Published online Jan 28, 2013. doi: 10.3748/wjg.v19.i4.516
Revised: September 12, 2012
Accepted: October 30, 2012
Published online: January 28, 2013
Processing time: 174 Days and 22.5 Hours
AIM: To determine the evolution of transient elastography (TE) in patients with alcoholic liver disease according to alcohol cessation or continuation.
METHODS: We retrospectively selected in our local database all patients who had two TE between June 2005 and November 2010 with chronic alcohol excessive consumption and excluded those with associated cause of liver disease. TE was performed at least one week apart by senior operator. TE examinations with less than ten successful measures or with an interquartile range above 30% were excluded. We retrospectively reviewed file of all patients to include only patient followed up by trained addictologist and for which definite information on alcohol consumption was available. Concomitant biological parameters [aspartate amino transferase (AST), alanine amino transferase and gamma-glutamyl transpeptidase (GGT)] within 4 wk of initial and final TE were recorded. Putative fibrosis score according to initial and final TE were determined with available cut-off for alcoholic liver disease and hepatitis C. Initial and final putative fibrosis score were compared according to alcohol consumption during follow-up.
RESULTS: During the study period 572 patients had TE examination for alcoholic liver disease and 79 of them had at least two examinations. Thirty-seven patients met our criteria with a median follow-up of 32.5 wk. At the end of the study, 13 (35%) were abstinent, and 24 (65%) relapsers. Eight patients had liver biopsy during follow-up. TE decreased significantly during follow-up in 85% of abstinent patients [median (range): -4.9 (-6.1,-1.9)], leading to a modification of the putative fibrosis stage in 28%-71% of patient according to different cut-off value. In relapsers TE increased in 45% and decreased in 54% of patient. There was no statistical difference between initial and final TE in relapsers. In the overall population, using 22.6 kPa as cut-off for cirrhosis, 4 patients had cirrhosis at initial TE and 3 patients had cirrhosis at final TE. Using 19.5 kPa as cut-off for cirrhosis, 7 patients had cirrhosis at initial TE and 5 patients had cirrhosis at final TE. Using 12.5 kPa as cut-off for cirrhosis, 16 patients had cirrhosis at initial TE and 15 patients had cirrhosis at final TE. Evolution of biological data was in accordance with the relapse or abstinent status: abstinence ratio (duration of abstinence/duration follow-up) was correlated with AST ratio (r = -0.465, P = 0.007) and GGT ratio (r = -0.662, P < 0.0001). GGT was correlated with initial (r = 0.488, P = 0.002) and final TE (r = 0.49, P < 0.005). Final TE was correlated with AST (r = 0.362, P < 0.05). Correlation between TE ratio and AST ratio (r = 0.44, P = 0.01) revealed that TE varied proportionally to AST for all patients irrespective of their alcohol status. The same relationship was observed between TE ratio and GGT ratio (r = 0.65, P < 0.0001). Evolution of TE was significantly correlated with the ratio of time of abstinence to observation time (r = -0.387, P = 0.016) and the evolution of liver enzymes.
CONCLUSION: TE significantly decreased with abstinence. Results of TE in alcoholic liver disease cannot be interpreted without taking into account alcohol consumption and liver enzymes.
- Citation: Bardou-Jacquet E, Legros L, Soro D, Latournerie M, Guillygomarc’h A, Lan CL, Brissot P, Guyader D, Moirand R. Effect of alcohol consumption on liver stiffness measured by transient elastography. World J Gastroenterol 2013; 19(4): 516-522
- URL: https://www.wjgnet.com/1007-9327/full/v19/i4/516.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i4.516
Alcohol excessive consumption is a major public health issue[1,2] as it may lead to liver fibrosis and cirrhosis with life threatening complications such as hepatocellular carcinoma[3], liver failure and death[4]. Early diagnosis of cirrhosis is an important goal as it may promote change in alcohol consumption by increasing motivation in patients and lead for specific screening of esophageal varices[5] or hepatocellular carcinoma[6]. Diagnosis of cirrhosis can be easy when patients have clinical[7] or biological signs of portal hypertension or liver failure. But at the early stage, these signs are for the most part absent, and liver biopsy is the only way to assess fibrosis[8]. Cost and complications of liver biopsy[9-11], leading to significant morbidity or mortality, urge the search for non invasive tools using clinical, biological or morphological finding to establish a probability of fibrosis[12-15].
Transient elastography (TE) is a reproducible and non-invasive test to assess liver fibrosis in chronic liver disease[16]. Initial studies in hepatitis C[17-19] had shown an accurate identification of patients with mild fibrosis or cirrhosis and TE is now part of the European Association for the Study of Liver (EASL) clinical guidelines for hepatitis C management[20].
Studies have also suggested correlation between TE and fibrosis in other chronic liver disease such as hepatitis B[21,22], primary biliary cirrhosis[23], primary sclerosing cholangitis[24], and non alcoholic steatohepatitis[25,26], but it then appears that disease-specific cut-off values for significant fibrosis or cirrhosis should be used[27,28].
Concerning alcoholic liver disease, two studies have shown that TE is correlated with fibrosis[27,28], but diagnosis of severe fibrosis or cirrhosis need the use of higher cut-off values than in other diseases, moreover there are some discrepancies between proposed cut-off values. Explanations offered are different spatial distribution of fibrosis and, that acute liver damage increases liver stiffness by different ways such as hepatocyte swelling, cholestasis, inflammation, and hepatocyte necrosis[29]. Thus excessive alcohol intake, with or without acute alcoholic hepatitis, could increase TE and lead to a false positive diagnosis of fibrosis. Of note, no information regarding patient alcohol consumption at time of fibrosis evaluation was available in the studies which proposed those cut-off values. Our hypothesis is that the alcohol consumption greatly influences TE and thus explains the difficulty to validate TE in alcoholic liver disease, but in the other hand it could make TE a useful tool in the follow-up of patient as an indicator of alcohol consumption beyond the sole fibrosis evaluation.
The aim of this study was to assess the evolution of TE during follow up of patients with alcohol excessive intake history in regards to alcohol withdrawal or continuation. Secondary objective was to determine correlation between biological parameters and TE modification.
This is a retrospective study conducted in the department of Hepatology and Addictology, University Hospital, Rennes, France. In this unit, a measure of TE is routinely performed in patients hospitalized for alcohol withdrawal or compensated alcoholic liver disease. TE is measured with the Fibroscan® (Echosens, Paris) by a senior operator.
Internal memory of our Fibroscan® was screened for patients who had two TE between June 2005 and November 2010 for alcoholic liver disease. The two different TE examinations had to be performed at an interval of more than one week, ten successful measures were required with an interquartile range ≤ 30% of the median value. Then we reviewed the patients files to collect information on their history, duration of alcohol withdrawal, and alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma glutamyltranspeptidase (GGT) at the time (or up to 4 wk before or after) of the two TE.
Patients were excluded if (1) they presented other causes of chronic liver disease (hepatitis B or C, extra-hepatic cholestasis, hemochromatosis); (2) information about alcohol consumption between the two TE were missing, or (3) liver tests at the time of the two TE were missing.
Patients’ alcohol consumption during follow-up was assessed by self-declaration and corroborated by biochemistry evolution. Only patients followed by trained addictologists with definite information regarding alcohol consumption were included. We defined abstinence ratio as the duration of abstinence/duration of observation. In this study, patients were considered as abstinent if they were totally sober at least 90% of the time between the two TE.
TE initial (TEi) corresponds to the first TE, TE final (TEf) to the second TE and ΔTE to the difference between TEf and TEi. To determine variation of TE, we also defined TE ratio as (TEf-TEi)/TEi.
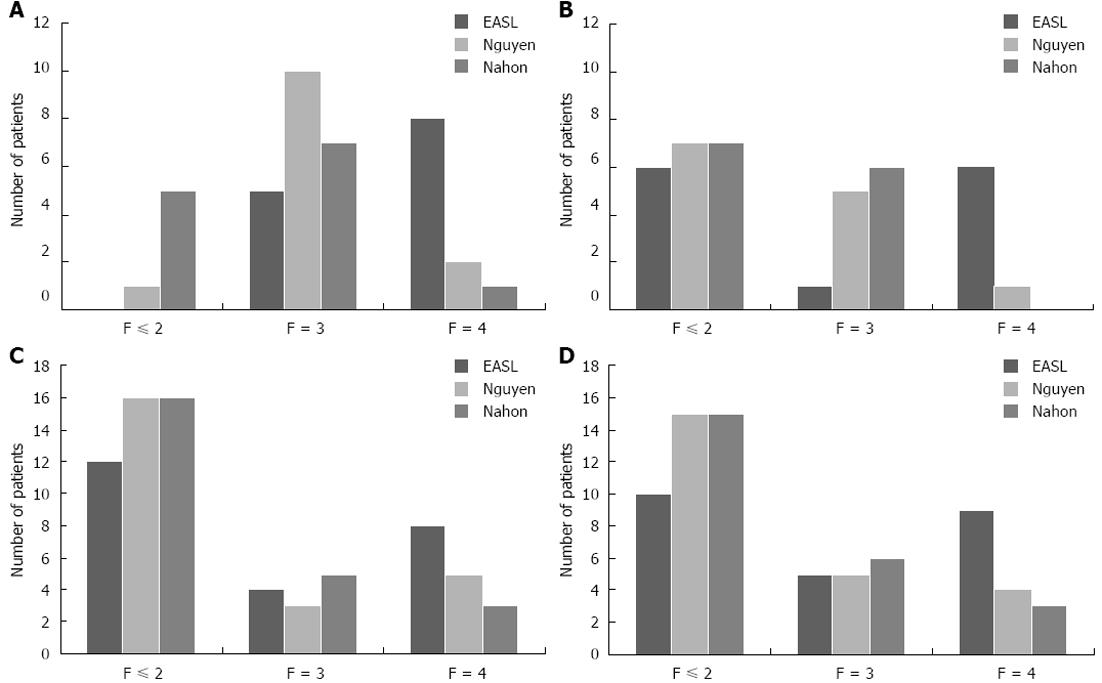
The liver fibrosis stages of the patients were calculated according to TE using cut-off values from different publications or guidelines: EASL recommendations for hepatitis C: 8 kPa for F ≥ 3 and 12.5 kPa for F = 4; Nahon[6]: 12.9 kPa for F ≥ 3 and 22.6 kPa for F = 4; and Nguyen[7]: 5.9 kPa for F ≥ 1, 7.8 kPa for F ≥ 2, 11 kPa for F ≥ 3 and 19.5 kPa for F = 4. Liver fibrosis stage referred to Metavir classification[9]: stage 0 corresponds to the absence of fibrosis, 1 to the presence of periportal fibrotic extension, 2 to periportal septa; severe fibrosis corresponds to stages 3 (porto-central septa) and 4 (cirrhosis). We defined Δstage as the difference between the fibrosis stages calculated at the first and the second TE, according to the different publications.
When liver biopsy had been performed, fibrosis was classified according to Metavir score[30].
We defined ASTi and GGTi as biological data related to the first TE, ASTf and GGTf to the second TE. The AST ratio [(ASTf-ASTi)/ASTi] and the GGT ratio [(GGTf-GGTi)/GGTi] were calculated to determine variation of biological markers.
Demographic data were expressed as median and inter quartile range. Qualitative variables were compared using the χ2 test or Fischer exact test, when appropriate. Quantitative variables were compared using the Mann Whitney U test or Wilcoxon test for paired values. Correlation between variables was assessed using Spearman’s rank correlation coefficient.
All tests were two sided with a significance of 5%. Tests were performed using JMP® software version 9.0 SAS, Cary, NC.
During the period of the study, 6160 TE examinations were performed, of which 572 for patients with alcohol liver disease and 79 of them had 2 examinations. Forty-two patients were excluded because of missing data (lack of biological data or definite information on alcohol intake). Thus 37 patients corresponded to our criteria and were considered for analysis.
There were 7 women and 30 men with a median age of 44 years (39-53.5 years). Median follow up time was 32.5 wk (15-85 wk). At the end of the observation time, 13 (35%) were considered as abstinent, and 24 as relapsers.
Results of TE measures and biochemistry are shown in Table 1. Patients had marked increase in liver enzymes. TEi was significantly correlated with GGTi (r = 0.488, P = 0.002), and TEf with ASTf (r = 0.362, P < 0.05) and GGTf (r = 0.49, P < 0.05).
| Abstinent (n = 13) | Relapser (n = 24) | |||
| Initial | Final | Initial | Final | |
| Normal AST | 8% | 75% | 8% | 15% |
| AST (IU/L) | 130 (39-374) | 42 (15-118) | 140 (16-472) | 119 (17-749) |
| ALT (IU/L) | 109 (34-387) | 44 (13-163) | 88 (18-266) | 76 (9-329) |
| GGT (IU/L) | 1414 (121-3405) | 301 (17-1040) | 874 (48-5749) | 536 (53-1858) |
| GGT (kPa) | 15.5 (10.9-24.3) | 11.7 (4.8-22.5) | 11.7 (4-34.8) | 13.5 (4.2-26.4) |
| Age, yr | 48 ± 9 | 44 ± 10 | ||
| Sex (M/F) | 11/2 | 4/20 | ||
Repartition of patient according to putative liver fibrosis stage was different depending on TE cut-off used (EASL[2], Nahon[6] or Nguyen[7]). Results are shown in Figure 1. Overall, 16 patients had a TE indicating liver cirrhosis according to EASL, 7 according to Nguyen and 4 according Nahon cut-off values during initial evaluation.
Liver biopsy was performed in 8 patients. Results are shown in Table 2. According to EASL cut-off, 2 relapsers were misclassified. Patient 5 had stage 1 fibrosis, with TEi indicating severe fibrosis and normal TEf; and patient 6 had stage 3 fibrosis with TEf indicating severe fibrosis and normal TEi.
| Patient | TEi (kPa) | EASL stage | Liver biopsy initial | TEf (kPa) | EASL stage | Liver biopsy final | Alcohol status |
| 1 | 33.8 | F4 | 26.4 | F4 | F4 | Relapser | |
| 2 | 34.8 | F4 | 75 | F4 | F4 | Relapser | |
| 3 | 10.2 | F3 | F4 | 20.6 | F4 | Relapser | |
| 4 | 21.3 | F4 | 14 | F4 | F3 | Relapser | |
| 5 | 9.9 | F3 | F1 | 4.6 | F ≤ 2 | Relapser | |
| 6 | 5.3 | F ≤ 2 | 16.9 | F4 | F3 | Relapser | |
| 7 | 20.3 | F4 | 14.3 | F4 | F4 | Abstinent | |
| 8 | 11.7 | F3 | 13.6 | F4 | F4 | Abstinent |
During the observation period, 85% of abstinent patients had a decrease of TE, whereas among relapsers 45% had an increase and 54% a decrease of TE (P = 0.05, χ2 test).
In the abstinent group, TE decreased significantly (Wilcoxon test for paired values, P = 0.0085) [median ΔTE = -4.9 kPa (-6.1, -1.9)] whereas TEf and TEi were not significantly different in relapsers [median ΔTE = -0.4 kPa (-4.8, +4)]. Δ TE were significantly different between abstinent and relapsers patients (P < 0.05).
There were variations in the putative staging of fibrosis according to TE during the observation time (Table 3). Putative fibrosis stage decreased in abstinent patients (in 28%-71% according to the cut-off used). In relapsers, stability, increase or decrease was observed. Δ stage according to EASL and Nguyen cut-offs were significantly different between relapsers and abstinent patients (P = 0.015 and P = 0.012 respectively).
| Cut-off value | Increase in one or more stage of liver fibrosis (%) | Stable (%) | Decrease in one or more stage of liver fibrosis (%) | |
| Abstinents | EASL | 7 | 36 | 57 |
| Nguyen | 7 | 22 | 71 | |
| Nahon | 7 | 65 | 28 | |
| Relapsers | EASL | 29 | 55 | 16 |
| Nguyen | 33 | 30 | 37 | |
| Nahon | 16 | 72 | 12 |
TE decreased proportionally to abstinence, as demonstrated by the correlation between abstinence ratio and TE ratio (r = -0.47, P = 0.0029).
As expected abstinence ratio was correlated with AST ratio (r = -0.465, P = 0.007) and GGT ratio (r = -0.662, P < 0.0001).
Correlation between TE ratio and AST ratio (r = 0.44, P = 0.01) revealed that TE varied proportionally to AST for all patients irrespective of their alcohol status. The same relationship was observed between TE ratio and GGT ratio (r = 0.65, P < 0.0001).
TE is a useful tool which has been extensively validated in hepatitis C, but despite the incomparable more important frequency of alcoholic liver disease, few studies have been performed to assess TE in alcoholics. Moreover, despite of this active research, few studies evaluated TE regarding the current alcohol consumption of patients and only one studied the evolution of TE after alcohol detoxification. Our study is the first to assess TE evolution with a medium term follow up according to the alcohol consumption of patients.
Our results confirm the important variation of TE with alcohol withdrawal: 85% of abstinent patients showed a significant decrease of TE during a median follow up period of 32.5 wk. Moreover we showed a likely risk of overestimation of the fibrosis stage according to the different cut off values using TEi. TE evolution was correlated to AST and GGT evolution, which are usually used as marker of alcoholic hepatitis.
The results of our study may suffer from the retrospective data collection and the low number of patient. This is in part induced by the stringent inclusion criteria regarding missing data about alcohol consumption. Indeed, to avoid bias induced by poor quality of data and follow up bias we excluded all patients that were not followed by an addictologist and who lack definite information on alcohol consumption. Moreover, albeit alcoholic liver disease is an extremely frequent disease, patients are seldom compliant with follow up in the absence of severe disease, making rare those completing our inclusion criteria as shown by the number of TE performed for alcoholic liver disease (572) contrasting with the low number of patient who had a second TE (79).
In chronic hepatitis C, TE cut off associated with fibrosis greater than stage 3 ranges from 8 to 9 kPa, and cirrhosis can be diagnosed with cut-off ranging from 14 to 15 kPa. Studies performed to assess TE diagnostic accuracy in other diseases than hepatitis C found higher cut off values for significant fibrosis or cirrhosis. Ganne-Carrié et al[31] studied 122 patients with non alcoholic or alcoholic steatohepatitis and found a cut off value for cirrhosis of 21.5 kPa. Nahon et al[27] studied 147 patients with alcoholic liver disease. Seventy-five percent of them had at least significant fibrosis, which is far more important than expected in alcoholic patients. They found a cut off value for significant fibrosis of 12.9 kPa and 22.6 kPa for cirrhosis. All the patients without cirrhosis and misclassified by TE had histological alcoholic hepatitis. In both studies, the patients were included if liver biopsy was indicated for chronic liver disease and there was no information regarding current alcohol consumption at the time of evaluation. Those data stressed the potential impact of alcohol induced liver modification beyond fibrosis on TE.
Coco et al[32] had reported that liver stiffness assessed by TE increased 1.3-to-3 fold during ALT flares in patients with viral hepatitis exacerbation. Studying 195 patients who had both liver biopsy and TE in acute liver damage, Fraquelli et al[33] showed that liver stiffness is increased in the acute phase with a correlation between TE and necroinflammatory activity. Recently Mueller et al[34] have showed that decrease of TE is correlated with the decrease of AST. Studying liver biopsies for alcoholic liver disease, they found that TE was constant if AST ≤ 100 UI/L and that accuracy of cirrhosis diagnosis by TE was improved in patient with AST ≤ 100 UI/L. Those data suggest that TE should be assessed differently regarding the current alcohol consumption and the presence or absence of acute liver modification or biochemical activity. In our study we used biochemical data gathered up to 4 wk from the date of TE, this is a long period which could have induced bias, however the rate of decrease or increase of TE in regard to the biochemical evolution remain to be determined in prospective study.
To date only one study assessed the evolution of TE with alcohol detoxification. Gelsi et al[35] studied in a population from an addictology unit the evolution of TE after alcohol weaning over a period of 60 d, and compared this evolution between relapser and abstinent patients. They found a rapid decrease of TE (-21% ± 27% at day 8) with detoxification in an increase proportion of patients if abstinence was sustained: 41% of patient had a decreased at day 8 and 66.7% at day 60. Relapsers were found to have a new increase in TE during follow up after alcohol relapse. As fibrosis is not likely to evolve during that short period of time, and albeit no liver biopsies were performed, one can assume that first TE measurement could have lead to overestimation of fibrosis.
Our results confirm those data, during a much longer follow up period (median 32.5 wk) with a precise addictologic follow up. TE decrease after alcohol cessation over a long period of time, and this was of particular importance in TEi ranging from 8 to 16 kPa, which could indicate significant fibrosis or cirrhosis in chronic hepatitis C, but should be interpreted with caution in alcoholic liver disease. However due to the retrospective design of the study which induced a variable timespan between TE examination, we could not assess the optimal time to perform TE after alcohol cessation.
Relapsers were found to have either an increase or a decrease of TE during follow up, this could be due to the level of alcohol consumption after relapse, as relapsers could have a lower alcohol consumption during follow up which could lead to a decrease of TE, as suggested by the correlation between Δ TE and GGT ratio. This point should be assessed in a prospective study with precise alcohol consumption amount, if confirmed TE could thus be a useful tool to monitor adherence during follow up and fluctuation in alcohol consumption.
TE was correlated with AST and GGT, and TE ratio was correlated with AST and GGT ratio, indicating that TE could be corrected by a calculated modifying factor based on liver enzymes value in order to increase the precision of this test in the diagnosis of fibrosis in alcoholic liver disease. This remains to be proven on large scale prospective studies of TE during alcohol withdrawal with liver biopsy as a gold standard. Such a study should also determine the optimal duration of alcohol cessation before initial increased TE could be controlled.
In conclusion our results show that TE decreased significantly after alcohol cessation over a long period of follow up. Thus TE in alcoholic liver disease should be interpreted with caution and assessed in regard to the current alcohol consumption. Variation of TE is correlated to AST and GGT suggesting that fibrosis could be more likely overestimated in patients with high biological perturbations. Large-scale prospective studies should be performed to determine the different optimal cut off values according to alcohol consumption and more data are required to determine the best delay of alcohol cessation for TE evaluation. TE examination could be a useful tool during the follow-up of alcoholic liver disease to assess actual alcohol consumption and maybe used as a prognosis tool as in chronic hepatitis C[36].
Excessive alcohol consumption is a major public health issue. Alcoholic liver disease can lead to liver fibrosis and cirrhosis with a risk of developing liver cancer or liver failure. Assessing the severity of liver fibrosis is thus an important landmark in alcoholic liver disease. Until recently the unique way to assess liver fibrosis was liver biopsy which is an invasive procedure with some risk of severe complication. Recently transient elastography (TE) was shown to be efficient in assessing liver fibrosis in chronic viral hepatitis C, it is now frequently used and acknowledged as a validated method by international hepatology society. Some studies have shown that TE could be used in alcoholic liver disease but with method differing from those in hepatitis C.
TE (Fibroscan®) is an innovative non invasive method, measuring liver stiffness which is correlated to the severity of liver fibrosis. It has been widely validated in hepatitis C, but many studies have shown that results cannot be transposed “as this” to other type of liver disease because liver stiffness can be modified by other liver anomaly than fibrosis. In the area of alcoholic liver disease and liver fibrosis assessment, the research hotspot is how to use liver stiffness measured by TE and what are the parameter influencing liver stiffness.
This study showed that if performed in patient with alcoholic liver disease but without biological inflammation, performance of TE to assess fibrosis was good, which led the auhors to study the evolution of liver stiffness in patient with alcoholic liver disease in regard to their alcohol consumption.They compared the evolution of liver stiffness and biological data in patient with continued or stopped alcohol consumption. They showed that liver stiffness significantly decreases in patient after alcohol cessation and that this decrease is proportional to the severity of the biological inflammation.
The study results suggest that transient elastrography could be used in alcoholic liver disease by interpreting stiffness values in regard to the ongoing or not alcohol consumption. This hypothesis needs prospective confirmation before clinical application.
Liver fibrosis is a “scar” that is developed in liver secondary to toxic (alcohol, iron overload) or viral (hepatitis C) damages. With increasing amount of fibrosis, cirrhosis appear thus leading to liver loss of function and an increased risk of liver cancer.
The enclosed manuscript aims to the effect of alcohol consumption on liver stiffness measured by TE. The aim of the manuscript is sound.This is an interesting study.
P- Reviewers Badea R, Sandahl TD S- Editor Jiang L L- Editor A E- Editor Xiong L
| 1. | Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol. 2008;23 Suppl 1:S2-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Cargiulo T. Understanding the health impact of alcohol dependence. Am J Health Syst Pharm. 2007;64:S5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Williams R. The pervading influence of alcoholic liver disease in hepatology. Alcohol Alcohol. 2008;43:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 6. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 7. | McCormick PA, Nolan N. Palpable epigastric liver as a physical sign of cirrhosis: a prospective study. Eur J Gastroenterol Hepatol. 2004;16:1331-1334. [PubMed] |
| 8. | Persico M, Palmentieri B, Vecchione R, Torella R, de SI. Diagnosis of chronic liver disease: reproducibility and validation of liver biopsy. Am J Gastroenterol. 2002;97:491-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Castéra L, Nègre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 11. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1736] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 12. | Sporea I, Sirli R, Deleanu A, Popescu A, Cornianu M. Liver stiffness measurement by transient elastography in clinical practice. J Gastrointestin Liver Dis. 2008;17:395-399. [PubMed] |
| 13. | Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:557-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Bedossa P. Assessment of hepatitis C: non-invasive fibrosis markers and/or liver biopsy. Liver Int. 2009;29 Suppl 1:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16:300-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 18. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 19. | Gómez-Domínguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. Transient elastography: a valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 21. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 22. | Viganò M, Paggi S, Lampertico P, Fraquelli M, Massironi S, Ronchi G, Rigamonti C, Conte D, Colombo M. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther. 2011;34:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Gómez-Dominguez E, Mendoza J, García-Buey L, Trapero M, Gisbert JP, Jones EA, Moreno-Otero R. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment Pharmacol Ther. 2008;27:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 26. | Abenavoli L, Beaugrand M. Transient elastography in non-alcoholic fatty liver disease. Ann Hepatol. 2012;11:172-178. [PubMed] |
| 27. | Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Lédinghen V, Douvin C, Marcellin P, Ganne-Carrié N, Trinchet JC, Beaugrand M. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, Brevet M, Grignon P, Lion S, Le Page L. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther. 2008;28:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 30. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 31. | Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 32. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 33. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 34. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [PubMed] |
| 35. | Gelsi E, Dainese R, Truchi R, Mariné-Barjoan E, Anty R, Autuori M, Burroni S, Vanbiervliet G, Evesque L, Cherikh F. Effect of detoxification on liver stiffness assessed by Fibroscan® in alcoholic patients. Alcohol Clin Exp Res. 2011;35:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-1979, 1979.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |