Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6228
Revised: June 12, 2013
Accepted: July 4, 2013
Published online: October 7, 2013
Processing time: 182 Days and 7.6 Hours
AIM: To isolate biliary lipid-carrying vesicles from isolated perfused rat livers after taurohyodeoxycholic acid (THDC) infusion. Biliary lipid vesicles have been implicated in hepatic disease and THDC was used since it increases biliary phospholipid secretion.
METHODS: Rat livers were isolated and perfused via the hepatic portal vein with THDC dissolved in Krebs Ringer Bicarbonate solution, pH 7.4, containing 1 mmol/L CaCl2, 5 mmol/L glucose, a physiological amino acid mixture, 1% bovine serum albumin and 20% (v/v) washed human erythrocytes at a rate of 2000 nmol/min for 2 h. The livers were then removed, homogenized and subjected to centrifugation, and the microsomal fraction was obtained and further centrifuged at 350000 g for 90 min to obtain subcellular fractions. These were analyzed for total phospholipid, cholesterol, protein and alkaline phosphodiesterase I (PDE).
RESULTS: No significant changes were observed in the total phospholipid, cholesterol and protein contents of the gradient fractions obtained from the microsomal preparation. However, the majority of the gradient fractions (ρ= 1.05-1.07 g/mL and ρ = 1.95-1.23 g/mL) obtained from THDC-infused livers had significantly higher PDE activity compared to the control livers. The low density gradient fraction (ρ = 1.05-1.07 g/mL) which was envisaged to contain the putative vesicle population isolated from THDC-perfused livers had relatively small amounts of phospholipids and protein when compared to the relevant control fractions; however, they displayed an increase in cholesterol and PDE activity. The phospholipids were also isolated by thin layer chromatography and subjected to fractionation by high performance liquid chromatography; however, no differences were observed in the pattern of the fatty acid composition of the phospholipids isolated from THDC and control perfused livers. The density gradient fractions (ρ = 1.10-1.23 g/mL) displayed an increase in all the parameters measured from both control and THDC-infused livers.
CONCLUSION: No significant changes in biliary lipids were observed in the fractions from THDC-infused livers; however, PDE activity was significantly increased compared to the control livers.
Core tip: Bile contains various constituents including cholesterol and phospholipid, mainly phosphatidylcholine with a unique fatty acid composition of 1-palmitoyl 2-linoleyl (16:0-18:2) phosphatidylcholine and 1-palmitoyl 2-oleoyl (16:0-18:1). These biliary lipids are transported in vesicles from a specific intra-hepatic pool and an increase in biliary lipid-carrying vesicles may have implications for hepatic diseases such as gallstone formation. Taurohyodeoxycholic acid (THDC) stimulates the secretion of biliary phospholipids; hence THDC-infused rat livers were subjected to ultracentrifugation in order to isolate these phospholipid-carrying vesicles. The isolation of these biliary lipid-carrying vesicles was not successful; however, vesicles enriched in PDE activity were obtained.
- Citation: Hismiogullari AA, Hismiogullari SE, Rahman K. Isolation and biochemical analysis of vesicles from taurohyodeoxycholic acid-infused isolated perfused rat livers. World J Gastroenterol 2013; 19(37): 6228-6236
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6228
Phospholipids and cholesterol are synthesized in the hepatocytes and are thought to be transferred into bile by vesicular and non-vesicular mechanisms. Biliary lipids mainly consist of cholesterol and phospholipids and their secretion into bile is effected by secretion of bile salts[1]. Hepatocytes acquire biliary lipid by three pathways namely biosynthesis, lipoproteins and existing lipid molecules drawn from intracellular membranes and newly synthesized biliary lipids; these account for less than 20% of the total lipids[2].
The majority of biliary phospholipid is phosphatidylcholine (PC) with distinct fatty acid composition, namely 1-palmitoyl 2-linoleyl (16:0-18:2) PC and 1-palmitoyl 2-oleoyl (16:0-18:1), whereas hepatocyte PC contains significant amounts of different phospholipid classes[3,4]. Very few studies have been performed on biliary lipid transport in hepatocytes[5,6], whereas numerous studies have been performed on bile, its formation and composition especially related to lipids, and these studies have identified several physical forms of lipid carriers, including biliary vesicles[7,8].
The main source of biliary lipid, before its appearance in bile, has been suggested to be the bile canalicular membrane where it is removed by the detergent action of bile salts. These lipids are then thought to be continuously replaced from within the cell, probably via vesicular transport, for biliary lipid secretion to continue without damage to the liver and canalicular membrane[9]. In support of this, inhibitors of microtubular function such as colchicine and vinblastine have been shown to reduce biliary lipid secretion[10-12]. Such vesicles supplying lipids to the plasma membrane have also been shown and isolated in other cell types, thus, it can be postulated that biliary lipid is probably supplied to the canalicular membrane via such vesicles. This is supported by the fact that increased numbers of vesicles have been observed accumulating near the bile canaliculus during extensive bile acid secretion[2,9,13-15]. The isolation of putative biliary lipid-carrying vesicles, however, is difficult due to the wide range of vesicle types in hepatocytes, and the difficulty of identifying them because of inadequate criteria.
Hepatic ATP-binding cassette half-transporter genes 5/8 (ABCG5 and ABCG8) are expressed in the canalicular membrane of hepatocytes and have an essential role in biliary cholesterol secretion[16-19]. However, the pathways involved in trans-hepatic cholesterol trafficking into bile are still not clear and a specific cholesterol transport protein has not been confirmed in hepatocytes. Biliary cholesterol secretion is important for the two important disease complexes of atherosclerotic cardiovascular disease (CVD) and gallstone disease[1]. In atherosclerotic CVD, biliary cholesterol secretion is thought to be the final step in the completion of the reverse cholesterol transport pathway which includes the transport of peripheral cholesterol back to the liver for excretion into bile. Increase in biliary cholesterol secretion can lead to the supersaturation of bile and under the right conditions this may lead to the formation of cholesterol gallstones[1]. Taurohyodeoxycholic acid (THDC) is a natural 6α-hydroxylated bile acid with hydrophilic properties, causing more secretion of PC into bile compared to tauroursodeoxycholic acid and taurocholic acid, whereas no significant differences were found in the biliary secretion of cholesterol[20]. Due to its relatively high hydrophilicity, THDC has been proposed for use instead of other bile acids for the treatment of cholesterol gallstone dissolution[21]. Angelico et al[20] showed by the use of electron microscopy that increased recruitment of vesicles and lamellar bodies around and within bile canaliculi in the liver occurred with THDC infusion. The identification of biliary lipid-carrying vesicles may have implications for the treatment of hepatic disorders such as cholesterol gallstone formation.
The aim of this study was to isolate these biliary lipid-carrying vesicles in hepatocytes by using a novel gradient centrifugation technique and to verify their origin by separating PC by thin layer chromatography (TLC) and measuring its unique fatty acid pattern by high-performance liquid chromatography (HPLC). Cholesterol was measured by gas liquid chromatography (GLC).
All chemicals were purchased from Sigma Chemical Co., Poole, Dorset, United Kingdom, except for cannulation tubing PP10 (internal diameter 0.28 mmol/L) which was obtained from Portex Ltd., Hythe, United Kingdom.
Animals used throughout this study were male Wistar rats (250-300 g), bred within Liverpool John Moores University, Life Services Support Unit, and they were allowed free access to standard laboratory diet in powdered form.
Rats were anaesthetized with sodium pentobarbitone (6 mg/100 g body weight, intraperitoneally) before starting the experiment. Once isolated, livers were perfused in the absence and presence of THDC infusion in situ using the method of Rahman and Coleman[22]. Heparin (2500 units/0.5 mL) was injected into the vena cava and after 2 min, the hepatic portal vein was cannulated with a Wallace 17.5 G cannula and the perfusion was commenced immediately with 150 mL of Krebs ringer bicarbonate buffer, pH 7.4, containing 1 mmol/L CaCl2, 5 mmol/L glucose, a physiological amino acid mixture, 1% bovine serum albumin and 20% (v/v) washed human erythrocytes, and the abdominal aorta was severed. The inferior vena cava was then cannulated with a Wallace 16 G cannula and a recycling perfusion commenced by returning the efferent perfusate to the original perfusate pool which was gassed continuously with O2/CO2 (19:1, v/v). The livers were maintained in a thermostatically controlled cabinet at 37 °C throughout the experiment. As soon as the liver perfusion was established, THDC infusion was commenced into the hepatic portal cannula at a rate of 2000 nmol/min for 2 h to stimulate delivery of lipid-carrying vesicles to the canalicular membrane.
At the end of perfusion livers were removed, weighed and transferred to 3 vol. (w/v) of ice-cold buffered sucrose (0.25 mol/L containing 1 mmol/L HEPES pH 7.4). They were then cut into several large pieces and swirled around in the buffer to remove as much blood as possible. The livers were then minced finely with sharp scissors, transferred to an ice-cold homogenizing vessel and were finally homogenized with about six strokes of the pestle at full speed. Finally, the homogenate was made up to 4 vol. (w/v) with sucrose buffer solution.
The homogenate from the liver was used to produce subcellular fractions based on the method of Ford and Graham[23]. A sample of homogenate (3-4 mL) was removed for analysis and the remainder was centrifuged in a fixed angle rotor at 4 °C for 10 min at 1000 g to pellet the nuclei and heavy mitochondria. The pellet was then suspended in sucrose buffer and stored frozen at -20 °C until analysis.
Further centrifugation was performed at 4000 g for 10 min to produce the mitochondrial fraction, followed by 15000 g for 20 min to produce the light mitochondrial and lysosome fraction. A final centrifugation step at 100000 g for 45 min was then performed and the microsomal fraction was obtained. All fractions were assayed for cholesterol, phospholipids, protein and PDE activity
The microsomal pellet was then dissolved in sucrose buffer solution up to 8 mL and then loaded onto 2 mL of OptiPrepTM (1.32 g/mL) in a Beckman Vti65 vertical tube rotor and centrifuged at 350000 g for 90 min at 4 °C. At the end of the centrifugation, the gradient was fractioned by upward displacement into 10 × 1 mL samples and these fractions were analyzed for cholesterol, phospholipids, protein and PDE activity.
Cholesterol was analyzed by GLC as trimethylsilyl ether derivatives as described by Zak et al[24]. Phospholipid was extracted from the liver fractions as described by Bligh and Dyer[25] in a method by which lipid is extracted into a chloroform-methanol-water mixture. Addition of further chloroform and water forms a biphasic system with non-lipids passing into the methanol-water phase. The phospholipid in the chloroform phase was then assayed by the method of Bartlett[26] in which organic phosphate is digested and the resulting orthophosphate is determined by converting it to phosphomolybdic acid, which is reduced to a blue complex allowing spectrophotometric measurement at 830 nm.
PDE (EC 3.1.4.1) was measured at 37 °C, essentially as described by Trams and Lauter[27].
Sample extraction: 200 μL of sample was added to 200 μL of distilled water in a 2 mL (microcentrifuge) tube followed by the addition of 750 μL of chloroform then methanol (1:2 v/v) to each tube, vortex mixed and left to stand for 20 min. After this time, 250 μL of chloroform and 250 μL of distilled water were added and the tubes were vortex mixed and then centrifuged for 1 min. The lower organic phase was then transferred to a clean tube and placed in a water bath at 37 °C to evaporate the chloroform[25]. Samples were finally redissolved in 30 mL of chloroform, vortex mixed and loaded on to the TLC plates.
Solvent for running phospholipid plates: The plates were developed in a solvent mixture containing chloroform/methanol/glacial acetic acid/water (75:45:12:1.5 by volume). These solvent ratios were poured into a tank containing a filter paper layered against the wall of the chamber and the lid was replaced; the tank was then left to saturate for at least 30 min prior to running the plates. The plates were left to run until solvent reached the scored solvent front line (approximately 75 min) and were then removed and air dried in a fume cupboard.
Developing the TLC plates: The dried plates were developed in an iodine tank and the position of the phospholipid was marked with a needle. The PC bands were scraped and the silica transferred to extraction tubes; at the same time silica was scraped from a similar area without any phospholipids to act as a control. Phospholipids were extracted with 2 × 1 mL methanol (HPLC grade) and vortex mixed for 5 min, vortexed again, centrifuged and the methanol extract was then transferred to clean glass tubes and dried at 37 °C under nitrogen. The dried lipids were redissolved in 1 mL of methanol (HPLC grade) and 2 × 10 μL aliquots were removed for phospholipids assay. The remainder was filtered, dried at 37 °C under nitrogen and stored cool and in the dark until required for HPLC analysis. At least 20 nmol of PC was injected onto the HPLC column.
HPLC analysis was performed using a Bio-Rad HRLC 2700, Series 8000 Gradient System V 2.30.1a liquid chromatograph equipped with an oven column module, and a spectrophotometric detector, Bio-Rad Model 1801 UV monitor. The sample was injected onto the column by a Rheodyne injector equipped with a 20 μL sample loop. An HPLC column of 100 mmol/L × 4.6 mmol/L id, packed with a Spherisorb ODS2 bonded phase, and with a 3 μL particle size was used and the mobile phase consisted of 20 mmol/L choline chloride in methanol/water/acetonitrile (90:8:3, by vol.). The operating conditions were: column temperature, 60 °C; chromatographic profile: initial flow, 1 mL/min, held for min and then a linear increase to 2 mL/min over 20 min; the final flow of 2 mL/min being held for 10 min.
Protein estimation was determined by the method of Winterbourne and all samples were assayed in duplicate[28]. A sheet of 3 mmol/L Whatman chromatography paper was divided into 1 cm × 1 cm squares. Standard protein concentration BSA ranged from 0.5-8 mg/mL and the standards were prepared by spotting corresponding amounts onto the center of the squares on the sheet. 3 μL of sample was carefully spotted onto the center of individual squares and blank squares were left for the determination of background staining. The standards and samples were then left to dry and were later fixed by immol/Lersing into 10% TCA solution for 15 min and the sheet was then transferred to a working dye solution (0.04% w/v Coomassie Blue, 25% v/v ethanol and 12% v/v acetic acid) and left to stain for 1 h. The sheet was then destained by immersing in three changes of destaining solution 10% (v/v) methanol and 5% (v/v) glacial acetic acid for 10 min, and it was then left to dry in the oven at 80 °C. The grid was then cut into its individual component squares and was placed into small plastic vials containing 1 mL eluent, 1 mol/L potassium acetate in 70% (v/v) ethanol for 1 h and finally the absorbance of the eluted dye solution was read at 590 nm against the background dye measurements.
Data were subjected to 2-tailed paired t test and P values ≤ 0.05 were considered as statistically significant.
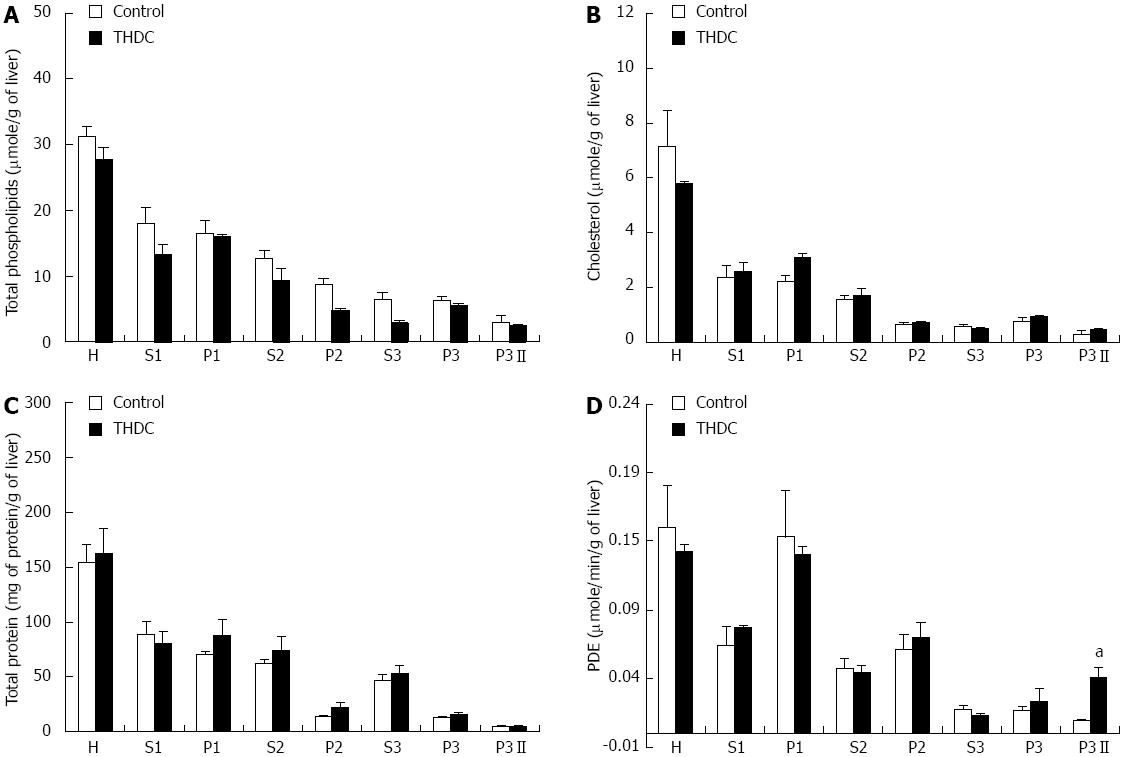
Total phospholipids and cholesterol in the subcellular fractions from isolated perfused rat livers in the absence and presence of THDC infusion are shown in Figure 1A and B. The total lipids have been expressed as μmol/g of liver due to the differences in liver weight of the animals. No significant differences were found in total phospholipids and cholesterol content of subcellular fractions from control and THDC-infused rat livers (Figure 1A and B).
Total protein and PDE activity in subcellular fractions of isolated perfused rat livers in the absence or presence of THDC infusion are depicted in Figure 1C and D. No significant differences were found in total proteins in the subcellular fractions of isolated perfused rat livers in the absence or presence of THDC infusion (Figure 1C). However, PDE activity was significantly higher in fraction P3II from THDC-infused livers (Figure 1D).
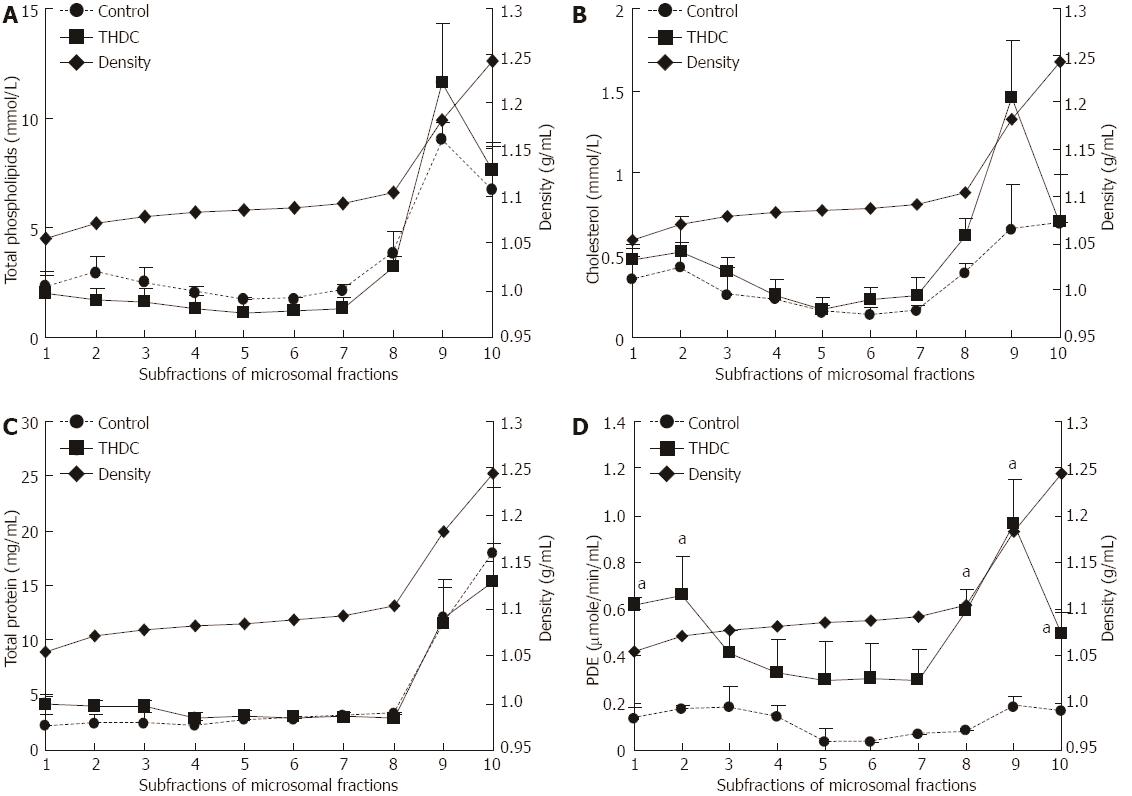
The microsomal fractions were subjected to self-generating density gradient centrifugation and the fractions were analyzed for total phospholipids, cholesterol, protein and PDE activity. The results are presented in Figure 2. Enzyme activity in THDC-infused liver fractions 1, 2, 8, 9 and 10 was significantly higher when compared to the control values. However, no significant differences were observed in total phospholipids, cholesterol and protein in the subcellular fractions of isolated perfused rat livers in the absence or presence of taurohyodeoxycholic acid infusion.
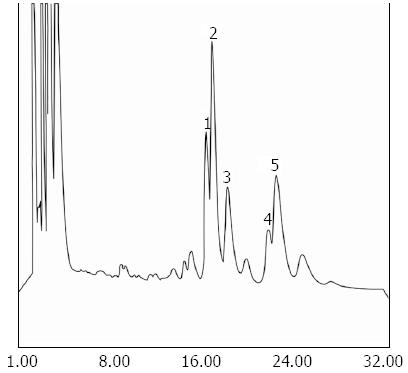
Biliary PC has a unique fatty acid pattern, 1-palmitoyl 2-linoleyl (16:0-18:2) PC, 1-palmitoyl 2-oleoyl (16:0-18:1) PC, which is distinct from that of membrane PC fatty acid pattern 1-stearoyl 2-arachidonyl (16:0-20:4). No differences in the level of subfractions of PC were found (Table 1). It was not possible to identify peak 1 due to lack of relevant standards (Figure 3).
| Fractions | PC (nmol) | C16:0 C20:4 | C16:0 C18:2 | C16:0 C18:1 |
| Control perfused fed rat livers | ||||
| 1 | 0.171 ± 0.001 | 41.147% ± 3.02% | 11.048% ± 0.8% | 8.327% ± 0.92% |
| 2 | 0.223 ± 0.003 | 42.219% ± 2.8% | 10.223% ± 0.7% | 6.49% ± 0.5% |
| 8 | 0.214 ± 0.007 | 41.545% ± 3.09% | 11.281% ± 0.8% | 6.436% ± 0.9% |
| 9 | 1.02 ± 0.05 | 43.59% ± 1.5% | 8.68% ± 1% | 6.61% ± 0.73% |
| 10 | 1.34 ± 1.1 | 44.64% ± 0.27% | 8.19% ± 0.64% | 6.84% ± 0.33% |
| THDC-perfused rat livers | ||||
| 1 | 0.193 ± 0.001 | 44.424% ± 3.29% | 7.864% ± 0.92% | 10.239% ± 1.52% |
| 2 | 0.208 ± 0.004 | 44.796% ± 2.09% | 8.177% ± 0.8% | 8.507% ± 0.93% |
| 8 | 0.444 ± 0.008 | 43.735% ± 0.98% | 9.373% ± 1.76% | 8.298% ± 1.35% |
| 9 | 1.47 ± 0.16 | 44.94% ± 1.2% | 8.07% ± 0.3% | 8.23% ± 0.95% |
| 10 | 1.17 ± 0.4 | 43.94% ± 2.8% | 7.94% ± 0.52% | 7.74% ± 1.7% |
The liver is the site of many important biochemical functions including formation of bile which contains many solutes including phospholipids and cholesterol, both of which are synthesized in the liver and have been implicated in liver and cholestatic disease[29]. Many studies have been performed in which the physical forms of lipids have been isolated in bile; however, very few studies have addressed this problem in the liver. Attempts to isolate vesicles containing biliary type PC and cholesterol have largely been unsuccessful in hepatocytes[5]. However, Gilat and Sömjen[7] and Sömjen et al[8] have identified three forms of biliary lipid carriers in bile, namely unilamellar vesicles, stacked lamellae and micelles. The sources of biliary phospholipids may be numerous: de novo synthesis, microsomes, Golgi, bile canalicular membrane and preformed hepatic and extrahepatic pool[30]. The extrahepatic pool may contribute about 40% of the biliary phospholipids secreted in the basal state in rats and is associated with high density lipoprotein[31], also bile acid activates a specific cytosolic PC transfer protein in the hepatocytes which then transfers PC to the canalicular membrane. It has also been reported that a PC transmembrane translocator (flippase) exists in the canalicular membrane and may be involved in the membrane translocation of specific PC to the biliary side of the canalicular membrane[32]. Most of the cholesterol secreted in bile is derived from circulating plasma lipoprotein, mainly low-density lipoprotein, high-density lipoprotein and chylomicron remnants. Cholesterol is transported probably in vesicles and binds to protein such as sterol carrier protein 2 present in the hepatocytes and, under physiological conditions, biliary bile acid secretion is the driving force behind the secretion of phospholipid and cholesterol in bile[33,34].
THDC is a hydrophilic bile acid, causing more secretion of biliary PC compared to tauroursodeoxycholic acid and taurocholic acid, whereas this bile acid does not significantly increase biliary secretion of cholesterol and protein when compared to the control[20]. It was thought that increased biliary PC carrier vesicles would be present in the hepatocytes in this experiment. This concept was initiated by the study of Angelico et al[20], who observed by electron microscopy that increased recruitment of vesicles and lamellar bodies around and within bile canaliculi in the liver occurred with THDC infusion. It is possible that mechanisms at a molecular level include stimulation by THDC of the PC transfer protein and/or of the phospholipid translocator involved in the transmembrane canalicular transport of phospholipids. It has also been reported from physical-chemical and imaging studies that bile salts stimulate the biliary secretion of unilamellar vesicles from the external hemileaflet of the canalicular membrane[31].
However, no significant differences were observed between control and THDC-infused rat liver sub-fractions in total phospholipids and cholesterol (Figure 1A and B). There was no significant difference in total proteins in the subcellular fractions of isolated perfused rat livers in the absence of THDC infusion (Figure 1C). However, PDE activity in the subcellular fraction P3II was significantly higher than in the corresponding fraction from the control experiment. Within the liver, PDE is a membrane-bound enzyme and would be expected to be associated with vesicles, hence this enzyme was assayed and results may indicate that there is more membrane material in this fraction. This is in contrast to the results reported by Lanzarotto et al[35] who observed that chronic administration of THDC in humans with intact enterohepatic circulation has little effect on biliary lipid composition and secretion. In contrast, Sinhal et al[36] and Cohen et al[37] showed that feeding THDC to hamsters and prairie dogs increased hepatic HMG-CoA reductase activity and thus an increase in vesicles carrying cholesterol. It is speculated that a similar increase in the activity of PDE is caused in this experiment by THDC.
Analysis of the microsomal fraction gave an interesting profile of total phospholipids, cholesterol, protein and PDE, as presented in Figure 2. The results indicate that there may be two different populations of the parameters measured. The first population isolated from fractions 1-2 (ρ = 1.05-1.07 g/mL) had relatively small amounts of cholesterol and PDE activity, whereas the population isolated from fractions 8-10 (ρ = 1.09-1.23 g/mL) had higher concentrations of phospholipids, cholesterol, protein and PDE activity (Figure 2A-D). Some putative vesicles may be present in fractions 8-10 (ρ = 1.09-1.23 g/mL) since these had more total phospholipids, cholesterol, protein and PDE activity. Enzyme activity in THDC-infused liver microsomal sub-fractions (ρ = 1.05-1.07 g/mL and ρ = 1.95-1.23 g/mL) was significantly higher than that observed in control values (Figure 2D). No significant difference was found in PC molecular species in the C16:0-C18:2, C16:0-C18:1 between control and THDC-infused liver subfractions.
In the experiments reported in this study, the isolation of biliary type vesicles was achieved by using the novel gradient medium, Iodixanol, which is a nonionic medium that has an advantage over sucrose in that it rapidly forms self-generated gradients in vertical or near-vertical rotors[38]. Increased lipid transfer vesicles might be present in the microsomal fraction[39,40]; however, subfractions of microsomal fraction on density gradient with Iodixanol failed to identify biliary transfer vesicles.
Crawford et al[32,41] reported that vesicles are secreted from the outer leaflet of the canalicular membrane by ABCB4 transporter and subsequently, bile salt/phospholipid micelles in bile extract cholesterol from these vesicles. Vesicular secretion is compatible with the function of ABCG5/ABCG8, and several studies[7,32,42,43] have suggested that vesicular secretion of cholesterol is one of the mechanisms by which sterols appear in bile.
According to the results of this study no significant changes in biliary lipid-carrying vesicles were observed; however, a significantly different profile of PDE was seen. However, the observation of increased vesicle accumulation during bile salt secretion by electron microscopy[44] and inhibition of the vesicle transport by colchicine, vinblastine and valproate still require explanation[45]. The changing of lipid content in any subcellular compartment might be prevented by analysis of the whole liver. However, the subcellular fraction of the livers which was also an initial fraction resulted in no significant difference between control and THDC-perfused rat livers (Figure 1A and B). Some putative biliary lipid transfer vesicles may exist but techniques used in this study have failed to identify them. The identification and regulation of biliary lipid-carrying vesicles may lead to an improvement in the treatment of hepatic disorders.
In conclusion, the present study failed to identify an increase in biliary lipid-carrying vesicles in THDC-infused rat livers probably due to the limitation of the techniques. However, PDE activity was significantly increased in the microsomal sub-fractions isolated from THDC-infused livers when compared to control values and needs further investigation.
Bile contains many constituents, including cholesterol and phospholipids, and these are reported to be transported from the hepatocytes to the bile canaliculus in vesicles. An increase in biliary lipid secretion can have implications for hepatic disorders such as cholesterol gallstone formation. Hence, the isolation of biliary lipid-carrying vesicles may lead to a better understanding of such hepatic disorders.
Taurohyodeoxycholic acid (THDC) is reported to increase biliary lipid-carrying vesicles (mainly phosphatidylcholine) and ultracentrifugation techniques are now available which can be used to separate vesicle type material relatively quickly.
Biliary phosphatidylcholine has a unique fatty acid composition compared to hepatic phosphatidylcholine and can be identified by high-performance liquid chromatography (HPLC). Since THDC induces an increase in biliary phosphatidylcholine it was thought that increased biliary phosphatidylcholine carrier vesicles would be present in the hepatocytes and could be separated by ultracentrifugation. Electron microscopy has confirmed the presence of increased vesicles and lamellar bodies around and within the bile canaliculus after THDC infusion.
Although the present study failed to identify an increase in biliary lipid-carrying vesicles in THDC-infused livers, probably due to the limitation of the techniques employed, vesicles enriched in phosphodiesterase I (PDE) activity were present in THDC livers compared to controls.
THDC is a natural 6α-hydroxylated bile acid displaying hydrophilic properties and causes more secretion of phosphatidylcholine into bile compared to other bile acids. The microsomal fraction was obtained and subjected to further ultracentrifugation using a novel gradient centrifugation technique. The phosphatidylcholine was separated and subjected to HPLC fractionation since biliary phosphatidylcholine has a unique fatty acid composition.
In this study the authors have isolated rat livers and have subjected them to THDC infusion, which is reported to increase biliary phospholipid secretion.The livers were then homogenized and the microsomal fraction was subjected to ultracentrifugation by using a novel gradient technique in order to isolate putative vesicles destined for biliary secretion. The results show that the density gradient fraction envisaged to contain the putative vesicle population isolated from THDC-perfused livers had relatively small amounts of phospholipids and protein when compared to the relevant control fractions. However, the vesicles isolated from the THDC-perfused livers displayed an increase in cholesterol and PDE activity.
P- Reviewers Muscarella P, Melek M S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN
| 1. | Dikkers A, Tietge UJ. Biliary cholesterol secretion: more than a simple ABC. World J Gastroenterol. 2010;16:5936-5945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 2. | Zanlungo S, Nervi F. The molecular and metabolic basis of biliary cholesterol secretion and gallstone disease. Front Biosci. 2003;8:s1166-s1174. [PubMed] |
| 3. | Booker ML, Scott TE, La Morte WW. Effect of dietary cholesterol on phosphatidylcholines and phosphatidylethanolamines in bile and gallbladder mucosa in the prairie dog. Gastroenterology. 1989;97:1261-1267. [PubMed] |
| 4. | White DA. In: Ansell GB, Hawthorne JN, Dawson RMC, editors. Form and Function of Phospholipids. New York: Elsevier Scientific 1973; 441-482. |
| 5. | Verkade HJ, Vonk RJ, Kuipers F. New insights into the mechanism of bile acid-induced biliary lipid secretion. Hepatology. 1995;21:1174-1189. [PubMed] |
| 6. | Nervi F, Marinović I, Rigotti A, Ulloa N. Regulation of biliary cholesterol secretion. Functional relationship between the canalicular and sinusoidal cholesterol secretory pathways in the rat. J Clin Invest. 1988;82:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Gilat T, Sömjen GJ. Phospholipid vesicles and other cholesterol carriers in bile. Biochim Biophys Acta. 1996;1286:95-115. [PubMed] |
| 8. | Sömjen GJ, Marikovsky Y, Wachtel E, Harvey PR, Rosenberg R, Strasberg SM, Gilat T. Phospholipid lamellae are cholesterol carriers in human bile. Biochim Biophys Acta. 1990;1042:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Crawford JM. Intracellular traffic and plasma membrane secretion of small organic solutes involved in hepatocellular bile formation. Comp Biochem Physiol. 1996;115B:341-354. |
| 10. | Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275-48282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Kamisako T, Ogawa H. Regulation of biliary cholesterol secretion is associated with abcg5 and abcg8 expressions in the rats: effects of diosgenin and ethinyl estradiol. Hepatol Res. 2003;26:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Hişmioğullari AA, Bozdayi AM, Rahman K. Biliary lipid secretion. Turk J Gastroenterol. 2007;18:65-70. [PubMed] |
| 13. | Bloks VW, Bakker-Van Waarde WM, Verkade HJ, Kema IP, Wolters H, Vink E, Groen AK, Kuipers F. Down-regulation of hepatic and intestinal Abcg5 and Abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia. 2004;47:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kamisako T, Ogawa H. Effect of obstructive jaundice on the regulation of hepatic cholesterol metabolism in the rat. Disappearance of abcg5 and abcg8 mRNA after bile duct ligation. Hepatol Res. 2003;25:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kosters A, Frijters RJ, Kunne C, Vink E, Schneiders MS, Schaap FG, Nibbering CP, Patel SB, Groen AK. Diosgenin-induced biliary cholesterol secretion in mice requires Abcg8. Hepatology. 2005;41:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kosters A, Kunne C, Looije N, Patel SB, Oude Elferink RP, Groen AK. The mechanism of ABCG5/ABCG8 in biliary cholesterol secretion in mice. J Lipid Res. 2006;47:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237-16242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Hazard SE, Patel SB. Sterolins ABCG5 and ABCG8: regulators of whole body dietary sterols. Pflugers Arch. 2007;453:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Angelico M, Baiocchi L, Nistri A, Franchitto A, Della Guardia P, Gaudio E. Effect of taurohyodeoxycholic acid, a hydrophilic bile salt, on bile salt and biliary lipid secretion in the rat. Dig Dis Sci. 1994;39:2389-2397. [PubMed] |
| 21. | Roda A, Piazza F, Baraldini M, Speroni E, Guerra MC, Cerré C, Cantelli Forti G. Taurohyodeoxycholic acid protects against taurochenodeoxycholic acid-induced cholestasis in the rat. Hepatology. 1998;27:520-525. [PubMed] |
| 22. | Rahman K, Coleman R. Output of lysosomal contents and cholesterol into bile can be stimulated by taurodehydrocholate. Biochem J. 1987;245:289-292. [PubMed] |
| 23. | Ford TC, Graham JM. An introduction to centrifugation. Oxford: BIOS, Scientific Publishers 1991; . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Zak B, Dickenman RC, White EG, Burnett H, Cherney PJ. Rapid estimation of free and total cholesterol. Am J Clin Pathol. 1954;24:1307-1315. [PubMed] |
| 25. | Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911-917. [PubMed] |
| 26. | Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466-468. [PubMed] |
| 27. | Trams EG, Lauter CJ. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974;345:180-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Winterbourne DJ. Chemical Assays for Proteins. Biomembrane Protocols. Totowa: Humana Press Inc 1993; . [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Abeysuriya V, Deen KI, Navarathne NM. Biliary microlithiasis, sludge, crystals, microcrystallization, and usefulness of assessment of nucleation time. Hepatobiliary Pancreat Dis Int. 2010;9:248-253. [PubMed] |
| 30. | Yousef IM, Bloxam DL, Phillips MJ, Fisher MM. Liver cell plasma membrane lipids and the origin of biliary phospholipid. Can J Biochem. 1975;53:989-997. [PubMed] |
| 31. | Chanussot F, Lafont H, Hauton J, Tuchweber B, Yousef I. Studies on the origin of biliary phospholipid. Effect of dehydrocholic acid and cholic acid infusions on hepatic and biliary phospholipids. Biochem J. 1990;270:691-695. [PubMed] |
| 32. | Crawford AR, Smith AJ, Hatch VC, Oude Elferink RP, Borst P, Crawford JM. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy. J Clin Invest. 1997;100:2562-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Barnwell SG, Tuchweber B, Yousef IM. Biliary lipid secretion in the rat during infusion of increasing doses of unconjugated bile acids. Biochim Biophys Acta. 1987;922:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Venneman NG, van Erpecum KJ. Pathogenesis of gallstones. Gastroenterol Clin North Am. 2010;39:171-83, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Lanzarotto F, Sosta S, Lanzini A. Effect of chronic administration of tauro-hyodeoxycholic acid on biliary bile acid composition and on biliary lipid secretion in humans. Scand J Gastroenterol. 2001;36:981-986. [PubMed] |
| 36. | Singhal AK, Cohen BI, Finver-Sadowsky J, McSherry CK, Mosbach EH. Role of hydrophilic bile acids and of sterols on cholelithiasis in the hamster. J Lipid Res. 1984;25:564-570. [PubMed] |
| 37. | Cohen BI, Mosbach EH, McSherry CK, Stenger RJ, Kuroki S, Rzigalinski B. Gallstone prevention in prairie dogs: comparison of chow vs. semisynthetic diets. Hepatology. 1986;6:874-880. [PubMed] |
| 38. | Billington D, Maltby PJ, Jackson AP, Graham JM. Dissection of hepatic receptor-mediated endocytic pathways using self-generated gradients of iodixanol (Optiprep). Anal Biochem. 1998;258:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Yamaguchi A, Tazuma S, Ochi H, Chayama K. Choleretic action of diosgenin is based upon the increases in canalicular membrane fluidity and transporter activity mediating bile acid independent bile flow. Hepatol Res. 2003;25:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Amigo L, Mendoza H, Zanlungo S, Miquel JF, Rigotti A, González S, Nervi F. Enrichment of canalicular membrane with cholesterol and sphingomyelin prevents bile salt-induced hepatic damage. J Lipid Res. 1999;40:533-542. [PubMed] |
| 41. | Crawford JM, Möckel GM, Crawford AR, Hagen SJ, Hatch VC, Barnes S, Godleski JJ, Carey MC. Imaging biliary lipid secretion in the rat: ultrastructural evidence for vesiculation of the hepatocyte canalicular membrane. J Lipid Res. 1995;36:2147-2163. [PubMed] |
| 42. | Sömjen GJ, Marikovsky Y, Lelkes P, Gilat T. Cholesterol-phospholipid vesicles in human bile: an ultrastructural study. Biochim Biophys Acta. 1986;879:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Poupon R, Poupon R, Dumont M, Erlinger S. Hepatic storage and biliary transport maximum of taurocholate and taurochenodeoxycholate in the dog. Eur J Clin Invest. 1976;6:431-437. [PubMed] |
| 44. | Barnwell SG, Lowe PJ, Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984;220:723-731. [PubMed] |
| 45. | Marzolo MP, Rigotti A, Nervi F. Secretion of biliary lipids from the hepatocyte. Hepatology. 1990;12:134S-141S; discussion 141S-142S. [PubMed] |