Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5250
Revised: June 12, 2013
Accepted: July 4, 2013
Published online: August 28, 2013
Processing time: 142 Days and 23.1 Hours
AIM: To investigate the role of human platelets in liver fibrosis.
METHODS: Severe combined immunodeficiency (SCID) mice were administered CCl4 and either phosphate-buffered saline (PBS group) or human platelet transfusions (hPLT group). Concentrations of hepatocyte growth factor (HGF), matrix metallopeptidases (MMP)-9, and transforming growth factor-β (TGF-β) in the liver tissue were compared between the PBS and the hPLT groups by enzyme-linked immunosorbent assay (ELISA) and Western blotting. The effects of a human platelet transfusion on liver fibrosis included the fibrotic area, hydroxyproline content, and α-smooth muscle actin (α-SMA) expression, which were evaluated by picrosirius red staining, ELISA, and immunohistochemical staining using an anti-mouse α-SMA antibody, respectively. Phosphorylations of mesenchymal-epithelial transition factor (Met) and SMAD3, downstream signals of HGF and TGF-β, were compared between the two groups by Western blotting and were quantified using densitometry. Hepatocyte apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling. Furthermore, the accumulation of human platelets in the liver 2 h after platelet transfusion was compared between normal and fibrotic livers by immunohistochemical staining using an anti-human CD41 antibody.
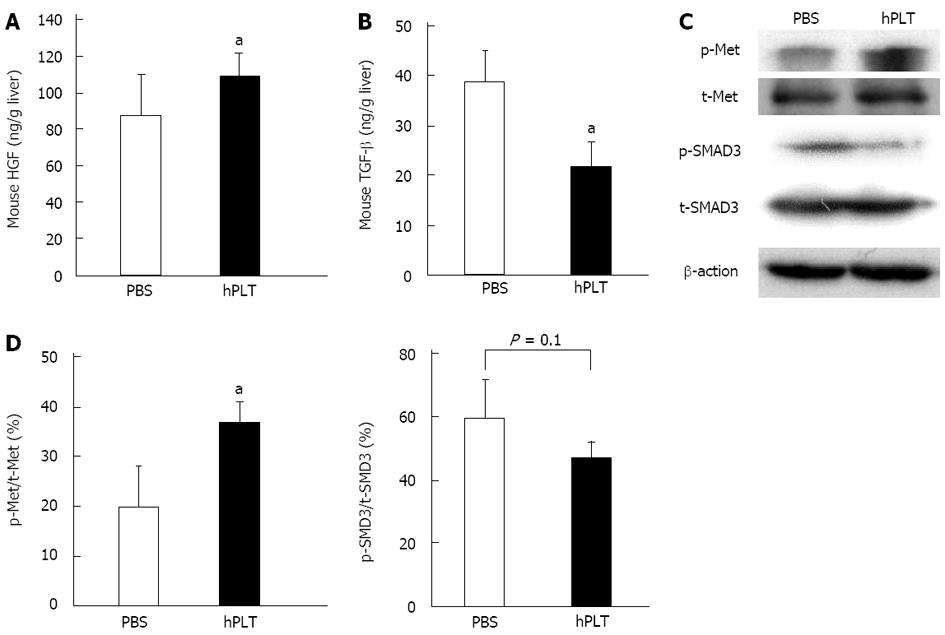
RESULTS: The fibrotic area and hydroxyproline content in the liver were both significantly lower in the hPLT group when compared to the PBS group (fibrotic area, 1.7% ± 0.6% vs 2.5% ± 0.6%, P = 0.03; hydroxyproline content, 121 ± 26 ng/g liver vs 156 ± 47 ng/g liver, P = 0.04). There was less α-smooth muscle actin staining in the hPLT group than in the PBS group (0.5% ± 0.1% vs 0.8% ± 0.3%, P = 0.02). Hepatic expression levels of mouse HGF and MMP-9 were significantly higher in the hPLT group than in the PBS group (HGF, 109 ± 13 ng/g liver vs 88 ± 22 ng/g liver, P = 0.03; MMP-9, 113% ± 7%/GAPDH vs 92% ± 11%/GAPDH, P = 0.04). In contrast, the concentration of mouse TGF-β in the liver tissue was significantly lower in the hPLT group than in the PBS group (22 ± 5 ng/g liver vs 39 ± 6 ng/g liver, P = 0.02). Phosphorylation of Met was more prevalent in the hPLT group than in the PBS group (37% ± 4%/GAPDH vs 20% ± 8%/GAPDH, P = 0.03). Phosphorylation of SMAD3 was weaker in the hPLT group than in the PBS group (60% ± 12%/GAPDH vs 84% ± 12%/GAPDH, P = 0.1), although this difference was not significant. Furthermore, a lower rate of hepatocyte apoptosis was observed in the hPLT group than in the PBS group (5.9% ± 1.7% vs 2.9% ± 2.1%, P = 0.02). Significant human platelet accumulation was observed in the fibrotic liver tissues, whereas few platelets accumulated in the normal liver.
CONCLUSION: Human platelets inhibit liver fibrosis in SCID mice. Increased concentration of HGF in the liver suppresses hepatic stellate cell activation, induces MMPs, and inhibits hepatocyte apoptosis.
Core tip: We assessed the effects of human platelet transfusion on liver fibrosis. Severe combined immunodeficiency (SCID) mice were administered CCl4 and either phosphate-buffered saline or human platelets. The effects of a human platelet transfusion on liver fibrosis and hepatocyte apoptosis were compared. The fibrotic area, hydroxyproline content, and α-smooth muscle actin expression were decreased in mice that received human platelet transfusions. Transfusion increased mouse hepatocyte growth factor (HGF) and matrix metallopeptidases (MMP)-9 levels in the liver and decreased mouse transforming growth factor-β. Furthermore, transfusion suppressed hepatocyte apoptosis. Human platelets inhibited liver fibrosis in SCID mice. Increased concentration of HGF in the liver suppresses hepatic stellate cell activation, induces MMPs, and inhibits hepatocyte apoptosis.
- Citation: Takahashi K, Murata S, Fukunaga K, Ohkohchi N. Human platelets inhibit liver fibrosis in severe combined immunodeficiency mice. World J Gastroenterol 2013; 19(32): 5250-5260
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5250
Chronic liver disease and liver cirrhosis are major causes of morbidity and mortality worldwide. In chronic liver disease, normal repair of hepatocyte damage and tissue remodeling is lost, resulting in fibrosis and ultimately cirrhosis, which leads to portal hypertension, hepatocellular carcinoma, and lethal hepatic failure[1]. The most common etiological factors in chronic liver disease are chronic hepatitis C virus infection, excessive alcohol consumption, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis. Liver transplantation is the only curative approach, and specific treatments that stop progressive fibrosis are currently unavailable[1].
Liver fibrosis is characterized by the excessive production and deposition of the extracellular matrix (ECM) proteins, such as collagen, proteoglycans, fibronectins, and hyaluronic acids[2]. Accumulation of the ECM results in remodeling of the hepatic structure. Among the deposited ECM proteins, collagen type I is a major constituent, which is mainly produced by hepatic stellate cells (HSCs). Matrix metallopeptidases (MMPs) are the key enzymes responsible for the degradation of all protein components of the ECM[3]. Recently, it has been reported that hepatocyte apoptosis in cirrhotic liver induces HSC activation, which promotes liver fibrosis[4].
Liver cirrhosis has traditionally been viewed as an irreversible state in which the normal hepatocellular structures and organization are destroyed and fibrosis is firmly established. However, several reports have opposed this conventional concept. Lang et al[5] reported that blocking transforming growth factor-β (TGF-β) with small interference RNA suppressed HSC activation and decreased liver fibrosis in mice. Iimuro et al[6] showed that the delivery of MMP-1 attenuated established liver fibrosis in rats. In recent years, platelets have been shown to exert both anti-fibrotic and fibrolytic effects on the liver[7-10].
In this study, we transfused human platelets into severe combined immunodeficiency (SCID) mice to examine the effects of human platelet transfusion on liver fibrosis. This model was used for the following two reasons: first, there is no direct evidence that human platelets inhibit liver fibrosis. Second, because in vivo human studies are difficult, xenotransfusion of human platelets into SCID mice has been used to examine the functions of human platelets[11,12]. Using this model, we evaluated the effects of human platelet transfusion on liver fibrosis and hepatocyte apoptosis.
Experiments were performed using 8-12-wk-old male C.B-17/lcr-scid/scid Jcl mice weighing 20-26 g (CLEA, Tokyo, Japan). Mice were maintained in a temperature- controlled room on a 12-h light-dark cycle with free access to water and standard chow. After an acclimation period of at least 7 d, mice were divided into two groups: CCl4 plus phosphate-buffered saline (PBS) administration (PBS group), and CCl4 plus human platelet transfusion (hPLT group). All experiments complied with the Guidelines for the Care and Use of Laboratory Animals (University of Tsukuba).
To induce liver fibrosis, each mouse received an intraperitoneal injection of CCl4 (200 μL/kg body weight) in a 1:3 ratio with corn oil twice a week for 8 wk. PBS or concentrated human platelets was transfused once a week from weeks 5 to 8. A 500-μL aliquot of PBS or concentrated human platelets was injected into the retro-orbital vein one day after the administration of CCl4. Mice were sacrificed 96 h after the final administration of PBS or human platelet transfusion, and livers were removed and divided into two samples; One liver section was fixed in 10% buffered formalin for subsequent immunohistochemical analysis, and the other section was snap-frozen in liquid nitrogen and kept at -80 °C until use.
Human whole blood was obtained from healthy volunteers. Platelet-rich plasma was obtained by centrifuging anticoagulated blood containing acid-citrate-dextrose at a 1:4 volume ratio at 120 g for 10 min. Samples were then centrifuged at 1000 g for 15 min, and resuspended in citrate buffer (120 mmol/L NaCl, 4.26 mmol/L NaHPO4, 5.5 mmol/L glucose, 4.77 mmol/L sodium citrate, and 2.35 mmol/L citric acid at pH 6.5). Platelets were then suspended in PBS and counted using a hematology analyzer (MICROS abc LC-152; Horiba Ltd., Kyoto, Japan).
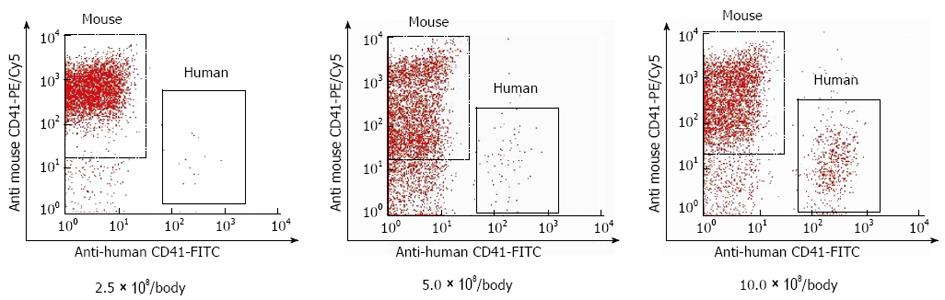
To determine the number of cells for transfusion, 2.5 × 108, 5.0 × 108, or 10.0 × 108 of human platelets were transfused into naive SCID mice, and the post-transfusion percentage of transfused platelets was measured after 6 h (n = 3). We examined at 6 h because a 10% increase in peripheral platelet count 6 h after platelet transfusion improved liver function of the patients with liver cirrhosis in our clinical study. Because it required approximately 15 mL of human whole blood to prepare 10.0 × 108 of human platelets, 10 × 108/body weight was determined to be the upper limit.
Peripheral blood was collected from the lateral tail vein. Blood samples were incubated for 30 min with a biotin-conjugated rat anti-mouse CD41 antibody (AbD Serotec, Oxford, United Kingdom) that specifically detected murine platelets. Samples were then washed in platelet HEPES buffer (137 mmol/L NaCl, 2 mmol/L KCl, 0.4 mmol/L NaH2PO4, 1 mmol/L MgCl2, 5.6 mmol/L glucose at pH 7.4) containing 10% acid-citrate-dextrose, and centrifuged at 500 g for 5 min. Supernatants were removed and the cells were resuspended in platelet HEPES buffer containing 10% acid-citrate-dextrose. Samples were incubated with a FITC-conjugated mouse anti-human CD41 antibody (Dako, Glostrup, Denmark) that specifically detected human platelets and streptavidin-phycoerythrin (PE)/Cy5 (Biolegend, San Diego, CA, United States) for 30 min and then analyzed using a flow cytometer (FACS Calibur, Becton Dickinson, Franklin Lakes, NJ, United States). The post-transfusion percentage of human platelets was defined as human platelets/(human platelets + murine platelets).
After 6 h, the post-transfusion percentages of human platelets in naive mice that received 2.5 × 108, 5.0 × 108, and 10.0 × 108 of human platelets were 0.6% ± 0.3%, 2.0% ± 1.6%, and 10.3% ± 1.4%, respectively (Figure 1). We used 10.0 × 108 of human platelets for each mouse in this study.
Blood samples were collected at the time of sacrifice. Platelet count was measured, and serum levels of asparatate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), albumin (Alb), and total cholesterol (T-CHO) were measured and compared between the PBS group and the hPLT group (Fuji Drichem; Fuji Film Inc, Tokyo, Japan) (n = 8).
Liver samples were fixed in 10% buffered formalin, and stained with picrosirius red solution, and the liver fibrotic area was quantified using the winROOF visual system (Mitani Co., Tokyo, Japan) (n = 8). In addition, specimens were immunostained with an anti-α-smooth muscle actin (SMA) antibody (Dako) and counterstained with hematoxylin. α-SMA expression was also quantified using the winROOF visual system (Japan) (n = 6). To assess the hepatocellular mitotic index, liver sections were stained with hematoxylin and eosin, and the number of hepatocytes undergoing mitosis was calculated. In addition, proliferating cell nuclear antigen (PCNA) staining was conducted using a PCNA staining kit (Invitrogen Co., Carlsbad, CA, United States). PCNA-positive hepatocytes and hepatocytes undergoing mitosis were counted in four randomly selected high-power fields (× 200). Liver sections were also incubated with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) antibody (Promega KK, Tokyo, Japan). TUNEL-positive hepatocytes were counted in four randomly selected high-power fields (× 200) on each slide, and calculated as TUNEL-positive hepatocytes/total hepatocytes (n = 6).
An enzyme-linked immunosorbent assay (ELISA) kit was used to measure mouse hepatocyte growth factor (HGF) (Institute of Immunology Co., LTD, Tokyo, Japan) and mouse TGF-β (R and D Systems, Minneapolis, MN, United States). ELISAs were used to measure levels of these proteins in 10% liver tissues lysates (n = 8).
Hydroxyproline content was determined as described previously[13]. Briefly, 50 mg liver samples were hydrolyzed in 6 mol/L HCl at 120 °C for 16 h. After centrifugation, the supernatant was removed and neutralized with 6 mol/L NaOH. The solution was oxidized with Chloramine T (Sigma-Aldrich Corp., St Louis, MO, United States) in acetate/citrate buffer, followed by the addition of Ehrlich’s solution (p-dimethylamino-benzaldehyde in 60% HCl4 with isopropanol). The final mixture was incubated at 60 °C for 30 min and then at room temperature for 10 min. Absorbance was determined at 560 nm. The value of the hepatic hydroxyproline concentration was expressed as μg/g wet tissue.
For Western blotting analysis, protein was obtained from liver tissues lysates, separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Millipore, Bedford, MA, United States). We used primary antibodies specific for α-SMA (Dako), MMP-9 (AB1916) (Chemicon International, Temecula, CA, United States), phosphoserine mesenchymal-epithelial transition factor (Met) (3127), Met (3135S), phosphotyrosine SMAD3 (9529S), SMAD3 (9513), caspase-3 (9662), cleaved caspase-3 (9962), Bcl-2 (2876), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (2118), and β-actin (4970) (Cell Signaling Technology, Beverly, MA, United States) and secondary mouse or rabbit antibodies conjugated with horseradish peroxidase (Invitrogen Co.). Immunoblots were analyzed using an enhanced chemiluminescence system. Protein band densities were quantified using densitometry. Band intensities were normalized to those of GAPDH, caspase-3, Met, or SMAD3 (n = 3).
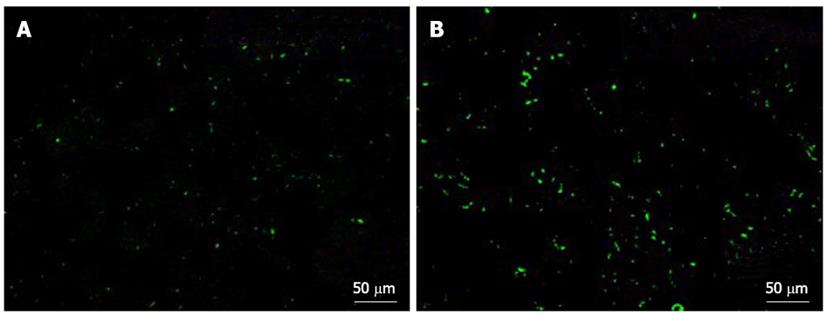
Human platelets were transfused to SCID mice with normal or fibrotic livers, and accumulation of the transfused human platelets in the liver 2 h after transfusion was measured and compared between the two groups.
Immunofluorescence staining was performed on 5 μm thick sections of tissue that had been fixed in 4% paraformaldehyde, immersed in OCT compound, and incubated with FITC-conjugated anti-human CD41 antibody (Dako). Stained sections were examined under a confocal laser-scanning microscope (BZ-9000, Keyence Co., Tokyo, Japan).
All data are expressed as means ± SD. Unpaired t-tests were used to compare two groups. P values < 0.05 were considered significant.
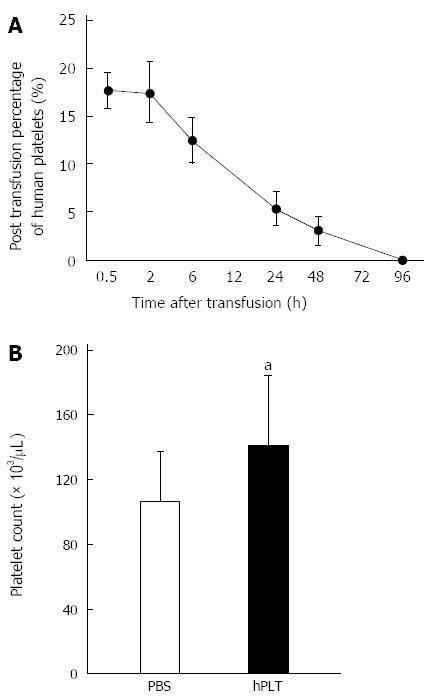
Human platelets disappeared from the peripheral blood 96 h after transfusion (Figure 2A). The peripheral platelet counts at the time of sacrifice, i.e., 96 h after transfusion, were significantly higher in the hPLT group than in the PBS group (P < 0.05) (Figure 2B).
There were no significant differences in the liver/body weight ratio, PCNA index, mitotic index, and spleen/body weight ratio between the hPLT and PBS groups (Table 1).
| Liver/body weight ratio | PCNA labeling index (/HPF) | Mitotic index (/HPF) | Spleen/body weight ratio | AST (U/mL) | ALT (U/mL) | T-bil (mg/mL) | Alb (g/mL) | T-CHO (g/mL) | |
| PBS | 6.2% ± 0.5% | 2.1 ± 0.9 | 0.6 ± 0.3 | 0.23% ± 0.04% | 50 ± 21 | 122 ± 56 | 1.0 ± 0.2 | 3.0 ± 1.0 | 77.8 ± 5.4 |
| hPLT | 6.7% ± 0.5% | 2.4 ± 0.9 | 0.6 ± 0.5 | 0.24% ± 0.05% | 50 ± 15 | 104 ± 64 | 0.8 ± 0.2 | 3.0 ± 1.5 | 82.7 ± 4.4a |
There were no significant differences in the serum AST, T-bil, and Alb levels between the PBS and hPLT groups. Despite the lack of statistically significant differences, there was a tendency for the serum ALT level to be lower in the hPLT group than in the PBS group (P = 0.3). The serum T-CHO level was significantly higher in the hPLT group than in the PBS group (P < 0.05) (Table 1).
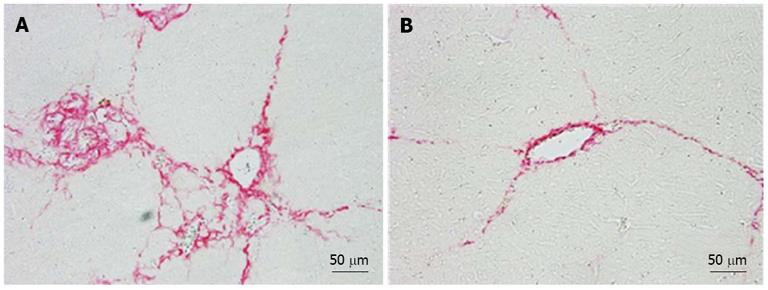
The fibrotic index, which was calculated based on the area stained with picrosirius red solution, was significantly lower in the hPLT group than in the PBS group (P < 0.05) (Figure 3A). In addition, the liver hydroxyproline content was significantly lower in the hPLT group than in the PBS group (P < 0.05) (Figure 3B).
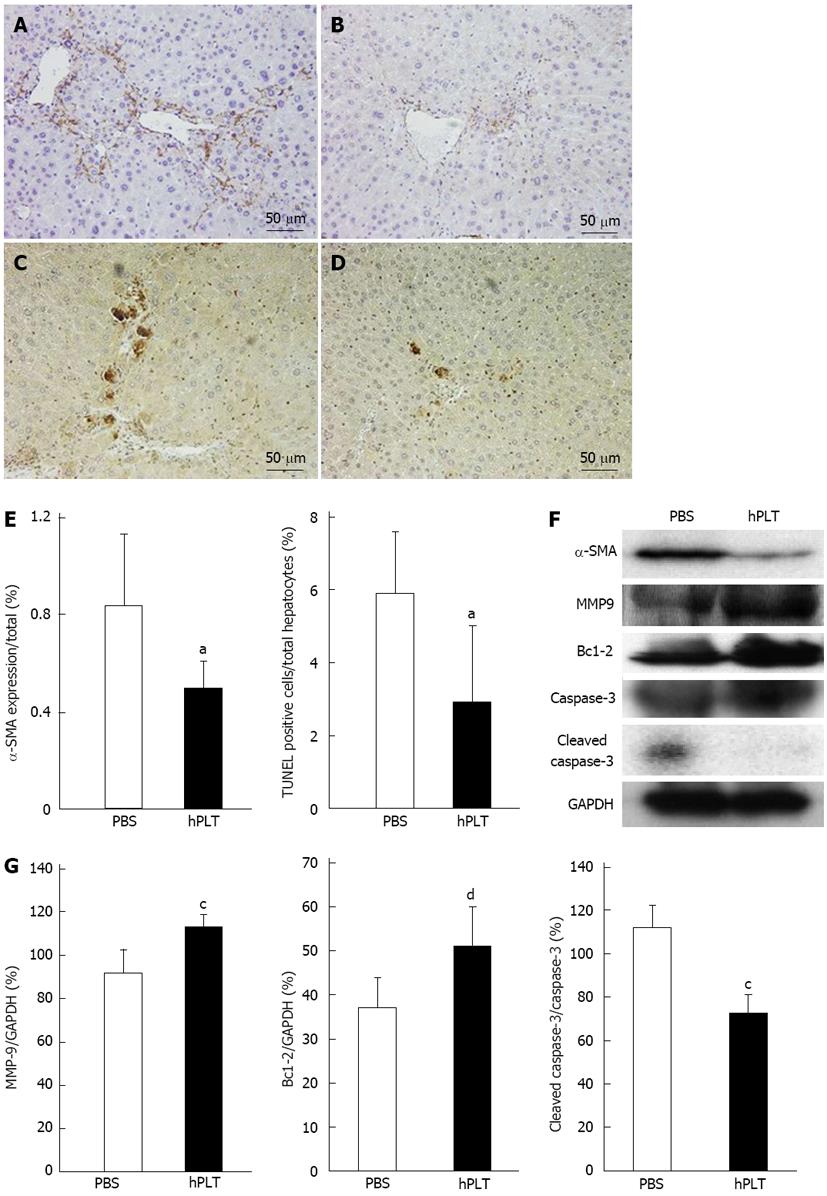
There was less α-SMA staining in the hPLT group compared to the PBS group (Figure 4A and B). TUNEL staining revealed only a few apoptotic cells in the hPLT group, whereas several apoptotic hepatocytes were observed in the PBS group (Figure 4C and D). α-SMA expression calculated based on the area stained by anti-α-SMA antibody and TUNEL positive hepatocytes/total hepatocytes were significantly lower in the hPLT group than in the PBS group (both P < 0.05) (Figure 4E).
MMP-9 expression was significantly higher in the hPLT group than in the PBS group (P < 0.05) (Figure 4F and G). Cleaved caspase-3 expression was significantly lower in the hPLT group than in the PBS group (P < 0.05), whereas Bcl-2 was more robustly expressed in the hPLT group as compared to the PBS group (P < 0.01) (Figure 4F and G).
Expression of mouse HGF in the liver tissue was significantly higher in the hPLT group than in the PBS group (P < 0.05) (Figure 5A). The concentration of mouse TGF-β was significantly lower in the liver tissues of the hPLT group than in the PBS group (P < 0.05) (Figure 5B).
There was increased Met phosphorylation in the hPLT group compared to the PBS group (P < 0.05) (Figure 5C and D). Although the difference was not statistically significant, SMAD3 phosphorylation was lower in the hPLT group than in the PBS group (P = 0.1) (Figure 5C and D).
Significant human platelet accumulation in the liver was observed in the fibrotic liver tissues, whereas fewer platelets accumulated in the normal liver (Figure 6).
We demonstrated that human platelets suppressed liver fibrosis in SCID mice. It was suspected that these anti-fibrotic effects were due to an increased concentration of HGF in the liver, resulting in decreased TGF-β concentrations and increased MMP-9 levels. Furthermore, inhibition of hepatocyte apoptosis by HGF may have suppressed HSC activation, resulting in decreased fibrotic changes. These results, together with recent reports showing that platelets contribute to liver regeneration[12,14-21], suggest that platelet increment therapy, such as thrombopoietin administration and platelet transfusions, may provide new clinical approaches for the treatment of liver diseases.
Platelets contain three types of secretory granules, notably α-granules, dense-granules, and lysosomal granules[22]. Each granule contains growth factors, such as platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), HGF, vascular endothelial growth factor, serotonin, ATP, and epidermal growth factor, among others[22]. The granule constituents of platelets exhibit species differences, i.e., although rodent platelets contain a large amount of HGF[16,23], human platelets do not[24]. Platelets accumulate in the liver in response to various conditions, such as ischemia and reperfusion[25], cirrhosis[26], cholestasis[27], and viral hepatitis[28]. Although most studies have evaluated platelets as promoters of inflammatory responses and liver injury[25,26,28], recent scientific[12,14-19] and clinical data[20,21] have revealed additional and different roles for platelets in the liver. We previously showed that platelets accelerate liver regeneration through three different mechanisms: a direct effect on hepatocytes[14,16], a cooperative effect with liver sinusoidal endothelial cells[18], and a collaborative effect with Kupffer cells[12]. Furthermore, platelets are reported to have anti-fibrotic and fibrolytic effects on the liver[7-10]. We have indicated that thrombopoietin-induced thrombocytosis attenuated fibrotic changes in rodents[7,8]. Kodama et al[9] reported that platelets exert an anti-fibrotic role by suppressing collagen type I expression via the HGF/Met signaling pathway. Ikeda et al[10] demonstrated that human platelet-derived ATP suppressed the activation of HSCs through the adenosine-cyclic 5’-adenosine monophosphate signaling pathway. In addition, Maruyama et al[29] reported that platelet transfusion once a week for 12 wk decreased serum hyaluronic acid concentrations, a fibrotic marker, in chronic hepatitis patients with Child-Pugh class A or B. In the present study, human platelet transfusion inhibited liver fibrosis in SCID mice. The elevated peripheral platelet counts and the higher serum T-CHO concentrations after transfusion were consequences of reduced liver cirrhosis. Furthermore, the increased number of platelets that accumulated in the fibrotic liver implied that transfused platelets accumulation was induced in the fibrotic liver and released biologically-active substances, such as ATP, which directly suppresses HSC activation and decreases fibrosis[10].
HSCs undergo a complex transformation and activation process during which the cells morphologically change from quiescent oval-shaped cells to activated spindle-shaped cells. The activation of HSCs correlates with α-SMA expression[30]. TGF-β is produced by HSCs and Kupffer cells and is recognized as the main pro-fibrogenic mediator that triggers HSC activation. Hepatic TGF-β concentrations have been shown to be increased among patients with liver cirrhosis[31]. The effects of TGF-β are mediated by intracellular signaling via SMAD proteins, which modulate the transcription of target genes[32]. Following ligand binding to the TGF-β type II receptors, the TGF-β type I receptor becomes activated. SMAD3 proteins associate with the activated receptor and become phosphorylated, allowing the formation of oligomeric complexes with SMAD4. This heterotrimeric complex translocates into the nucleus and binds to specific nucleotide motifs to regulate transcription of target genes such as COL1A2, which encodes the collagen α-2 (1) chain in HSCs[32]. In the present study, although there were no significant differences in the liver/body weight ratio, spleen/body ratio, and liver regeneration indexes, fibrogenic markers such as the fibrotic index, hydroxyproline content, and expression of α-SMA were decreased upon human platelet transfusion. In addition, TGF-β concentration decreased with subsequent suppression of SMAD3 phosphorylation after platelet transfusion. These results indicated that human platelet transfusion might have suppressed liver fibrosis by reducing the TGF-β concentration in the liver.
HGF is predominantly produced by Kupffer cells[33]. HGF is known for its major roles in liver development and regeneration by exerting mitogenic and morphogenic effects on hepatocytes. After HGF binds to Met, Met is phosphorylated and intracellular adapter proteins activate distinct intracellular signals, such as the PI3K, Ras, and ERK pathways, and execute pro-mitogenic and anti-apoptotic functions[34]. HGF contributes to the resolution of fibrosis by regulating TGF-β and MMP levels[35]. Giebeler et al[36] reported that hepatocyte-specific Met knockout mice exhibited increased expression of TGF-β, α-SMA, and collagen-1α messenger RNA, and enhanced collagen fiber staining. Kanemura et al[37] reported that up-regulated HGF expression after human HGF gene delivery induced higher MMP activities. In the present study, the mouse HGF concentration in the liver tissue was elevated after human platelet transfusion. Because human platelets do not contain significant amounts of HGF[24], it was suspected that the expression of HGF in the liver might be elevated because of enhanced release from Kupffer cells or an increased amount of mouse platelet accumulation in the liver, leading to a reduction in the TGF-β concentration and attenuated HSC activation. Furthermore, HGF might have enhanced the production of MMP-9, which promotes fibrinolysis in the liver.
In recent years, liver fibrosis has been considered to be associated with hepatocyte apoptosis[4]. Hepatic fibrosis was shown to be significantly reduced when Fas-mediated apoptosis was impaired or when caspases were inhibited[38]. Moreover, persistent hepatocyte apoptosis has been shown to lead to liver fibrosis due to hepatocyte disruption of Bcl-xL[39]. Engulfment of apoptotic bodies by Kupffer cells has been demonstrated to promote TGF-β production, and phagocytosis of apoptotic bodies by HSCs leads to their activation and increased production of TGF-β and collagen type I. Hisakura et al[40] reported that platelets protect against hepatocyte apoptosis and induce immediate activation of the Akt pathway, followed by an increase in Bcl-xL and a decrease in cleaved caspase-3 in hepatocytes. In the present study, hepatocyte apoptosis and expression of cleaved caspase-3 were suppressed and Bcl-2, an inhibitor of caspase-3, was increased by human platelet transfusion. It was hypothesized that inhibition of apoptosis by human platelet transfusion might help suppress liver fibrosis. Specifically, because HGF has an anti-apoptotic effect[34], elevated HGF levels may contribute to the inhabitation of hepatocyte apoptosis.
However, several questions remain. First, there are several types of growth factors in platelets that exert pro-fibrotic or anti-fibrotic effects. For example, platelet-derived chemokine ligand 4[26] and PDGF[41] induce HSC activation, whereas ATP[10] and IGF-1[42] suppress HSC activation. It is difficult to explain the pro-fibrotic or anti-fibrotic effects by one or two substances within platelets. In addition, there are many cell types in the liver, such as hepatocytes, Kupffer cells, HSCs, and liver sinusoidal endothelial cells, that are involved in liver fibrogenesis. Therefore, it is important to view these results from a comprehensive perspective. Second, in this study, there were no differences in liver regeneration between the PBS and hPLT groups, which differed from our previous study[7]. It has been reported that a higher dose of CCl4 is necessary to induce liver fibrosis in SCID mice compared to wild-type mice[43]. In this study, the degree of liver fibrosis was reduced compared to the previous study. The reduced fibrosis in the current model may have contributed to the low PCNA labeling index and hepatocyte mitosis in the hPLT group. Furthermore, in our previous study, we induced thrombocytosis using thrombopoietin, which resulted in higher peripheral platelet counts than those observed in this study. These differences in the degree of fibrosis and peripheral platelet counts may underlie the discrepancies in the results related to the requirement for the hepatocyte cell cycle and mitosis. Third, HGF and TGF-β are both produced by Kupffer cells, and the discrepancy in the dynamics of these growth factors was not clear. Because TGF-β is also produced by HSCs, it is possible that the increased HGF levels resulting from human platelet transfusion mainly suppressed HSC activity and down-regulated TGF-β expression in the liver. Fourth, although there was a significant difference in hepatocyte apoptosis as evaluated by TUNEL staining, serum AST and ALT concentrations were not significantly different. In our fibrosis model using CCl4 with this duration and dose, it was difficult to induce strong fibrosis and apoptosis of hepatocytes in SCID mice. Despite statistically significant differences in the number of apoptotic hepatocytes between the PBS and hPLT groups, the difference was small considering the damage to the entire liver. Therefore, the damage did not reflect the serum AST and ALT concentrations.
The authors thank Dr. Kozuma Y (Department of Medical Science, University of Tsukuba) for his support in flow cytometric analysis and Ms. Takahashi A (Department of Surgery, University of Tsukuba) for her support in Western blotting.
Liver cirrhosis is the ultimate stage of liver fibrosis, and there are currently no specific treatments that inhibit progressive fibrosis. Hepatocyte growth factor (HGF) helps resolve fibrosis by regulating transforming growth factor-β (TGF-β), matrix metallopeptidases (MMPs), and hepatocyte apoptosis.
Platelets have been conventionally regarded as an exacerbating factor to inflammatory response and injury in the liver. However, recent studies have demonstrated the role of platelets in promoting liver regeneration, improving liver fibrosis, and attenuating hepatitis. In this study, authors assessed the effects of human platelet transfusion on liver fibrosis.
Platelets contain three types of secretory granules: α-granules, dense-granules, and lysosomal granules. Each granule contains growth factors. The granule constituents of platelets exhibit species differences, i.e., human platelets do not contain significant amounts of HGF. This is the first study to show that human platelets have a role in suppressing liver fibrosis.
By demonstrating that human platelets suppress liver fibrosis, this study represents a potential future strategy for platelet therapy in the treatment of patients with liver cirrhosis.
HGF is known for its major roles in liver development and regeneration. After HGF binds to mesenchymal-epithelial transition factor (Met), Met is phosphorylated, and intracellular adapter proteins activate distinct intracellular signals, and execute pro-mitogenic and anti-apoptotic functions. HGF is known to contribute to the resolution of fibrosis by regulating TGF-β and MMP levels.
The authors examined the role of human platelets on liver fibrosis. It was revealed that increased concentrations of HGF in the liver suppressed hepatic stellate cell activation, induced MMPs, and inhibited hepatocyte apoptosis. The results are interesting and may provide new clinical approaches for the treatment of liver cirrhosis.
P- Reviewers Mohanakumar T, Theret N S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Friedman SL, Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] |
| 2. | Arthur MJ, Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Lang Q, Liu Q, Xu N, Qian KL, Qi JH, Sun YC, Xiao L, Shi XF. The antifibrotic effects of TGF-β1 siRNA on hepatic fibrosis in rats. Biochem Biophys Res Commun. 2011;409:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Watanabe M, Murata S, Hashimoto I, Nakano Y, Ikeda O, Aoyagi Y, Matsuo R, Fukunaga K, Yasue H, Ohkohchi N. Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol. 2009;24:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kodama T, Takehara T, Hikita H, Shimizu S, Li W, Miyagi T, Hosui A, Tatsumi T, Ishida H, Tadokoro S. Thrombocytopenia exacerbates cholestasis-induced liver fibrosis in mice. Gastroenterology. 2010;138:2487-2498, 2498. e1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Ikeda N, Murata S, Maruyama T, Tamura T, Nozaki R, Kawasaki T, Fukunaga K, Oda T, Sasaki R, Homma M. Platelet-derived adenosine 5’-triphosphate suppresses activation of human hepatic stellate cell: In vitro study. Hepatol Res. 2012;42:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Newman PJ, Aster R, Boylan B. Human platelets circulating in mice: applications for interrogating platelet function and survival, the efficacy of antiplatelet therapeutics, and the molecular basis of platelet immunological disorders. J Thromb Haemost. 2007;5 Suppl 1:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Takahashi K, Kozuma Y, Suzuki H, Tamura T, Maruyama T, Fukunaga K, Murata S, Ohkohchi N. Human platelets promote liver regeneration with Kupffer cells in SCID mice. J Surg Res. 2013;180:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Lee HS, Shun CT, Chiou LL, Chen CH, Huang GT, Sheu JC. Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: Emphasis on sampling variability. J Gastroenterol Hepatol. 2005;20:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 595] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 16. | Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Kawasaki T, Murata S, Takahashi K, Nozaki R, Ohshiro Y, Ikeda N, Pak S, Myronovych A, Hisakura K, Fukunaga K. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J Hepatol. 2010;53:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (3)] |
| 21. | Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Russell WE, McGowan JA, Bucher NL. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984;119:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1628] [Cited by in RCA: 1626] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 25. | Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, Myronovych A, Tadano S, Hisakura K, Ikeda O. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Zaldivar MM, Pauels K, von Hundelshausen P, Berres ML, Schmitz P, Bornemann J, Kowalska MA, Gassler N, Streetz KL, Weiskirchen R. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Laschke MW, Dold S, Menger MD, Jeppsson B, Thorlacius H. Platelet-dependent accumulation of leukocytes in sinusoids mediates hepatocellular damage in bile duct ligation-induced cholestasis. Br J Pharmacol. 2008;153:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Maruyama T, Murata S, Takahashi K, Tamura T, Nozaki R, Ikeda N, Fukunaga K, Oda T, Sasaki R, Ohkohchi N. Platelet transfusion improves liver function in patients with chronic liver disease and cirrhosis. Tohoku J Exp Med. 2013;229:213-220. [PubMed] |
| 30. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Baer HU, Friess H, Abou-Shady M, Berberat P, Zimmermann A, Gold LI, Korc M, Büchler MW. Transforming growth factor betas and their receptors in human liver cirrhosis. Eur J Gastroenterol Hepatol. 1998;10:1031-1039. [PubMed] |
| 32. | Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 494] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 33. | Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-α and TGF-beta1. J Hepatol. 1999;30:48-60. [PubMed] |
| 34. | Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1992] [Cited by in RCA: 2088] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 35. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 471] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Giebeler A, Boekschoten MV, Klein C, Borowiak M, Birchmeier C, Gassler N, Wasmuth HE, Müller M, Trautwein C, Streetz KL. c-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in mice. Gastroenterology. 2009;137:297-308, 308.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Kanemura H, Iimuro Y, Takeuchi M, Ueki T, Hirano T, Horiguchi K, Asano Y, Fujimoto J. Hepatocyte growth factor gene transfer with naked plasmid DNA ameliorates dimethylnitrosamine-induced liver fibrosis in rats. Hepatol Res. 2008;38:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Takehara T, Tatsumi T, Suzuki T, Rucker EB, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189-1197. [PubMed] |
| 40. | Hisakura K, Murata S, Takahashi K, Matsuo R, Pak S, Ikeda N, Kawasaki T, Kohno K, Myronovych A, Nakano Y. Platelets prevent acute hepatitis induced by anti-fas antibody. J Gastroenterol Hepatol. 2011;26:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Pañeda A, Juanarena N, Arcelus S, Razquin N, Guembe L. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663-10668. [PubMed] |