Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4146
Revised: May 27, 2013
Accepted: June 1, 2013
Published online: July 14, 2013
Processing time: 178 Days and 17.5 Hours
AIM: To investigate the role of T helper 17 cells (Th17) and regulatory T cells (Treg) in hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF).
METHODS: We enrolled 79 patients with HBV infection into the study, 50 patients with HBV-related ACLF and 29 patients with chronic hepatitis B (CHB), from the First Affiliated Hospital of Medical College from January 2009 to June 2012. The ACLF patients were diagnosed according to the criteria recommended by The 19th Conference of the Asian Pacific Association for the Study of the Liver in 2009. Twenty healthy individuals with a similar gender and age structures to the two patient groups were also included as the normal controls (NC). Of the 50 ACLF patients, 28 were subsequently classified as non-survivors: 19 patients died from multi-organ failure, 3 underwent liver transplantation, and 6 discontinued therapy during follow-up because of financial reasons. The remaining 22 ACLF patients whose liver and anticoagulation function recovered to nearly normal levels within the next 6 mo were classified as survivors. The number of circulating Treg and Th17 cells was determined upon diagnosis and during the 8th week of follow-up through flow cytometry.
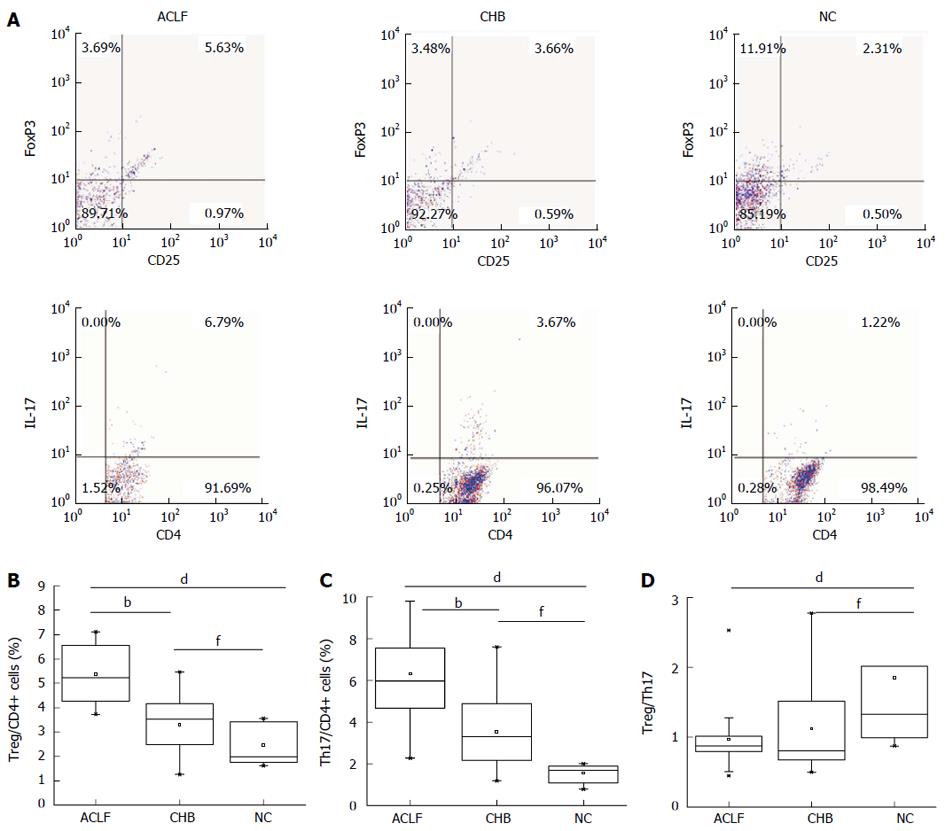
RESULTS: The percentage of circulating Treg cells in the ACLF group was significantly higher than that in the CHB group (5.50% ± 1.15% vs 3.30% ± 1.13%, P < 0.01). The percentages of circulating Th17 cells in the ACLF and the CHB groups were significantly higher than that in the NC group (6.32% ± 2.22% vs 1.56% ± 0.44%, P < 0.01; 3.53% ± 1.65% vs 1.56% ± 0.44%, P < 0.01). No significant difference in Treg cell to Th17 cell ratio was observed between the ACLF group and the CHB group (0.98 ± 0.44 vs 1.12 ± 0.64, P = 0.991), whereas those in the two HBV infection groups were significantly lower than that in the NC group (1.85 ± 1.22; both P < 0.01). The percentage of Treg cells in the survivors during the 8th week of follow-up was significantly lower than that during peak ACLF severity [total bilirubin (TBIL) peak] (3.45% ± 0.97% vs 5.18% ± 1.02%, P < 0.01). The percentage of Th17 cells in survivors during the 8th week of follow-up was significantly lower than that during the peak TBIL (2.89% ± 0.60% vs 5.24% ± 1.46%; P < 0.01). The Treg cell to Th17 cell ratio during the 8th week of follow-up was significantly higher than that during the TBIL peak (1.22 ± 0.36 vs 1.10 ± 0.54; P < 0.05).
CONCLUSION: Restoring the Treg cell to Th17 cell ratio during the follow-up phase of ACLF could maintain the immune system at a steady state, which favours good prognosis.
Core tip: In this study, the expression of circulating Treg and Th17 cells in hepatitis B virus-related acute-on-chronic liver failure (ACLF), chronic hepatitis B (CHB), and normal controls (NC) was measured using flow cytometric analysis. The percentages of circulating Treg and Th17 cells in ACLF group increased significantly compared with that in CHB group and NC group. Furthermore, the ratio of Treg to Th17 cells increased significantly upon recovery. Our study suggests that the reverting ratio of Treg to Th17 cells at the follow-up phase of ACLF could maintain the immune system at a steady state in favour of good prognosis.
- Citation: Niu YH, Yin DL, Liu HL, Yi RT, Yang YC, Xue HA, Chen TY, Zhang SL, Lin SM, Zhao YR. Restoring the Treg cell to Th17 cell ratio may alleviate HBV-related acute-on-chronic liver failure. World J Gastroenterol 2013; 19(26): 4146-4154
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4146
Acute-on-chronic liver failure (ACLF) is defined as an acute hepatic insult that manifests as jaundice and coagulopathy complicated by ascites and/or encephalopathy within four weeks among patients with chronic liver disease. The major aetiologic agents of ACLF are alcohol and drugs in the West and infectious agents in the East[1]. A characteristic feature of ACLF is its rapid progression and high incidence of short- and medium-term mortality, ranging from 50% to 90%[2]. Recent advances in medical therapy have only slightly decreased the mortality rate of HBV-related ACLF. Antiviral treatment improves the survival of patients with HBV-related ACLF[3,4]. Administering granulocyte-colony stimulating factor improves survival of patients with ACLF[5]. Liver transplantation can also improve outcomes, even in critically ill patients with multi-organ failure[6]. However, these advances have not significantly decreased the mortality associated with HBV-related ACLF.
In China, hepatitis B virus (HBV) infections account for 82% of all ACLF[7]. The exact mechanism of HBV-related ACLF is currently unclear. HBV is not directly cytopathic[8] and the hepatocellular injury caused by HBV infection is predominantly immune-mediated[9,10]. Wasmuth et al[11] demonstrated that the immunopathology of ACLF is similar to “sepsis-like” immune paralysis. Cytokines also play an important role in ACLF[12,13]. Evidence shows that circulating IL-17+ T cells accumulate in large numbers in the liver of CHB patients, increasing with progression from CHB to ACLF[14,15]. By contrast, Treg cells suppress immune responses and inflammatory diseases[16,17], and they regulate chronic inflammatory responses that contribute to the pathologic events in the liver during HBV infection[18]. Several research groups have demonstrated increased Th17 cells in the peripheral blood and liver tissues, as well as changes in the balance between Th17 and Treg cells in ACLF patients[19,20].
In this study, we focus on the balance between CD4+CD25+FoxP3+ Treg cells and CD4+IL17+ Th17 cells in HBV-related ACLF and examine the effects of this balance on patient responses to therapy and outcomes.
We enrolled 79 patients with HBV infection into the study, 50 patients with HBV-related ACLF and 29 patients with CHB, from the First Affiliated Hospital of Medical College, Xi’an Jiaotong University (Xi’an, China) from January 2009 to June 2012. The ACLF patients were diagnosed according to the criteria recommended by The 19th Conference of the Asian Pacific Association for the Study of the Liver in 2009[1]. Patients were excluded if their liver disease was caused by conditions other than HBV infection. No patient received steroids or other immunosuppressive drugs within 6 mo before sampling. The normal controls (NC) consisted of 20 healthy individuals with similar gender and age structures to the two patient groups.
Blood samples were collected from the ACLF patients 1 wk after diagnosis and again on the 2nd, 4th, 6th, 8th, and 12th week. The blood samples of the CHB patients were collected upon diagnosis and before receiving antiviral therapy. The clinical and biochemical details of ACLF patients at the time of their highest total bilirubin (TBIL), as well as those of the CHB patients are listed in Table 1. The Model for End-stage Liver Disease (MELD) score was calculated using the following formula: 3.8 × loge[bilirubin (mg/dL)] + 11.2 × loge(INR) + 9.6 × loge[creatinine (mg/dL)] + 6.4 × (aetiology: 0 if cholestatic or alcoholic, 1 otherwise)[21].
| ACLF (n = 50) | CHB (n = 29) | NC (n = 20) | |
| Male/female | 42/8 | 24/5 | 16/4 |
| Age (yr) | 40.2 ± 12.0 | 34.4 ± 12.0 | 34.5 ± 9.2 |
| ALT (IU/L) | 176.6 ± 430.0 | 328.4 ± 386.8 | 22.3 ± 6.7 |
| TBIL (μmol/L) | 566.3 ± 133.5 | 68.8 ± 90.9 | 12.2 ± 2.3 |
| INR | 1.83 ± 0.44 | 1.09 ± 0.10 | 0.99 ± 0.03 |
| HBeAg | 17 (34) | 18 (62.1) | NA |
| Anti-HBe | 30 (60) | 8 (27.6) | NA |
| HBVDNA (Log10 IU/mL) | 5.25 ± 1.30 | 6.11 ± 1.28 | NA |
| MELD score | 22.5 ± 4.7 | 4.6 ± 6.5 | -2.7 ± 1.9 |
We classified 28 patients as non-survivors: 19 patients died from multi-organ failure, 3 underwent liver transplantation, and 6 discontinued therapy during follow-up because of financial reasons. The remaining 22 patients whose liver function and coagulation recovered to nearly normal within the next 6 mo were classified as survivors.
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Medical College, Xi’an Jiaotong University, and written informed consent was obtained from each subject prior to enrolment in the study.
Heparinized whole blood (200 μL) from study subjects was incubated for 4 h in phorbol 12-myristate 13-acetate (PMA) (final concentration 25 ng/mL) and ionomycin (final concentration 1 μg/mL) with monensin (end concentration 1.4 μg/mL) at 37 °C under a 5% CO2 atmosphere.
Then, the whole blood was separated and incubated with anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD25-phycoerythrin conjugate (PE) or anti-CD4-FITC. After simultaneous fixation and permeabilization, the cells were incubated for 30 min with anti-Foxp3-phycoerythrin-cyanine 5 conjugate (PE-Cy5) or anti-IL17A-PE. Then, the cells were washed again and were resuspended in PBS for flow cytometric analysis.
To detect the expression of circulating Th17 and Treg cells, the whole blood was subjected to flow cytometry on a CyFlow® SL machine (PARTEC Company, Germany) using FloMax software.
The levels of HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HCV, anti-HDV, anti-HEV, and anti-HIV antibodies were detected via qualitative enzyme immunoassays. The serum HBV DNA levels were measured using a real-time polymerase chain reaction (PCR) assay with a detection limit of 1 × 103 IU/mL. All tests were performed in a clinical laboratory according to standardized methods.
Results are expressed as mean ± SD. Statistical comparisons between groups were performed using a Mann-Whitney non-parametric U test. A Spearman’s correlation analysis was performed to evaluate the relationship between variables. The data were analyzed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL). Differences with P values < 0.05 were considered statistically significant in all analyses.
The characteristics of survivors and non-survivors in the ACLF group are listed in Table 2. The biochemical parameters and MELD scores at the time of peak TBIL level of the survivors and non-survivors are listed in Table 3.
| Variceal bleeding | Infection | Drug | Alcohol | Indeterminant reasons | |||
| Gastrointestinal tract | Upper respiratory tract | Ascites | |||||
| Non-survivors (n = 28) | 2 (7.1) | 5 (17. 8) | 4 (14.3) | 2 (7.1) | 7 (25.0) | 2 (7.1) | 8 (28.6) |
| Survivors (n = 22) | 0 | 7 (31.8) | 5 (22.7) | 0 | 5 (22.7) | 1 (4.5) | 4 (18.2) |
| Non-survivors (n = 28) | Survivors (n = 22) | P value | |
| Male/female | 24/4 | 18/4 | ND |
| Age (yr) | 40.8 ± 11.0 | 39.5 ± 13.3 | 0.570 |
| TBIL (μmol/L) | 607.6 ± 117.5 | 513.7 ± 136.7 | 0.006 |
| INR | 2.00 ± 0.44 | 1.62 ± 0.33 | 0.088 |
| MELD scores | 23.7 ± 4.6 | 20.9 ± 4.4 | 0.021 |
The percentages of CD4+CD25+FoxP3+ (Treg) cells and CD4+IL-17+ (Th17) cells and the ratio of Treg to Th17 cells between groups are relative to CD4+ T cells (Figure 1A). The percentages of circulating Treg cells in the two HBV infection groups were significantly higher than that in the NC group [2.46% ± 0.78%, P = 0.000 (vs ACLF), P = 0.008 (vs CHB)]. The percentage of Treg cells in the ACLF group was also significantly higher than that in the CHB group (5.50% ± 1.15% vs 3.30% ± 1.13%, P = 0.001) (Figure 1B). Strikingly, the percentages of circulating Th17 cells in the ACLF and CHB groups were significantly higher than those in the NC group (6.32% ± 2.22% vs 1.56% ± 0.44%, P = 0.000; 3.53% ± 1.65% vs 1.56% ± 0.44%, P = 0.000) (Figure 1C). No significant difference in the Treg cell to Th17 cell ratio was observed between the ACLF group and the CHB group (0.98 ± 0.44 vs 1.12 ± 0.64, P = 0.991). However, the Treg cell to Th17 cell ratios in the two HBV infection groups were significantly lower than that in the NC group [1.85 ± 1.22; P = 0.000 (vs ACLF), P = 0.004 (vs CHB)] (Figure 1D). In summary, the percentages of Th17 and Treg cells in the peripheral blood of ACLF patients increased, whereas the ratio of Treg to Th17 cells was lower in the ACLF and CHB groups compared with that in the NC group.
The percentage of Treg cells in the ACLF group was correlated with ALT (r = 0.381 P = 0.006), TBIL (r = 0.378, P = 0.007), and INR (r = 0.381, P = 0.006) (Figure 2A-C). The percentage of Th17 cells was positively correlated with ALT (r = 0.360, P = 0.012), TBIL(r = 0.323, P = 0.025), and MELD scores (r = 0.293, P = 0.043) (Figure 2D-F). The percentage of Treg cells was significantly correlated with the percentage of Th17 cells (r = 0.425, P = 0.003; Figure 2G). The ratio of Treg to Th17 cells was not significantly correlated with any of the biochemical parameters. Neither the percentages of Treg and Th17 cells, nor the ratio of Treg to Th17 cells, was correlated with serum HBV DNA levels.
In CHB group, the percentage of Treg cells was positively correlated with TBIL (r = 0.431, P= 0.02), whereas the percentage of Th17 cells was significantly correlated with ALT (r = 0.367, P = 0.05). Neither the percentages of Treg and Th17 cells, nor the ratio of Treg to Th17 cells, showed a relationship with serum HBV DNA levels.
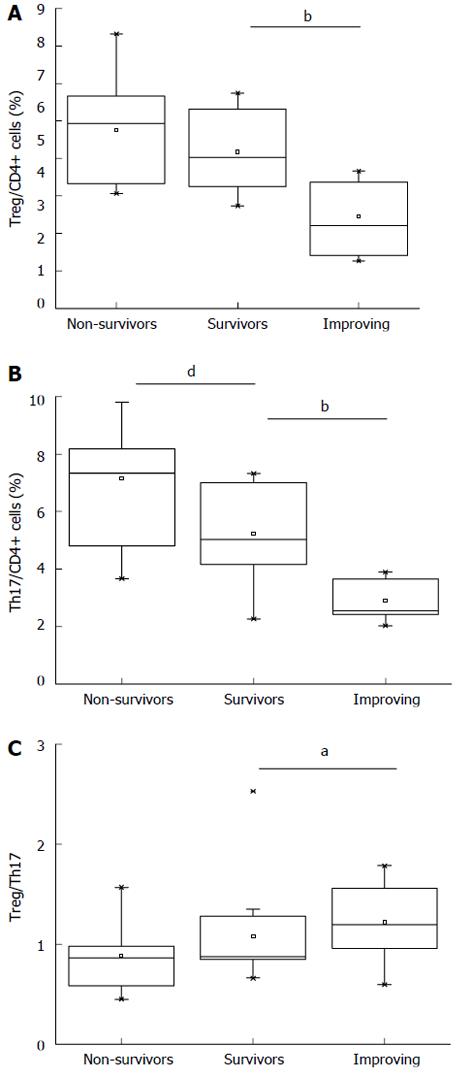
The percentages of Treg and Th17 cells were measured serially in the ACLF group during a follow-up phase, which lasted for 8 wk. During TBIL peak, the percentage of Treg cells in non-survivors increased slightly compared with that in survivors (5.76% ± 1.21% vs 5.18% ± 1.02%), but the difference was not statistically significant (P = 0.103; Figure 3A). The percentage of Th17 cells in non-survivors was significantly higher than that in survivors (7.17% ± 2.37% vs 5.24% ± 1.46%; P = 0.002) (Figure 3B). No significant difference in Treg cell to Th17 cell ratio was observed between non-survivors and survivors (0.88 ± 0.32 vs 1.10 ± 0.54; P = 0.233; Figure 3C).
During 8th week follow-up, the percentages of Treg and Th17 cells and the Treg cell to Th17 cell ratio of the survivors were compared with those at the time of TBIL peak. The percentage of Treg cells during the 8th week was significantly lower than that during the TBIL peak (3.45% ± 0.97% vs 5.18% ± 1.02%, P = 0.000) (Figure 3A), whereas the percentage of Th17 cells during the 8th week was significantly lower than that during the TBIL peak (2.89% ± 0.60% vs 5.24% ± 1.46%; P =0.000) (Figure 3B). The Treg cell to Th17 cell ratio was significantly higher during the 8th week than that during the TBIL peak (1.22 ± 0.36 vs 1.10 ± 0.54; P = 0.039) (Figure 3C).
We examined changes in the percentage of Treg cells and Th17 cells, as well as the Treg cell to Th17 cell ratios, of 6 ACLF survivors (5 males and 1 female) who exhibited decreased TBIL with obvious clinical improvement. The percentage of Treg cells (Figure 4A) decreased together with the percentage of Th17 cells (Figure 4B) during the follow-up period, whereas the Treg cell to Th17 cell ratio increased gradually (Figure 4C).
Th17 and Treg cells are subsets of CD4+ T helper cells with developmental pathways that contribute significantly to immune responses[22-27]. Th17 cells are implicated in host defence against a number of microorganisms[28,29]and they participate in autoimmune and chronic inflammatory diseases[30,31]. By contrast, Treg cells display suppressive and surveillance functions in immune responses and inflammatory diseases[16,17]. A study suggested that Th17 cells mediate airway inflammatory responses whereas antigen-specific Treg cells suppress Th17-mediated lung inflammation[32]. Yang et al[33] reported that an imbalance between Th17 and Treg cells contributes to the pathogenesis of systemic lupus erythematosus (SLE) and that regulating the balance between Treg and Th17 cells may be a promising strategy for SLE treatment. However, our aim is to determine whether an imbalance in Treg and Th17 cells contributes to the pathogenesis of acute hepatocellular injury in HBV-related ACLF and its mechanism.
Treg and Th17 cells were significantly higher in ACLF patients than in CHB patients and the normal controls. Furthermore, in ACLF patients, the percentage of Treg cells was significantly correlated with the percentage of Th17 cells, as well as with ALT and TBIL. Several recent studies demonstrated that the percentages of circulating Th17 and Treg cells increase with disease progression and are parallel to the severity of liver inflammation as CHB progresses to ACLF[14,18,19]. These results suggest that Th17 cells are a potential marker for the degree of liver injury in ACLF, whereas Treg cells may contribute to the suppression of the immune system[11].
We then analyzed the Treg and Th17 cell counts of ACLF survivors and non-survivors to determine their correlation with their clinical outcomes. The percentage of Th17 cells in ACLF non-survivors was significantly higher than that in survivors, but the percentage of Treg cells and the Treg cell to Th17 cell ratio were not significantly different between the two groups. Our findings are consistent with those of Zhai et al[20] who also found increased Th17 cells and Treg cells in ACLF patients. However, they found no significant difference in Th17 and Treg cell counts between ACLF survivors and non-survivors and they observed significantly lower Th17 cell to Treg cell ratios in the survivors than in non-survivors. These differences between the two studies may be attributed to differences in the severity of hepatic injury, as well as differences in the timing of sample acquisition. However, both studies found that patients with ACLF have higher Treg and Th17 cell counts and that higher Th17 cell to Treg cell ratios may predict poorer prognosis.
In China, ACLF occurs mainly in patients with CHB or HBV-related cirrhosis. Spontaneous or treatment-induced inflammatory flare ups are frequently observed in chronic hepatitis B[34]. Several researchers in Asia have demonstrated that early intervention using antiviral therapy improves the short- and long-term outcomes of HBV-related ACLF by aggressively targeting the precipitating events to prevent multi-organ failure[3,4,35]. Entecavir-induced suppression of HBV replication in nine CHB patients showed rapid increases in Th17 cells and decreases in Treg cells, which significantly reduced the Treg cell to Th17 cell ratio[36]. Thus, the effects of antiviral therapies with ACLF will affect Treg and Th17 cells.
We measured the Treg and Th17 cell counts in ACLF survivors during follow-up to determine whether antiviral therapy affects the balance between Treg and Th17 cells. The percentages of Treg and Th17 cells decreased significantly during follow-up, whereas the Treg cell to Th17 cell ratio increased significantly compared with that during the peak of illness, which coincides with the peak serum total bilirubin. Restoring the Treg cell to Th17 cell ratio could help maintain the immune system in a steady state, favouring good outcomes among patients with HBV-related ACLF.
In conclusion, Th17 cell counts may reflect the degree of liver injury, where Treg cells may regulate the protective suppression of the immune system of patients with ACLF. Treg cells and Th17 cells increased in patients with ACLF, but higher Th17 cell to Treg cell ratios may be correlated with poorer prognosis. Restoring the Treg cell to Th17 cell ratio during ACLF could help maintain the immune system in a steady state, which may improve patient prognosis.
Acute-on-chronic liver failure (ACLF) is an acute hepatic insult with rapid progression and high short- and medium-term mortality. In China, hepatitis B virus (HBV) infections account for 82% of all ACLF cases. Studies have demonstrated that the hepatocellular injury caused by HBV infection is predominantly immune-mediated.However, the mechanism of HBV-related ACLF is currently unclear.
Several studies showed that Treg and Th17 cells may contribute to the pathologic events in the liver during HBV infection and the balance between Treg cells and Th17 cells may be disrupted during ACLF progression.
The authors found that ACLF patients have significantly increased Treg cell to Th17 cell ratios. The Treg cells and Th17 cells decreased significantly with improvement of the ACLF, but the Treg cell to Th17 cell ratio significantly increased. Thus, Th17 cells may be a marker for the degree of liver injury and poor prognosis in ACLF. Restoring the Treg cell to Th17 cell ratio during the aggravating phase of ACLF could maintain the immune system at a steady state, which favours good prognosis.
Th17 cells and Treg cells are subsets of CD4+ T helper cells with related developmental pathways. The balance between Th17 cells and Treg cells is important for maintaining human immune homeostasis. The balance between Th17 cells and Treg cells should be maintained before chronic hepatitis B progresses to ACLF. Once CHB has progressed to ACLF, restoring the Treg cell to Th17 cell ratio becomes even more significant to the outcome.
ACLF is defined as an acute hepatic insult that manifests as jaundice and coagulopathy in a patient with chronic liver disease, complicated within 4 wk by ascites and/or encephalopathy. The major aetiologic agents of ACLF are alcohol and drugs in the West and infectious agents in the East. A characteristic feature of ACLF is its rapid progression and high short- and medium-term mortality (50% to 90%).
The manuscript focuses on the balance between CD4+CD25+FoxP3+ Treg cells and CD4+IL17+ Th17 cells in HBV-related ACLF. The authors examined the effects of this balance on patient responses to therapy and outcomes. This manuscript is interesting. The references used in the study are updated.
P- Reviewers Fung SK, Lampertico P S- Editor Wen LL L- Editor O’Neill M E- Editor Zhang DN
| 1. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 2. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 3. | Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Chen T, He Y, Liu X, Yan Z, Wang K, Liu H, Zhang S, Zhao Y. Nucleoside analogues improve the short-term and long-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Clin Exp Med. 2012;12:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505-512.e1. [PubMed] |
| 6. | Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation. 2011;92:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Du WB, Li LJ, Huang JR, Yang Q, Liu XL, Li J, Chen YM, Cao HC, Xu W, Fu SZ. Effects of artificial liver support system on patients with acute or chronic liver failure. Transplant Proc. 2005;37:4359-4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 639] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 9. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 611] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 10. | Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 394] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Ambrosino G, Naso A, Feltracco P, Carraro P, Basso SM, Varotto S, Cillo U, Zanus G, Boccagni P, Brolese A. Cytokines and liver failure: modification of TNF- and IL-6 in patients with acute on chronic liver decompensation treated with Molecular Adsorbent Recycling System (MARS). Acta Biomed. 2003;74 Suppl 2:7-9. [PubMed] |
| 13. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 324] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Ye Y, Xie X, Yu J, Zhou L, Xie H, Jiang G, Yu X, Zhang W, Wu J, Zheng S. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J Clin Immunol. 2010;30:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 962] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 17. | Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739-747. [PubMed] |
| 19. | Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, Zhang S, Lin S, Zhao Y. The balance between intrahepatic IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Zhai S, Zhang L, Dang S, Yu Y, Zhao Z, Zhao W, Liu L. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol. 2011;24:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 22. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1541] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 23. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5475] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 24. | Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 1242] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 26. | Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883-889. [PubMed] |
| 27. | Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1590] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 28. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3360] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 29. | Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3407] [Cited by in RCA: 3594] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 30. | Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1224] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 31. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1164] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 32. | Jaffar Z, Ferrini ME, Girtsman TA, Roberts K. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B-cell recruitment and polymeric IgA and IgM levels in the airways. Eur J Immunol. 2009;39:3307-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Yang J, Yang X, Zou H, Chu Y, Li M. Recovery of the immune balance between Th17 and regulatory T cells as a treatment for systemic lupus erythematosus. Rheumatology (Oxford). 2011;50:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 35. | Cui YL, Yan F, Wang YB, Song XQ, Liu L, Lei XZ, Zheng MH, Tang H, Feng P. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci. 2010;55:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One. 2010;5:e13869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |