Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4262
Revised: April 24, 2013
Accepted: May 7, 2013
Published online: July 14, 2013
Processing time: 111 Days and 0.6 Hours
The endoscopic findings of gastric hyperplastic polyps (HPs) with dysplasia have not been well-defined, and the clinical significance of these lesions, including their malignant potential, is unclear. In this report, we describe a case of a white opaque substance (WOS)-positive gastric HP with dysplasia. A 76-year-old woman was referred to our hospital for endoscopic resection of a gastric HP. Upper endoscopy revealed a 25-mm whitish and reddish polypoid lesion on the greater curvature in the lower third of the stomach. The whitish part was diagnosed as a WOS using conventional and magnifying endoscopy with narrow band imaging. An examination of the biopsy specimen indicated that the lesion was a typical gastric HP. However, because of its color and the presence of a WOS, we suspected that this lesion was an atypical gastric HP. Therefore, we performed a polypectomy. Histopathologically, diffuse low- to high-grade dysplasia was found on the surface of the polyp. We performed immunohistochemical staining using a monoclonal antibody specific for adipophilin as a marker of lipid droplets (LDs). LDs were detected in approximately all of the neoplastic cells, especially in the surface epithelium of the intervening apical parts and were located in the subnuclear cytoplasm of the neoplastic cells. According to endoscopic and histopathological findings, the WOS-positive epithelium indicated dysplasia of the gastrointestinal phenotype, which could absorb lipids. The presence of a WOS in a gastric HP may be considered an endoscopic finding that is predictive of the neoplastic transformation of a gastric HP. We suggest that a WOS-positive gastric HP should be resected endoscopically to investigate its neoplastic transformation.
Core tip: In this report, we present the first case of a white opaque substance (WOS)-positive gastric hyperplastic polyp (HP) with dysplasia. We performed immunohistochemical staining using a monoclonal antibody specific for adipophilin as a marker of lipid droplets. According to endoscopic and histopathological findings, the WOS-positive epithelium corresponded to the dysplasia in this lesion. The presence of a WOS in a gastric HP may be considered an endoscopic finding that is predictive of the neoplastic transformation of a gastric HP. We suggest that patients with a WOS-positive gastric HP should be treated by endoscopic resection to investigate the neoplastic transformation of the HP.
- Citation: Ueyama H, Matsumoto K, Nagahara A, Gushima R, Hayashi T, Yao T, Watanabe S. A white opaque substance-positive gastric hyperplastic polyp with dysplasia. World J Gastroenterol 2013; 19(26): 4262-4266
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4262
With the widespread use of digestive endoscopy in recent years, gastric polyps are now diagnosed more frequently and can be easily studied after a biopsy or polypectomy. Gastric hyperplastic polyps (HPs) are among the most common type of benign epithelial gastric polyps[1-6]. Gastric HPs are usually considered to be benign lesions similar to adenomas; however, neoplastic transformation can occur but rarely. Moreover, endoscopic findings of gastric HPs with dysplasia have not been well-defined, and the clinical significance of these lesions, including their malignant potential, is unclear. A white opaque substance (WOS) is a finding from magnifying endoscopy (ME) with narrow band imaging (NBI), which was first reported by Yao et al[7-9] to be a substance in the superficial area of gastric neoplasias that obscures the subepithelial microvascular architecture. However, the presence of a WOS in gastric lesions other than adenomas and adenocarcinomas has not been reported. We report a rare case of a WOS-positive gastric HP with dysplasia.
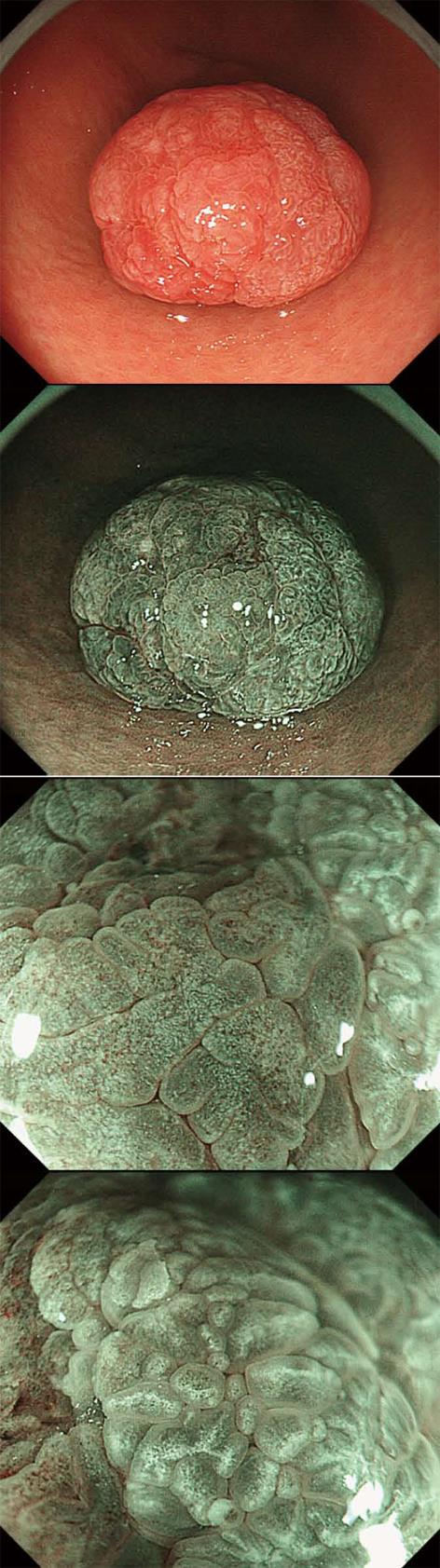
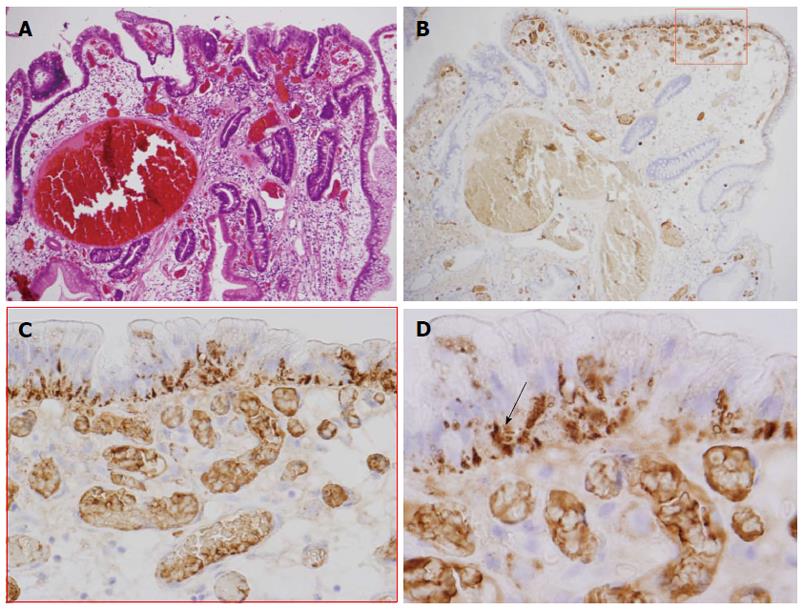
A 76-year-old woman was referred to our hospital for further investigation and treatment of a gastric HP. Excluding the existence of the gastric HP, she had no specific symptoms and the results of the physical examination were normal. Her medical history included hyperlipidemia and diabetes mellitus, and there was no family history of gastrointestinal polyposis. She had not undergone proton pump inhibitor therapy. An assessment of Immunoglobulin G antibodies and a histological examination were negative for Helicobacter pylori infection. Upper endoscopy revealed a 25-mm polypoid lesion on the greater curvature in the lower third of the stomach (Figure 1A). The entire lesion was reddish with scattered whitish areas. The whitish parts were determined to be a WOS using conventional endoscopy and ME with NBI (Figure 1). The WOS in the lesion was comprised of two morphological types (Figure 1C and D). One type had a symmetrical distribution of a regular dotted pattern (Figure 1C), and the other type had an asymmetrical distribution of an irregular speckled and linear pattern (Figure 1D). An examination of the biopsy specimen revealed findings that were typical of a gastric HP without dysplasia. However, we suspected that this lesion was an atypical gastric HP because of its color and the irregular distribution of the WOS. Therefore, we performed a polypectomy, which was without complications. Histopathologically, the findings for the entire lesion were typical of a gastric HP, and diffuse low- to high-grade dysplasia was found on the surface of the lesion (Figure 2A-D). Immunohistochemically, the lesion had diffuse positivity for MUC5AC, focal positivity for mucin 2 (MUC2) and villin, and negative staining for MUC6 and CD10 (Figure 2E-I). This lesion was classified as having the gastrointestinal (GI) phenotype according to combinations of the expression of MUC2, MUC5AC, MUC6, CD10 and villin. The GI phenotype was detected in approximately all of the neoplastic cells, whereas an examination of the other cells indicated a gastric phenotype. The Ki-67 labeling index of dysplasia was slightly higher than that of a typical HP, and the positive cells were irregularly distributed. The overexpression of the p53 protein was not observed. In addition, adipophilin was detected in approximately all of the neoplastic cells, especially in the surface epithelium of the intervening apical parts and was located in the subnuclear cytoplasm of the neoplastic cells (Figure 3). This lesion was finally diagnosed as a WOS-positive gastric hyperplastic polyp with dysplasia. Surveillance endoscopy with biopsy specimens is planned for 6 mo after the endoscopic resection.
The endoscopic findings for gastric HP with dysplasia have not been well-defined. Typical HPs are markedly reddish polypoid lesions with a smooth surface, which occasionally has erosions. In this case, the entire lesion was reddish and was scattered with whitish areas, which differs from typical HPs. The whitish areas were determined to be a WOS using conventional and ME with NBI. Histopathologically, low- to high-grade dysplasia was diffusely present on the surface of the gastric HP. Adipophilin was detected in approximately all of the neoplastic cells, especially in the surface epithelium of the intervening apical parts. These findings suggested that the WOS-positive epithelium corresponded to the dysplasia in this lesion. Yao et al[10] reported that the hallmark of a WOS is the presence of lipid droplets (LDs) that accumulate in the superficial part of the epithelial neoplasia within the stomach. Using immunohistochemistry and immunoelectron microscopy, Ueo et al[11] found that the WOS resulted from an accumulation of LDs with adipophilin. These findings supported a relationship between the WOS and adipophilin in this case. In addition, the WOS in this lesion was comprised of two morphological types: one type with a symmetrical distribution of a regular dotted pattern and the other type with an asymmetrical distribution of an irregular speckled and linear pattern. We could not discriminate between these patterns pathologically. Yao et al[7] reported that the WOS in adenomas was regular and homogeneous, whereas the WOS in adenocarcinomas was irregular and speckled. In this case, we speculated that the findings for a WOS may be based on the differences in the shape, the intraepithelial and intracytoplasmic density and the distribution of the LDs between low-grade and high-grade dysplasia. In our case, most of the reddish area indicated low-grade dysplasia. Endoscopically, we determined that these areas were reddish because a slight accumulation of LDs may not allow these areas to be visualized as a WOS. We concluded that a WOS may be visualized only in the dysplastic areas of gastric HPs. The presence of a WOS in a gastric HP may be considered an endoscopic finding that is predictive of the neoplastic transformation of a gastric HP.
The neoplastic transformation of HPs has not been well-defined, and their clinical significance, including their malignant potential, is unclear. Kang et al[12] reported that the neoplastic transformation of gastric HPs was significantly associated with the postgastrectomy state and lesions that were 1 cm in diameter, pedunculated, and synchronous neoplastic lesion. Daibo et al[13] reported that cancer cells arose from the dysplastic area in HPs rather than directly from nondysplastic hyperplastic epithelium, which is consistent with the histogenesis of the malignant transformation of HPs. Endoscopic resection should be considered for these HPs to avoid the risk of missing HPs with neoplastic potential. In our case, the dysplasia was observed on the surface of the resected HP; however, an examination of the biopsy specimen indicated a typical HP without dysplasia. Regarding this discrepancy, we speculate that when the biopsy specimen was collected, a small sample was unintentionally taken from the part of the lesion that did not exhibit dysplasia. Using the biopsy specimen that was obtained, we could not clearly determine whether the lesion was a typical HP or an HP with low-grade dysplasia. Therefore, in gastric HPs, WOS positivity may be considered an endoscopic finding that indicates endoscopic resection.
The mechanism of the accumulation of LDs in gastric epithelium is unknown. Yao et al[10] proposed the following two possible mechanisms: the absorption hypothesis and the production hypothesis. In addition, they reported that the WOS in gastric neoplasms with an intestinal phenotype was caused by the accumulation of lipids and WOS-positive gastric neoplasms may be able to absorb lipids. Matsubara et al[14] demonstrated that the expression of adipophilin may be induced during the process of early colorectal carcinogenesis, which supports the production hypothesis that neoplastic cells synthesize LDs. In our case, the neoplastic cells that were positive for adipophilin were of the GI phenotype. This finding suggests that the neoplastic transformation of gastric epithelium with a phenotypic change to the intestinal phenotype may require the ability to absorb lipids. However, further investigation is needed to elucidate the mechanism of LD accumulation.
In this report, we present the first case of a WOS-positive gastric HP with dysplasia. We suggest that patients with a WOS-positive gastric HP should be treated by endoscopic resection to investigate the neoplastic transformation of the HP.
P- Reviewers Sipos F, Zhang XC S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Cao H, Wang B, Zhang Z, Zhang H, Qu R. Distribution trends of gastric polyps: an endoscopy database analysis of 24 121 northern Chinese patients. J Gastroenterol Hepatol. 2012;27:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 2. | Deppisch LM, Rona VT. Gastric epithelial polyps. A 10-year study. J Clin Gastroenterol. 1989;11:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Morais DJ, Yamanaka A, Zeitune JM, Andreollo NA. Gastric polyps: a retrospective analysis of 26,000 digestive endoscopies. Arq Gastroenterol. 2007;44:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 135] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Carmack SW, Genta RM, Schuler CM, Saboorian MH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 7. | Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Yao K, Iwashita A, Matsui T. White opaque substance within superficial-elevated gastric neoplasia as visualized by magnification endoscopy (ME) with narrow-band imaging (NBI): A new useful marker for discriminating adenoma from carcinoma. Endoscopy. 2007;39:A16. |
| 9. | Yao K, Iwashita A, Nagahama T. White opaque substance as visualized by magnifying endoscopy with narrow-band imaging: A new useful sign for differentiating high-grade dysplasia/early gastric carcinoma from low-grade dysplasia in gastric neoplastic lesions. Endoscopy. 2008;40:A61. |
| 10. | Yao K, Iwashita A, Nambu M, Tanabe H, Nagahama T, Maki S, Ishikawa H, Matsui T, Enjoji M. Nature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imaging. Dig Endosc. 2012;24:419-425. [PubMed] |
| 11. | Ueo T, Yonemasu H, Yada N, Yano S, Ishida T, Urabe M, Takahashi K, Nagamatsu H, Narita R, Yao K. White opaque substance represents an intracytoplasmic accumulation of lipid droplets: immunohistochemical and immunoelectron microscopic investigation of 26 cases. Dig Endosc. 2013;25:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Kang HM, Oh TH, Seo JY, Joen TJ, Seo DD, Shin WC, Choi WC, Kim JY. [Clinical factors predicting for neoplastic transformation of gastric hyperplastic polyps]. Korean J Gastroenterol. 2011;58:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. Am J Gastroenterol. 1987;82:1016-1025. [PubMed] |
| 14. | Matsubara J, Honda K, Ono M, Sekine S, Tanaka Y, Kobayashi M, Jung G, Sakuma T, Nakamori S, Sata N. Identification of adipophilin as a potential plasma biomarker for colorectal cancer using label-free quantitative mass spectrometry and protein microarray. Cancer Epidemiol Biomarkers Prev. 2011;20:2195-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |