (P = 0.007), 7% vs 50% (P = 0.003), 6% vs 53% (P = 0.001), and 0% vs 64% (P = 0.000) when comparing patients with high and normalized ALT levels at week 4, 8, 12, 24, 48 and 72, respectively. The multiple logistic regression analysis revealed that RVR (OR = 7.05; 95%CI: 3.1-16.05, P = 0.000), complete early virological response (cEVR) (OR = 17.55; 95%CI: 6.32-48.76, P = 0.000), normalization of ALT at 8 wk (OR = 3.04; 95%CI: 1.31-7.06, P = 0.008), and at 12 wk (OR = 4.21; 95%CI: 1.65-10.76, P = 0.002) were identified as independent significant predictive factors for SVR.
CONCLUSION: Normalization of ALT at 8 wk may predict viral response during peginterferon-ribavirin treatment in genotype-1 CHC patients especially without RVR.
Core tip: Rapid virological response (RVR) has been acknowledged as a powerful on-treatment predictor of sustained virological response (SVR) in the treatment of chronic hepatitis C (CHC). However, RVR rates are relatively low and a new predictor is needed for CHC patients; especially those without RVR. In this context, on-treatment alanine aminotransferase (ALT) changes may be a new predictor for SVR. In this study, we found that ALT normalization at the 8 wk may be an important on-treatment predictor for CHC.
- Citation: Dogan UB, Akin MS, Yalaki S. Alanine aminotransferase normalization at week 8 predicts viral response during hepatitis C treatment. World J Gastroenterol 2013; 19(46): 8678-8686
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8678.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8678
The sustained virological response (SVR) after combined peginterferon and ribavirin treatment in chronic hepatitis C (CHC) patients is heterogeneous[1]. Thus, for the treatment of CHC, clinicians would like to establish predictive factors for SVR[2]. Pretreatment predictive viral factors are hepatitis C virus (HCV) genotype and serum HCV RNA levels at baseline, and many host factors including age, sex, weight, race, liver fibrosis, insulin resistance and recently acknowledged presence of interleukin-28 polymorphism[1,3,4].
Once treatment is initiated, rapid virological response (RVR) is acknowledged as a powerful on-treatment predictor of SVR[5]. However, RVR rates are relatively low and a new predictor is needed for CHC patients; especially those without RVR. In this context, on-treatment alanine aminotransferase (ALT) changes may be a new predictor for SVR. There are few data evaluating the relationship between on-treatment ALT changes and SVR during combination treatment with peginterferon and ribavirin in patients with CHC.
The purpose of this study was to investigate the relationship between on-treatment ALT changes and SVR in genotype 1 CHC patients during peginterferon-ribavirin treatment.
Medical records of patients with CHC, who were treated between 2008 and 2012 at the Adana Numune Training and Research Hospital, Turkey, were retrospectively reviewed. Eligible patients had chronic HCV genotype 1 infection with compensated liver disease and a detectable plasma HCV RNA level, and had not been previously treated for hepatitis C. Patients who were on treatment or had withdrawn because of adverse events, or were lost during follow-up were excluded from the study. Patients were also excluded if they had co-infection with hepatitis B or HIV, any other cause of liver disease such as alcohol abuse or autoimmune hepatitis, morbid obesity (Body Mass Index > 40), poorly controlled diabetes mellitus (glycated hemoglobin value > 8.5%), severe depression or a severe psychiatric disorder, or active substance abuse. Finally, 151 patients who were followed up for at least 6 mo after completion of treatment were included in the study. Most patients had undergone liver biopsy within 6 mo before screening. The liver histology was graded by the histological activity index according to the criteria of Ishak et al[6], which comprise two major components namely Histological Activity Index and fibrosis. The study was approved by our Institutional Review Board and was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
We first categorized 151 patients into two main groups. Group 1 included 52 patients with RVR, and Group 2 included 99 patients without RVR. Each group was then subdivided into two according to the initial ALT level: Group 1h, patients who had initial abnormal ALT levels with RVR; Group 1n, patients who had initial normal ALT levels with RVR; Group 2h, patients who had initial abnormal ALT levels without RVR; and Group 2n, patients who had initial normal ALT levels without RVR. ALT patterns were analyzed throughout the course of treatment and follow-up period.
Patients with genotype 1 infection were administered peginterferon α-2a at a dose of 180 μg/wk or peginterferon α-2b at the standard dose of 1.5 μg/kg per week; both in combination with oral ribavirin at a dose of 1000-1200 mg/d, according to body weight (< 75 kg, 1000 mg/d; ≥ 75 kg, 1200 mg/d). Patients underwent treatment for 48 wk and were followed-up for 24 wk.
Patients were followed up by blood sample analysis and measurement of biochemical variables. Blood samples were tested for complete blood counts, serum ALT levels, HCV genotype (baseline only) and serum HCV RNA. Serum ALT levels were obtained from all patients at baseline and at weeks 4, 8, 12, 24 and 48 of combined peg-interferon and ribavirin treatment, and 24 wk after completing therapy. According to the results of ALT, patients were included in either the follow-up abnormal or follow-up normalized ALT groups at each interval. Patients with high and normal ALT levels were compared at weeks 4, 8, 12, 24, and 48 of treatment; and follow-up week 24 in terms of SVR. The upper normal limit for serum ALT was 40 IU/L in our laboratory.
HCV RNA levels were measured with the use of the Cobas TaqMan assay (Roche Diagnostics, Milan, Italy), which has a lower limit of quantitation of 20 IU/mL. Real-time polymerase chain reaction with Rotor Gene Q (Qiagen, Milan, Italy) was used for genotype determination. Measurements were obtained at screening visits (baseline); weeks 4, 12, 24 and 48 during the treatment period; and 24 wk follow-up. The primary endpoint of efficacy was SVR (undetectable serum HCV RNA levels at 24 wk after completing treatment). RVR was defined as undetectable serum HCV RNA level at the end of 4 wk. Patients with detectable HCV RNA at week 4 (no RVR) who had undetectable HCV RNA at week 12 were said to have a complete early virological response (cEVR). End of treatment response (ETR) was defined as the undetectable serum HCV RNA level at the end of treatment.
Data management and statistical analyses were performed with SPSS for Windows Release 18.0.0 (SPSS Inc., Chicago, IL, United States). Results are expressed as the mean ± SD. Student’s t test or analysis of variance was used to assess the significance of SVR rates. Univariate analysis and multiple logistic regression analysis were used to identify predictive factors for sustained response. In the multiple logistic regression analysis, we determined the strength of the influence of possible variables (RVR, cEVR, normalization of ALT at 8 and 12 wk) for sustained response. P < 0.05 was considered as statistically significant.
RVR was achieved in 52 (34.4%) patients and cEVR was achieved in 110 (72.9%) patients. The remaining 41 patients who did not achieve a cEVR at week 12 had undetectable HCV RNA at 24 wk. Comparison of baseline characteristics and virological responses in patients with and without RVR are summarized in Table 1. The initial ALT level was higher in patients with RVR than patients without RVR, although there was no significant difference in the number of patients with initial abnormal ALT level between the groups. Initial HCV RNA (log10 IU/mL) was significantly lower and the SVR rate was significantly higher in patients with RVR compared to patients without RVR. The overall SVR rate was 55%.
| Patients with RVR | Patients without RVR | P1value | |
| (Group 1,n= 52) | (Group 2,n= 99) | ||
| Age (yr) | 55.9 ± 12.2 | 57.9 ± 11.1 | 0.312 |
| Male | 24 (46.2) | 51 (51.5) | 0.534 |
| Initial ALT (IU/L) | 87.3 ± 109.8 | 52.2 ± 40.4 | 0.005 |
| Initial abnormal ALT level | 33 (63.5) | 49 (49.5) | 0.103 |
| Initial HCV RNA (log10 IU/ mL) | 5.4 ± 1.1 | 6.1 ± 0.8 | 0.000 |
| cEVR | 52 (100) | 58 (59) | 0.000 |
| ETR | 48 (92) | 56 (57) | 0.000 |
| SVR | 43 (83) | 40 (40) | 0.000 |
| ISHAK score, mean ± SD | |||
| Biopsy of receipt | 31 (59.6) | 63 (63.6) | 0.631 |
| HAI | 8.9 ± 3.8 | 8.2 ± 2.8 | 0.356 |
| Fibrosis score | 2.9 ± 1.3 | 2.7 ± 1.4 | 0.681 |
Baseline characteristics and virological responses according to the initial ALT level in patients with and without RVR were similar (Table 2).
| Patients with RVR (Group 1) | P1value | Patients without RVR (Group 2) | P1value | |||
| Initial abnormal | Initial normal | Initial abnormal | Initial normal | |||
| ALT level | ALT level | ALT level | ALT level | |||
| (Group 1h,n= 33) | (Group 1n,n= 19) | (Group 2h,n= 49) | (Group 2n,n= 50) | |||
| Age, yr | 54.3 ± 13.8 | 58.6 ± 8.3 | 0.222 | 56.6 ± 12.6 | 59.2 ± 9.2 | 0.246 |
| Male | 18 (54.6) | 6 (31.6) | 0.114 | 26 (53.1) | 25 (50.0) | 0.763 |
| Initial ALT (IU/ L) | 122.4 ± 125.3 | 26.4 ± 8.2 | 0.002 | 78.1 ± 43.9 | 26.8 ± 7.4 | 0.000 |
| HCV RNA (log10 IU/mL) | 5.6 ± 0.9 | 5.1 ± 1.4 | 0.139 | 6.2 ± 0.9 | 6.1 ± 0.7 | 0.748 |
| cEVR | 33 (100) | 19 (100) | NA | 29 (59) | 29 (58) | 0.906 |
| ETR | 30 (91) | 18 (95) | 0.626 | 26 (53) | 30 (60) | 0.491 |
| SVR | 43 (82) | 40 (84) | 0.83 | 18 (37) | 22 (44) | 0.466 |
| ISHAK Score, mean ± SD | ||||||
| Biopsy of receipt | 17 (51.5) | 14 (73.7) | 0.121 | 33 (67.4) | 30 (60) | 0.453 |
| HAI | 9.6 ± 3.7 | 8.0 ± 3.9 | 0.257 | 8.7 ± 2.6 | 7.7 ± 3.0 | 0.164 |
| Fibrosis score | 2.9 ± 1.2 | 2.9 ± 1.4 | 0.957 | 3.0 ± 1.4 | 2.4 ± 1.4 | 0.100 |
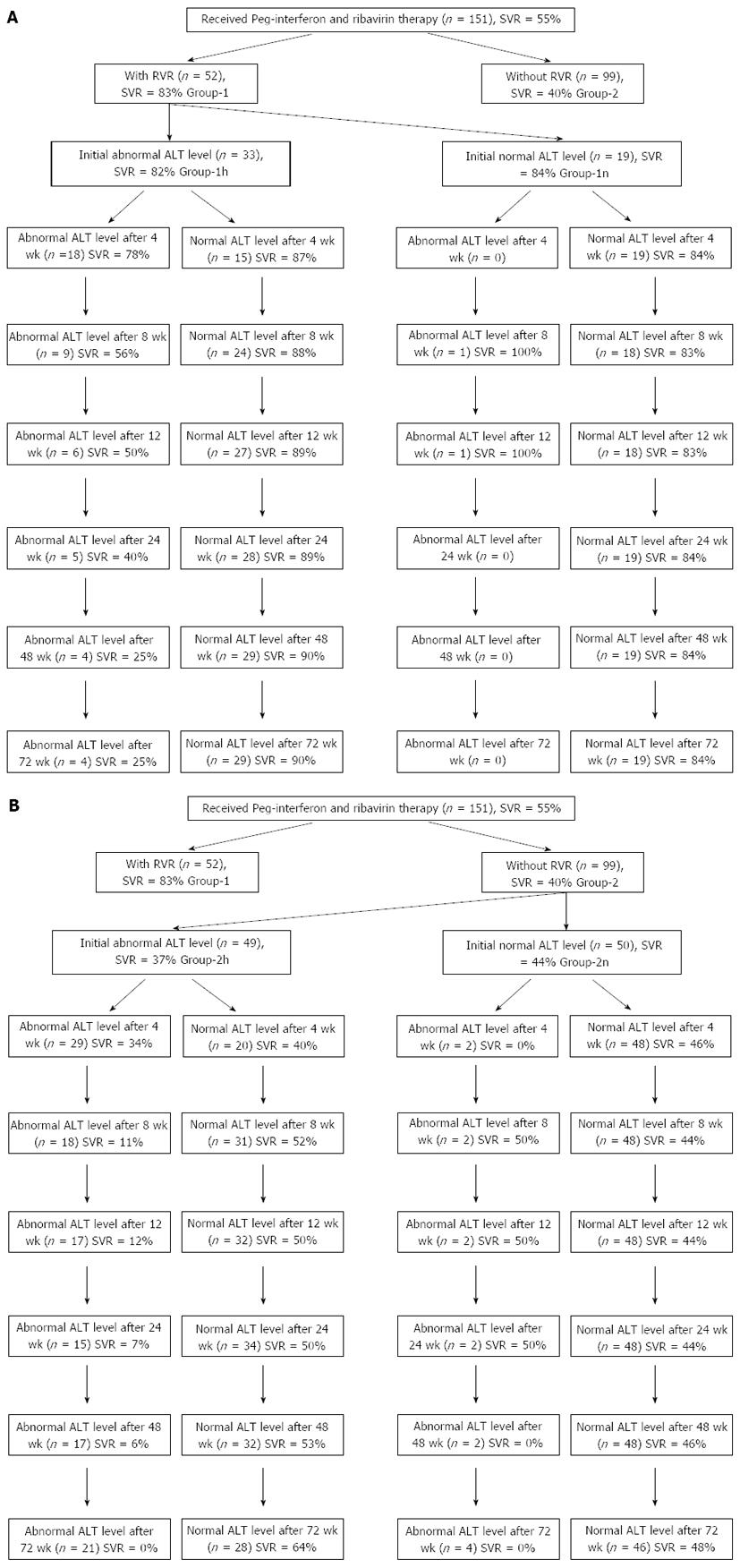
Schematic diagrams showing patient group flow according to initial ALT level and subsequent pattern of changes in patients with and without RVR are shown in Figure 1.
Patients who had normal initial ALT levels showed nearly sustained normal ALT levels during treatment. Only one patient in Group 1n (Figure 1A) and two patients in Group 2n (Figure 1B) had variable ALT abnormality during treatment. SVR rates were 84% and 44% in Group 1n and 2n, respectively.
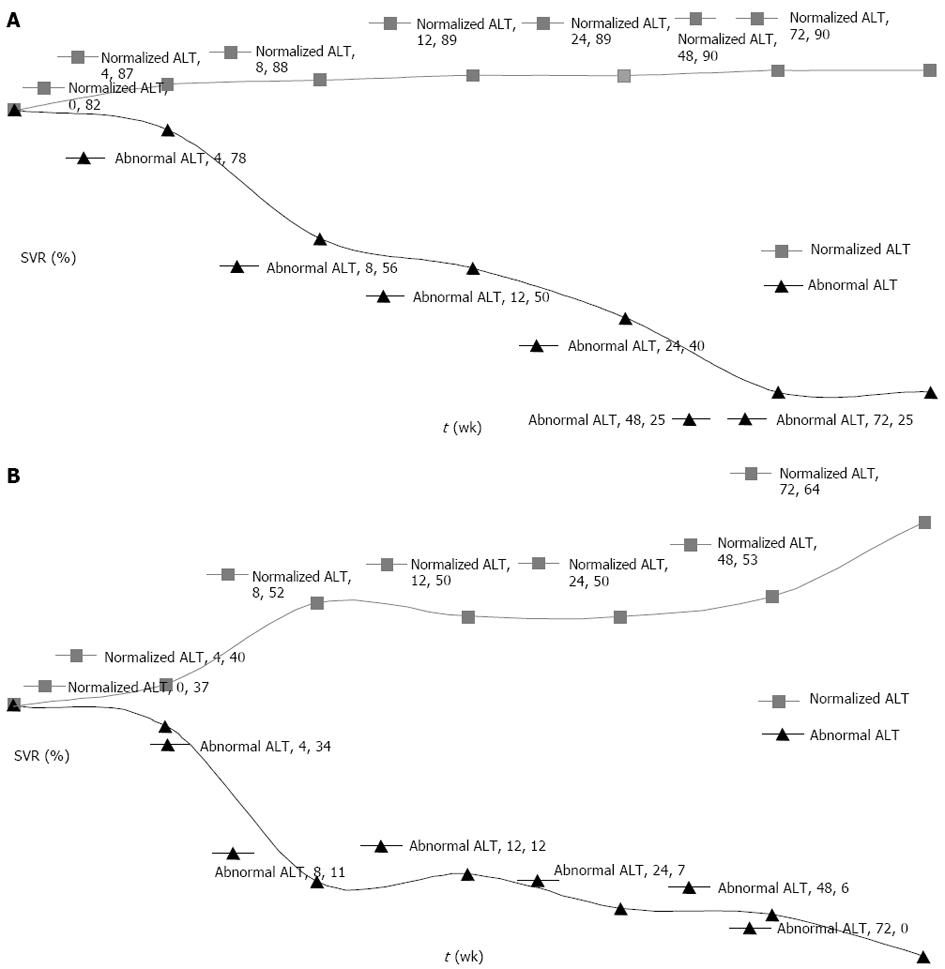
Comparison of SVR rates in patients with high and normalized ALT levels at weeks 4, 8, 12, 24, 48 and 72 in the initial abnormal ALT level groups with and without RVR are summarized in Table 3 and illustrated in Figure 2. At 8 wk, normalization of ALT became significant in terms of SVR in both groups.
| Initial abnormal ALT level in patients | P2value | Initial abnormal ALT level in patients | P1value | |||
| with RVR (Group 1h,n= 33) | without RVR (Group 2h,n= 49) | |||||
| Follow-up | Follow-up | Follow-up | Follow-up | |||
| abnormal ALT | normalized ALT | abnormal ALT | normalized ALT | |||
| After 4 wk | ||||||
| No. of patients | 18 | 15 | 29 | 20 | ||
| SVR rate | 78 | 87 | 0.525 | 34 | 40 | 0.701 |
| After 8 wk | ||||||
| No. of patients | 9 | 24 | 18 | 31 | ||
| SVR rate | 56 | 88 | 0.049 | 11 | 52 | 0.004 |
| After 12 wk | ||||||
| No. of patients | 6 | 27 | 17 | 32 | ||
| SVR rate | 50 | 89 | 0.028 | 12 | 50 | 0.007 |
| After 24 wk | ||||||
| No. of patients | 5 | 28 | 15 | 34 | ||
| SVR rate | 40 | 89 | 0.001 | 7 | 50 | 0.003 |
| After 48 wk | ||||||
| No. of patients | 4 | 29 | 17 | 32 | ||
| SVR rate | 25 | 90 | 0.006 | 6 | 53 | 0.001 |
| After 72 wk | ||||||
| No. of patients | 4 | 29 | 21 | 28 | ||
| SVR rate | 25 | 90 | 0.006 | 0 | 64 | 0.000 |
We performed univariate analysis using the χ2 test to investigate the association of SVR with various factors. In the multiple logistic regression for the strength of influence factors, RVR (OR = 7.05; 95%CI: 3.1-16.05, P = 0.000), cEVR (OR = 17.55; 95%CI: 6.32-48.76, P = 0.000), normalization of ALT at week 8 (OR = 3.04; 95%CI: 1.31-7.06, P = 0.008), and at week 12 (OR = 4.21; 95%CI: 1.65-10.76, P = 0.002) were identified as independent significant predictive factors for SVR.
Treatment with pegylated interferon-α and ribavirin is a well-accepted standard of care for patients with CHC[2]. Although this approach appears to be highly effective for patients with HCV genotypes 2 or 3, who have a SVR of about 80%, the treatment algorithm is less effective for patients with HCV genotype 1, because these patients have SVR rates of just 40%-50%[7,8]. There are some pretreatment factors related to SVR. Clinicians need to know these factors for predicting SVR, to determine non-responders as early as possible in order to avoid prolonged treatment without benefit[2,9]. The viral factors are HCV genotype and serum HCV RNA levels at baseline and numerous host factors include age, sex, race, weight, liver fibrosis, and insulin resistance[1]. Recently, an interleukin-28 polymorphism has been acknowledged as a powerful pretreatment predictor of SVR[3,4].
Once treatment is initiated, the monitoring of viral responses such RVR and early virological response (EVR) can further aid in predicting treatment response[5]. As for the response-guided approach, RVR is regarded as the most important predictor for SVR[10-12]. In a recent retrospective analysis of 1383 patients, it was shown that achieving RVR correlated with a high probability (86%-100%) of SVR to peginterferon-ribavirin combination therapy, regardless of genotype[13]. In another retrospective analysis, it was shown that the SVR rate was 42% in the absence of RVR at week 48[14]. Unfortunately, RVR rates are small and range from 7.4%-37%[15]. Also, there is a positive correlation between the magnitude of the decrease in HCV RNA at week 4 and the probability of SVR[16]. We previously demonstrated that patients with a ≥ 3 log10 drop in HCV RNA at week 4 have a high probability of achieving an SVR when treated with either peginterferon α-2a-ribavirin or peginterferon α-2b-ribavirin[17]. In addition, EVR is an important parameter for the decision to terminate or continue treatment because patients without EVR can hardly achieve SVR[18]. RVR seems to be the single important on-treatment factor for SVR. Consequently, there is a need for a new on-treatment predictor for SVR; especially in patients without RVR. In this context, on-treatment ALT changes may be a new predictor for SVR.
In general, a decreased pattern of ALT level is the accepted basic indicator of interferon therapeutic effect in CHC, and several studies have shown that delayed normalization of ALT levels may indicate poor response to interferon therapy[9,19], although the viral response was not always associated with biochemical response[6,20].
Serum ALT, a surrogate marker of hepatocyte damage or death, decreases during antiviral treatment, and shows the lowest activity at the end of treatment[21]. The mechanism of decline of ALT level is not clear; however it can be explained by a reduction in infected cells, a non-cytolytic cure, or cell removal irrelevant of ALT dynamics. However, a decreased ALT level at the early phase of treatment is not related to apoptotic activity[22]. Theoretically, the rapid declines in ALT may reflect a rapid decrease of ongoing inflammation in the same manner as removal of the virus. The pattern of viral elimination shows a rapid decrease in the first month. Ribeiro et al[21] showed that the RVR significantly correlated with the decline in ALT levels at week 4 of treatment. A retrospective study of 111 patients with chronic HCV infection treated by conventional interferon and ribavirin also demonstrated that the larger decline in ALT level within the first 2 and 4 wk was a predictor of SVR[23]. These correlations suggest that ALT dynamics can be presented as a possibility to reflect rapid virological changes; especially in patients with elevated baseline ALT levels.
Kim et al[24] reported that, instead of RVR, the rapid normalization of serum ALT level after initiation of treatment may play an additional role in predicting SVR. They retrospectively analyzed changes in ALT levels between baseline and week 4 of treatment in 168 patients with chronic HCV infection. Rapid normalization of ALT within 1.5 times of the normal range after treatment was found to be significantly associated with improved SVR in patients with genotype I HCV infection (34.1% vs 20.0%, P = 0.01) and non-genotype-1 infection (88.1% vs 66.7%, P = 0.11) who had initially high ALT levels. This result suggests that rapid normalization of ALT at week 4 of treatment could be used as a strategy for predicting SVR in patients with elevated baseline ALT levels; however, its use is limited because of the paucity of knowledge about RVR and the difficulty of application in normal ALT levels.
A recent report suggested that mild ALT elevations (peak ALT value 1-1.5 × baseline value) during treatment may reflect ongoing viral activity in non-responders, but a more significant rise may reflect a good virological response due to an immunomodulating effect of interferon[25]. However, it was difficult to use these data to analyze the reason for on-treatment ALT elevation and to elucidate the relationship between on-treatment ALT elevation and SVR; especially at week 4 of treatment.
In our study, patients with genotype 1 CHC were divided into two groups as those with or without RVR, because RVR is the most important on-treatment predictor of SVR. The SVR rate was also found to be high in patients with RVR (83% vs 40.0%, P < 0.001) in our study. Each group was further subdivided into two according to the initial ALT level being high or normal. The SVR rates were similar between patients with high and normal ALT levels at baseline and at week 4 in patient with and without RVR. SVR rates were found to be significantly higher in patients with normalized ALT at week 8 and thereafter.
In the patient group with RVR, SVR starts at 82% at baseline in patients with initially abnormal ALT level. SVR declines in patients with continuing abnormal ALT levels and increases in patients with normalized ALT levels. However, this difference becomes significant, with 56% vs 88%, only after 8 wk treatment. Later, this difference increases but at a slower rate, reaching 25% vs 90% at week 48 (Figure 2A). However, it is difficult to comment on patients with continuing abnormal ALT levels because of the lower number of patients.
In the patient group without RVR, the decrease in SVR is larger in patients with continuing abnormal ALT levels. SVR starts at 37% at baseline in patients with initial abnormal ALT levels, and declines in patients with continuing abnormal ALT levels and increases in those with normalized ALT levels, as in patients with RVR. The difference in SVR levels in those groups becomes significant at 8 wk, reaching 11% vs 52%. The difference in SVR continues to increase slightly, reaching 6% vs 53% at week 48 (Figure 2B).
Although SVR was found to be significantly correlated with the decline of ALT level at week 4 of treatment in a few studies[21,23,24], high levels of ALT may also reflect a good virological response due to an immunomodulating effect of interferon[25]. Clinicians must also know the baseline ALT level in order to be able to predict SVR. Furthermore, RVR is already the most important predictor at week 4 of treatment and it is still unclear whether the use of serum ALT levels, instead of RVR, is helpful for predicting SVR in clinical practice. The main problem is to predict SVR in patients without RVR. In our study, SVR was found to be higher in patients with normalized ALT at week 4 of treatment; however, the difference was not significant at that stage (34% vs 40.0%, P = 0.701). SVR rates continued to increase and became significant at 8 wk in non-RVR patients with normalized ALT. At week 12 of treatment and later, SVR rates were found to be higher in these patients; however, cEVR was already a more important criterion for SVR at this stage, compared to the ALT (OR = 17.55 vs 4.21). Therefore determination of ALT levels at 8 wk would be better than at 4 and 12 wk.
In our opinion, if a patient with initial abnormal ALT without RVR still has abnormal ALT level at 8 wk, peginterferon–ribavirin treatment may be discontinued because SVR is expected to be only 11%.
In conclusion, normalization of ALT at the 8 wk may predict viral response during peginterferon-ribavirin treatment in patients with genotype 1 CHC; especially without RVR.
Rapid virological response (RVR) is acknowledged as a powerful on-treatment predictor of sustained virological response (SVR) during peginterferon-ribavirin treatment of chronic hepatitis C (CHC). However, RVR rates are relatively low and a new predictor is needed for CHC patients; especially those without RVR.
The authors investigated the relationship between on-treatment alanine aminotransferase (ALT) changes and SVR in patients with genotype 1 CHC during peginterferon–ribavirin treatment.
The authors found that normalization of ALT at 8 wk may predict viral response during peginterferon-ribavirin treatment in patients with genotype 1 CHC; especially without RVR.
If the patients with initial abnormal ALT without RVR still had abnormal ALT level at 8 wk, peginterferon-ribavirin treatment may be discontinued because SVR is expected to be only 11%.
This study investigated the relationship between on-treatment ALT changes and SVR in patients with genotype 1 CHC during peginterferon-ribavirin treatment, and demonstrated that on-treatment ALT changes may be a new predictor for SVR.
P- Reviewers: Carlos NF, Leardkamolkarn V, Yu B S- Editor: Qi Y L- Editor: Kerr C E- Editor: Wang CH
| 1. | Jang JY, Chung RT. Chronic hepatitis C. Gut Liver. 2011;5:117-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 3. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 4. | Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120-129.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 5. | Poordad FF. Review article: the role of rapid virological response in determining treatment duration for chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:1251-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3783] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 7. | Dogan UB, Atabay A, Akin MS, Yalaki S. The comparison of the efficacy of pegylated interferon α-2a and α-2b in chronic hepatitis C patients with genotype 1. Eur J Gastroenterol Hepatol. 2013;25:1082-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Hung CH, Lee CM, Lu SN, Wang JH, Tung HD, Chen TM, Chen CH, Changchien CS. Is delayed normalization of alanine aminotransferase a poor prognostic predictor in chronic hepatitis C patients treated with a combined interferon and ribavirin therapy? J Gastroenterol Hepatol. 2002;17:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, Vinelli F, Scotto G, Bacca D, Annese M. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 450] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, Sayed K, Moustafa A, Hakem SA, Ibrahiem A. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic response. Hepatology. 2007;46:1732-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Gevers TJ, Slavenburg S, van Oijen MG, Drenth JP. Treatment extension benefits HCV genotype 1 patients without rapid virological response: a systematic review. Neth J Med. 2011;69:216-221. [PubMed] |
| 15. | Coppola N, Pisaturo M, Tonziello G, Sagnelli C, Sagnelli E, Angelillo IF. Efficacy of Pegylated interferon α-2a and α-2b in patients with genotype 1 chronic hepatitis C: a meta-analysis. BMC Infect Dis. 2012;12:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 886] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 17. | Dogan UB, Akin MS, Yalaki S. Sustained virological response based on the week 4 response in hepatitis C virus genotype 1 patients treated with peginterferons α-2a and α-2b, plus ribavirin. Eur J Gastroenterol Hepatol. 2013;25:1317-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 575] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 19. | Davis GL, Lindsay K, Albrecht J, Bodenheimer HC, Balart LA, Perrillo RP, Dienstag JL, Tamburro C, Schiff ER, Carey W. Clinical predictors of response to recombinant interferon-alpha treatment in patients with chronic non-A, non-B hepatitis (hepatitis C). The Hepatitis Interventional Therapy Group. J Viral Hepat. 1994;1:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Civeira MP, Prieto J. Early predictors of response to treatment in patients with chronic hepatitis C. J Hepatol. 1999;31 Suppl 1:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ribeiro RM, Layden-Almer J, Powers KA, Layden TJ, Perelson AS. Dynamics of alanine aminotransferase during hepatitis C virus treatment. Hepatology. 2003;38:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Kronenberger B, Zeuzem S, Sarrazin C, Mihm U, von Wagner M, Hofmann WP, Piiper A, Herrmann E. Dynamics of apoptotic activity during antiviral treatment of patients with chronic hepatitis C. Antivir Ther. 2007;12:779-787. [PubMed] |
| 23. | Turbide C, Soulellis C, Deschênes M, Hilzenrat N. Does a rapid decline in the hematological and biochemical parameters induced by interferon and ribavirin combination therapy for the hepatitis C virus predict a sustained viral response? Can J Gastroenterol. 2008;22:149-152. [PubMed] |
| 24. | Kim YJ, Jang BK, Kim ES, Park KS, Cho KB, Chung WJ, Hwang JS. Rapid normalization of alanine aminotransferase predicts viral response during combined peginterferon and ribavirin treatment in chronic hepatitis C patients. Korean J Hepatol. 2012;18:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Thurairajah PH, Thorburn D, Hubscher S, White A, Lai WK, O’Donnell K, Mutimer D. Incidence and characterization of serum transaminases elevations in pegylated interferon and ribavirin treated patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |