Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3272
Revised: April 15, 2013
Accepted: April 18, 2013
Published online: June 7, 2013
Processing time: 195 Days and 17.6 Hours
AIM: To investigate whether matrix metalloproteinases-9 (MMP-9) or trypsinogens could serve as histological markers for an aggressive disease course in pediatric ulcerative colitis (UC).
METHODS: We identified 24 patients with pediatric onset (≤ 16 years) UC who had undergone surgery during childhood/adolescence a median of 2.1 years (range 0.1-7.4 years) after the diagnosis (between 1990 and 2008) in Children’s Hospital, Helsinki, Finland. We also identified 27 conservatively treated UC patients and matched them based on their age at the time of diagnosis and follow-up at a median of 6 years (range 3-11 years) to serve as disease controls. Twenty children for whom inflammatory bowel disease (IBD) had been excluded as a result of endoscopy served as non-IBD controls. Colon biopsies taken by diagnostic endoscopy before the onset of therapy were stained using immunohistochemistry to study the expression of MMP-9, trypsinogen-1 (Tryp-1), Tryp-2, and a trypsin inhibitor (TATI). The profiles of these proteases and inhibitor at diagnosis were compared between the surgery group, the conservatively treated UC patients and the non-IBD controls.
RESULTS: The proportions of Tryp-1 and Tryp-2 positive samples in the colon epithelium and in the inflammatory cells of the colon stroma were comparable between the studied groups at diagnosis. Interestingly, the immunopositivity of Tryp-1 (median 1; range 0-3) was significantly lower in the epithelium of the colon in the pediatric UC patients undergoing surgery when compared to that of the conservatively treated UC patients (median 2; range 0-3; P = 0.03) and non-IBD controls (median 2; range 0-3; P = 0.04). For Tryp-2, there was no such difference. In the inflammatory cells of the colon stroma, the immunopositivities of Tryp-1 and Tryp-2 were comparable between the studied groups at diagnosis. Also, the proportion of samples positive for TATI, as well as the immunopositivity, was comparable between the studied groups in the colon epithelium. In the stromal inflammatory cells of the colon, TATI was not detected. In UC patients, there were significantly more MMP-9 positive samples and a higher immunopositivity in the stromal inflammatory cells of the colon when compared to the samples from the non-IBD patients (P = 0.006 and P = 0.002, respectively); the immunopositivity correlated with the histological grade of inflammation (95%CI: 0.22-0.62; P = 0.0002), but not with the other markers of active disease. There were no differences in the immunopositivity or in the proportions of MMP-9 positive samples when examined by epithelial staining. The staining profiles in the ileal biopsies were comparable between the studied groups for all of the studied markers.
CONCLUSION: For pediatric UC patients who require surgery, the immunopositivity of Tryp-1 at diagnosis is lower when compared to that of patients with a more benign disease course.
Core tip: The risk factors for aggressive pediatric ulcerative colitis (UC) were studied in 24 patients who had undergone surgery by staining diagnostic tissue samples for matrix metalloproteinase-9 and trypsinogen-1 (Tryp-1) and Tryp-2, as well as a trypsin inhibitor. In the UC group, there were significantly more samples that were matrix metalloproteinase-9 positive in comparison to the samples from non-inflammatory bowel disease patients. UC patients undergoing colectomy showed lower immunopositivity of Tryp-1 in the colon epithelium in their diagnostic biopsies when compared to that of conservatively treated patients and non-inflammatory bowel disease patients. The discovery of a low trypsinogen level at diagnosis warrants further study.
- Citation: Piekkala M, Hagström J, Tanskanen M, Rintala R, Haglund C, Kolho KL. Low trypsinogen-1 expression in pediatric ulcerative colitis patients who undergo surgery. World J Gastroenterol 2013; 19(21): 3272-3280
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3272
Inflammatory bowel disease (IBD) is a common name for Crohn’s disease (CD), ulcerative colitis (UC), and unclassified colitis. Some patients may have only one episode of active disease and remain in remission for the rest of their lives, while others will suffer from a relapsing or continuously active disease and require colectomy[1-3]. The studied risk factors for an aggressive disease course (steroid dependency[4], pancolitis[4,5], extraintestinal manifestations[5], severe disease at diagnosis[6], or disease extension from the primary site of the active disease) are related to the clinical presentation and thus it is impossible to foresee the phenotype of the disease at diagnosis.
Since many pediatric UC patients present with aggressive disease during their disease course, it would be beneficial to find biomarkers that at the onset of disease could distinguish patients with complicated disease behavior and a high risk for surgery from those with a more benign course. The current opinion, however, is that immunosuppressive treatment is needed for a patient with a complicated disease. If it was possible to recognize an aggressive disease with biomarkers in the early disease phase, then such patients could be introduced to immunosuppressive treatment aiming to improve the disease outcome. Previous pediatric data show the benefits of immunosuppressive treatment[7-9], although biological treatment with a tumor necrosis factor-α (TNF-α) antagonist has, disappointingly, not reduced the surgery rates[10].
Matrix metalloproteinases (MMPs), a family of 24 zinc-dependent enzymes, comprise a group of proteinases[11] that degrade the extracellular matrix and basement membrane proteins in tissue remodeling processes both in normal and in pathological conditions[11]. In IBD the most abundantly expressed MMP is MMP-9[12]. Previously, we have shown that the immunopositivity of MMP-9 in the colon decreases after TNF-α antagonist treatment in adult CD[13]. Trypsinogens are serine proteases that are capable of degrading extracellular matrix proteins and the pro-forms of acute phase reaction proteins[14], such as TNF-α, which cause damage to the mucosal barrier and UC-like inflammation[15]. The exact role of trypsinogens in IBD for the most part remains unknown, but it is known that trypsins activate promatrix metalloproteinases (proMMPs), especially the IBD-related proMMP-9[16-19]. Tumor associated trypsin inhibitor (TATI), also called pancreatic secretory trypsin inhibitor[20], inhibits trypsin in a 1:1 molar ratio[21]. Extrapancreatically secreted TATI is assumed to play additional roles in ulcer healing and tissue regeneration[22]. TATI also takes part in preventing the excessive digestion of gastrointestinal (GI) mucus[22,23]. As with trypsinogens, the role of TATI in IBD-related inflammation is mostly unknown.
Matrix and serine proteases may be regarded as “regulators” of the barrier and inflammation cascade of the gut[14]. Accordingly, we hypothesized that the presence of expression of such proteases would be associated with the severity of the course of IBD. The hypothesis was tested by comparing the results of immunohistochemical stainings with MMP-9, trypsinogen-1 (Tryp-1), Tryp-2, and TATI on the biopsy material of pediatric patients who underwent surgery vs conservatively treated patients and subjects without IBD.
We reviewed all pediatric onset (≤ 16 years old) UC patients from the IBD patient registry of Children’s Hospital, Helsinki University Central Hospital who had been diagnosed between 1990 and 2008. From this database, we identified 24 UC patients who had undergone surgery (time from diagnosis to surgery, maximum 7 years) and 27 conservatively treated disease controls. The latter group had been diagnosed at the same age as the operated patients and the follow-up took place within the period of time that had elapsed between the diagnosis and the surgery in the index case. None of the disease controls underwent an operation during follow-up (median 6 years). All of the patients had undergone diagnostic ileocolonoscopy and upper gastrointestinal endoscopy, and during follow-up the diagnosis remained consistent for the UC patients. Table 1 presents the background data of the study groups. The data on the indications and type of surgical therapy of the patients is shown in Table 2. Twenty children who had undergone ileocolonoscopy with biopsies and who did not suffer from IBD served as non-IBD controls. The indications for endoscopy in these children were as follows: suspected IBD (n = 12), abdominal pain (n = 4), colorectal bleeding (n = 3), and pancreatic insufficiency (n = 1).
| Patient groups | Surgery | Disease | Non-IBD |
| controls | controls | ||
| No. of patients (male) | 24 (11) | 27 (15) | 20 (10) |
| Age at diagnosis (yr), median (range) | 13.1 (3.1-16.0) | 12.1 (2.8-16.6) | 13.5 (2.7-16.8) |
| Time from diagnosis to surgery (yr), median (range) | 2.1 (0.1-7.4) | - | - |
| Follow-up (yr), median (range) | - | 6.3 (2.8-10.7) | 1.0 (0.0-4.0) |
| Endoscopic disease extension | |||
| Proctitis | 0 | 1 | - |
| Left-sided colitis | 9 | 6 | - |
| Pancolitis | 15 | 20 | - |
| No inflammation | - | - | 20 |
| Histological inflammation1 | |||
| No inflammation | - | - | 18 |
| Mild | 6 | 10 | 1 |
| Moderate to severe | 18 | 17 | - |
| Laboratory markers, median (range) | |||
| Hb (g/L) | 112 (86-135) | 116 (86-140) | 131.0 (93-153) |
| ESR (mm/h) | 25 (7-61) | 22 (7-63) | 8 (2-23) |
| CRP (mg/L) | 20 (< 3-92) | 9 (< 5-36) | < 3 (< 3-< 5) |
| WBC (E9/L) | 9.6 (6.7-26.3) | 7.5 (4.1-15.9) | 5.1 (3.0-16.3) |
| Alb (g/L) | 34.6 (28.0-40.0) | 36.2 (23.6-42.6) | 42.3 (39.8-47.2) |
| Age at first surgery (yr), median (range) | 15.3 (5.5-19.8) |
| Time from diagnosis to surgery (yr), median (range) | 2.1 (0.1-6.6) |
| Indication for primary operation | |
| Stricture | 1 (perianal) |
| Refractory disease to medication | 20 |
| Steroid-dependency | 18 |
| No response to TNF-alpha antagonist | 2 |
| Fulminant colitis | 3 |
| Primary operation | |
| Colectomy (IRA) | 1 |
| Proctocolectomy (IPAA) | 23 |
For all of the patients and controls, tissue samples from the diagnostic ileocolonoscopy (colonic and ileal biopsies) were stained with immunohistochemistry to test for MMP-9, Tryp-1, Tryp-2, and TATI antibodies (see below). Based on the patient records, we reviewed the laboratory values of albumin, C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), the white blood cell count (WBC), and the haemoglobin at the time of the ileocolonoscopy.
The disease was considered to be acute and severe if the patient required hospitalization at diagnosis and the PUCAI score was over 65[24]. The endoscopic distribution of the disease at the time of the diagnostic colonoscopy was scored as 0, indicating no inflammation, as 1 for proctitis, as 2 for left-sided colitis, and as 3 for pancolitis. The degree of histological inflammation was recorded based on the primary diagnostic histological examination reports provided by experienced pathologists. The samples were graded according to the presence of ulceration, the frequency of acute and chronic inflammatory cells, crypt distortion, and goblet cell depletion recorded on a scale of 0 to 2 (0 no inflammation, 1 mild inflammation, 2 moderate to severe inflammation) (modified from Beattie et al[25]). The presence of backwash ileitis in the diagnostic ileocolonoscopy was also recorded.
Formalin-fixed, paraffin-embedded tissue sections (4-μm) were deparaffinized in xylene and rehydrated in graded concentrations of ethanol and water. To block endogenous peroxidase activity, the sections were treated with 0.3% Dako REAL Peroxidase-Blocking Solution (DAKO, Glostrup, Denmark) for 5 min. For the antigen retrieval tissue, the sections were treated in the pretreatment module of the Autostainer (LabVision UK Ltd) in a Tris-HCl buffer (pH 8.5) for 20 min at 98 °C (Tryp-1 and MMP), a 0.1% pepsin solution for 15 min at 37 °C (Tryp-2), or a 0.01% trypsin solution for 30 min at 37 °C (TATI). After pretreatment, a Lab Vision Autostainer TM 480 (LabVision, Fremont, CA, United States) was used for immunohistochemistry. The sections were incubated with antibodies against Tryp-1 at a dilution of 1:500 (MAB 1482, Chemicon, Temecula, CA, United States), against MMP-9 (RB-1539-PO, LabVision Fremont, CA) at a dilution of 1:1000, and against Tryp-2 (8F7)[26] and TATI (6E8)[27] at dilutions of 1:200 and 1:500, respectively. The incubation times were one hour for Tryp-1, MMP-9, and TATI and overnight for Tryp-2. Subsequently, the sections were incubated with a peroxidase-conjugated Dako REAL EnVision/HRP, Rabbit/Mouse (ENV) reagent for 30 min. The final visualization was performed by incubating the sections in DAKO REAL DAB+ Chromogen for 10 min. Between the staining steps, the slides were washed with 0.04% PBS. Mayer’s hematoxylin was used for counterstaining. Finally, the sections were rinsed in tap water for 10 min and mounted in an aqueous mounting medium (Aguamount, BDH, Poole, United Kingdom). As positive controls, we used formalin-fixed, paraffin-embedded sections of pancreatic papillary carcinoma (Tryp-1), pancreatic adenocarcinoma (Tryp-2 and TATI), and ventricular adenocarcinoma (MMP-9). A tissue sample without a primary antibody served as a negative control during each staining.
The immunohistochemical specimens were scored independently by two investigators (Piekkala M and Hagström J) in a semi-quantitative fashion under a light-field microscope at × 100 magnification using a marking and staining immunopositivity scale. This was done as follows: negative immunoreactivity was scored as 0, a diffuse weak positivity was scored as 1, a moderately positive or focally strongly positive immunopositivity was scored as 2, and a homogeneously strong immunopositivity was scored as 3 (modified from Böckelman et al[28]). An experienced pathologist (Hagström J) confirmed the identity of the cell types producing each Tryp-1, Tryp-2, TATI, or MMP-9.
The National Supervisory Authority for Welfare and Health gave its permission for use of the tissue samples. In accordance with Finnish regulations, no informed consent is required for this kind of study.
Because of the small number of patients, the non-parametric Mann-Whitney’s test and Kruskal-Wallis test were used to compare the significance of the differences in the level of immunopositivities between the studied groups. An independent samples t test (Spearman), and Fisher’s exact test were performed to investigate the significance of the association between the level of immnopositivities and the clinical markers. A P < 0.05 was considered significant.
The endoscopic distribution of inflammation was comparable between the UC groups (P = 0.7). During the diagnostic biopsies, we detected mild inflammation in 16 of 51 (31%) and moderate to severe inflammation in 35 of 51 UC patients (69%), without major differences between surgical and conservative groups (P = 0.2). Of the 24 patients requiring surgery (median two years after diagnosis), 46% (n = 11) had an acute severe disease at diagnosis. This was significantly more than in the disease controls (15%, n = 4) (P = 0.03). In total, 15 of 51 UC patients (30%) had an acute severe disease at diagnosis (73% of them underwent surgery).
Table 3 shows the proportions of Tryp-1, Tryp-2, TATI, and MMP-9 positive samples, the median levels of immunopositivity, and the P values when comparing the proportions of positive samples and the level of immunopositivity between the surgery group and the conservatively treated or non-IBD groups.
| Surgery (n = 24) | Conservative treatment controls (n = 27) | Non-IBD controls (n = 20) | P valueSurgery vs conservative treatment controls | P valueSurgery vs non-IBD controls | |
| Number of positive samples n (%) | |||||
| Tryp-1 | |||||
| Epithelium | 13 (57) | 19 (76) | 15 (79) | 0.22 | 0.19 |
| Stroma; inflammatory cells | 11 (48) | 8 (32) | 5 (26) | 0.38 | 0.21 |
| Tryp-2 | |||||
| Epithelium | 21 (95) | 21 (81) | 18 (95) | 0.20 | 0.22 |
| TATI | |||||
| Epithelium | 18 (78) | 24 (92) | 15 (75) | 0.23 | 1.00 |
| MMP-9 | |||||
| Epithelium | 4 (18) | 2 (8) | 0 (0) | 0.40 | 0.17 |
| Stroma; inflammatory cells | 11 (50) | 12 (48) | 1 (5) | 1.00 | 0.0061 |
| Median immunopositivity | |||||
| Tryp-1 | |||||
| Epithelium | 1.0 | 2.0 | 2.0 | 0.032 | 0.042 |
| Stroma; inflammatory cells | 0.0 | 0.0 | 0.0 | 0.16 | 0.10 |
| Tryp-2 | |||||
| Epithelium | 1.0 | 1.0 | 1.0 | 0.56 | 0.38 |
| TATI | |||||
| Epithelium | 1.0 | 1.0 | 1.0 | 0.81 | 0.97 |
| MMP-9 | |||||
| Epithelium | 0.0 | 0.0 | 0.0 | 0.33 | 0.17 |
| Stroma; inflammatory cells | 0.5 | 0.0 | 0.0 | 0.89 | 0.0033 |
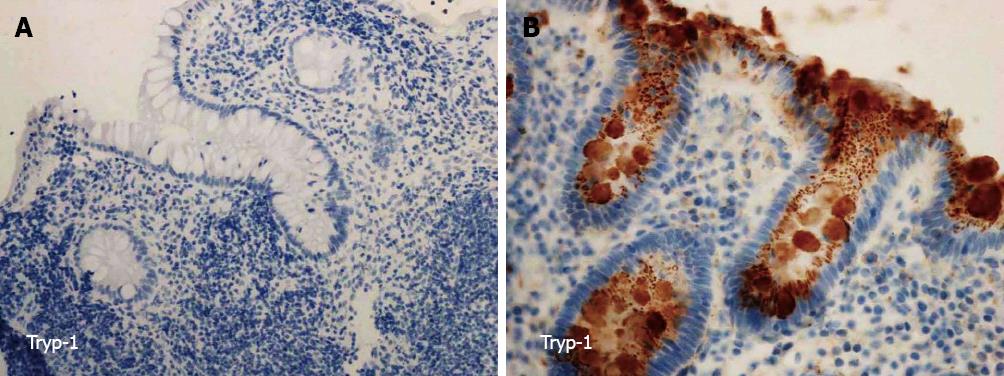
Tryp-1: Tryp-1 was stained in the colon epithelium and in the inflammatory cells of the stroma. The staining of Tryp-1 was granular. The immunopositivity in the colon epithelium was significantly lower in the samples from the surgery group (Figure 1A) when compared to that of the conservatively treated (Figure 1B) and non-IBD controls (Table 3). No difference in the immunopositivity of the colon epithelium was found between the conservatively treated and non-IBD controls. The proportion of positive samples and the level of immunopositivity in the inflammatory cells of the stroma were similar in the studied groups (Table 3).
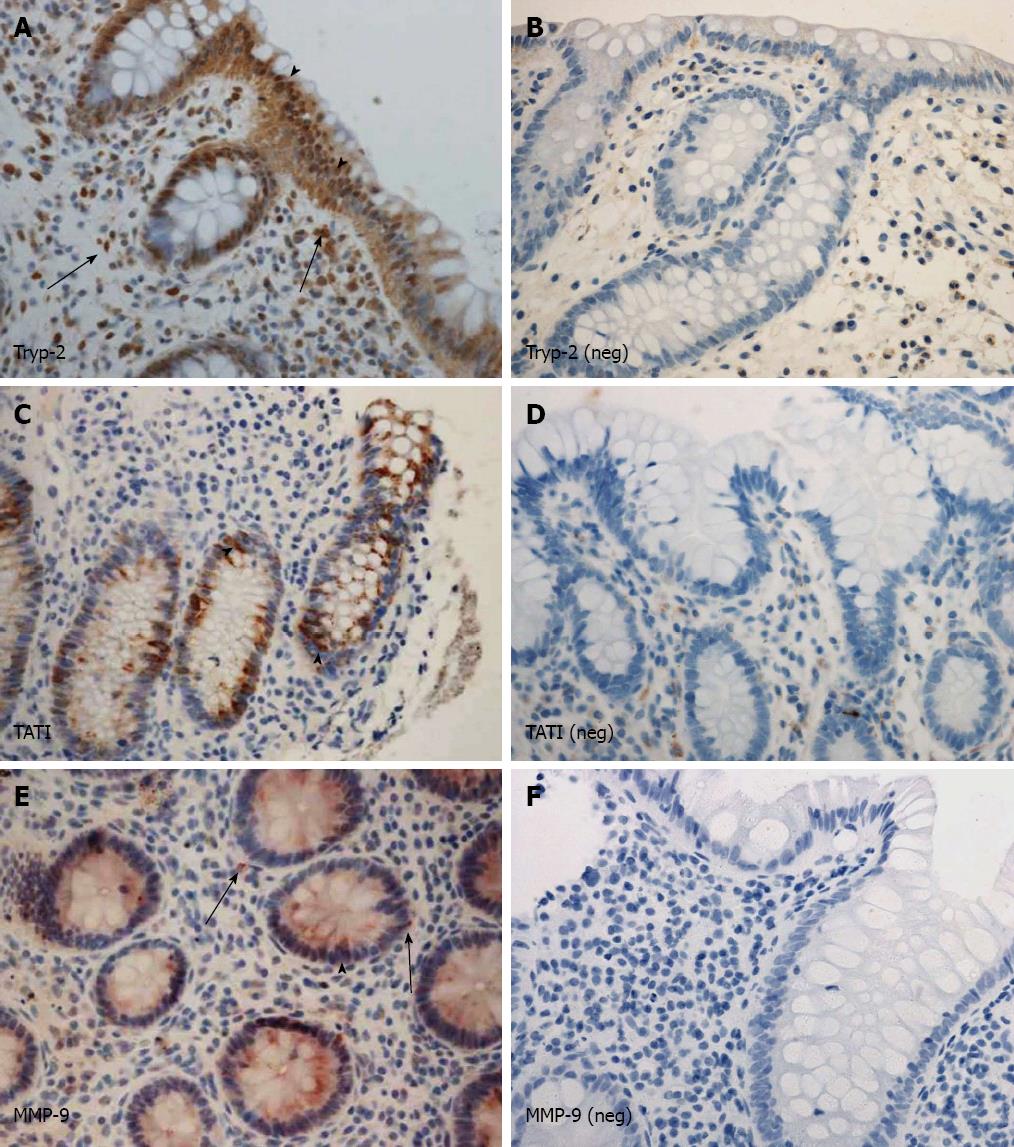
Tryp-2: Tryp-2 was stained in the epithelium. All of the samples, regardless of the study group, had the same level of Tryp-2 immunopositivity in the inflammatory cells of the colon stromal tissue (Figure 2A and B). The proportion of positive samples and the level of immunopositivity in the epithelium were comparable between the surgery group, the conservatively treated, and non-IBD controls (Table 3). In total, 83% (39 of 47) of the samples that were Tryp-1 positive in the colon epithelium were also Tryp-2 positive.
TATI: TATI was only detected in the colon epithelium, the inflammatory cells of the colon stroma being negative for TATI (Figure 2C and D). The proportion of TATI positive samples and the level of immunopositivity were comparable between the surgery group and the conservative and non-IBD groups (Table 3). The immunopositivity of TATI did not relate to the expression of Tryp-1 or Tryp-2 (data not shown).
MMP-9: In the colon, MMP-9 was mainly found in the stromal inflammatory cells and positive staining was detected in the epithelium (Figure 2E and F). The proportion of MMP-9 immunopositive samples and the level of immunopositivity in both the inflammatory cells and the epithelium were similar for the surgery group and the conservative group (Table 3). When compared to non-IBD patients, the proportion and immunopositivity of MMP-9 in the inflammatory cells was higher in the UC patients, but we detected no differences when considering the epithelial positivity (Table 3). There was no association between the expression of MMP-9 and the expression of Tryp-1, Tryp-2, or TATI (data not shown).
In 59% of the ileal samples (available for 36 UC patients and 14 non-IBD controls), the epithelium was Tryp-1 positive. Of the inflammatory cells in the stroma, 33% were Tryp-1 positive. The comparative staining proportions were 100% for Tryp-2 (positivity of the epithelium and inflammatory cells), 82% for TATI (positivity of the epithelium, inflammatory cells negative) and 28% and 26% for MMP-9 (positivity of the epithelium and of the inflammatory cells of the stroma, respectively). The proportions of positively stained samples for Tryp-1, Tryp-2, and TATI were comparable between the studied groups. All UC patients, regardless of treatment outcome, had significantly more MMP-9 positivity in the ileal epithelium than non-IBD patients (P = 0.005). The staining intensities of the studied markers were similar in all of the study groups (data not shown).
The immunopositivity of MMP-9 in the inflammatory cells was positively associated with the grade of inflammation in the colon (95%CI: 0.22-0.62; P = 0.0002). However, there was no association with MMP-9 immunopositivity and the other markers of active disease. The staining intensities of the other markers in the epithelium of the colon or in the inflammatory cells of the stroma did not associate with the initial disease severity at diagnosis, the grade of inflammation at diagnosis, the endoscopic distribution of the disease at diagnosis, or the presence of backwash ileitis.
All of the primary laboratory values in the surgery group and disease controls were comparable. The blood inflammatory markers (CRP, ESR, WBC) were significantly higher and the albumin level lower in UC patients compared to the non-IBD controls (data not shown).
The exact roles of the active inflammation markers trypsinogens, TATI, and MMP-9 have not been studied in pediatric UC patients in relation to the disease outcome. Therefore, we compared the immunohistochemical positivity of Tryp-1, Tryp-2, TATI, and MMP-9 in diagnostic colonoscopic tissue samples from pediatric UC patients who required surgery with those of conservatively treated patients and non-IBD controls.
For the first time serine proteases Tryp-1 and Tryp-2 were demonstrated in the epithelium and in the inflammatory cells of the colon and ileum and TATI was found in the epithelium of the colon and ileum in pediatric UC patients. At the time of the diagnosis, the immunopositivity of Tryp-1 in the colon epithelium is lower when comparing patients that have aggressive disease requiring surgery with those who have conservative treatment. We could also confirm that MMP-9 expression correlated with the grade of inflammation of the colon similarly, as reported earlier in adult and pediatric IBD patients[12].
It is known that serine proteases regulate the gut barrier and inflammation cascade[14], and that they also take part in protease-activated receptor signaling, which has been shown to activate intestinal inflammation[29]. Thus, our finding of a low Tryp-1 level in the colon epithelium of the surgery group is important because as many as 75% of the patients had more than mild inflammation and nearly half had acute severe disease at the time of diagnosis. Acute inflammation is, however, not the only background factor explaining the low expression of Tryp-1 in patients undergoing surgery; in addition, the immunopositivity did not associate with the degree of inflammation at diagnosis. The expression of Tryp-2 was also low in all of the studied groups. These findings, when taken together, indicate that trypsinogens are weakly expressed in the colon and not upregulated by inflammation in UC patients. Since they are also expressed in the healthy colon, their role might be mainly physiological. It may be speculated that severe inflammation suppresses the physiological secretion rate of Tryp-1 but not Tryp-2 and TATI. At the moment, however, the exact role of trypsins Tryp-1 and Tryp-2 in intestinal inflammation remains unknown.
In healthy subjects proteases are expressed in the GI tract at low concentrations. An increase in concentrations is regarded as destructive; thus the balance between the proteases and their inhibitors is considered important[14]. It is known that TATI inhibits both Tryp-1 and Tryp-2 equally[21]. We found no correlation between the expression of the major trypsin inhibitor TATI and trypsinogens. Since the expression of TATI was low, the weak expression of trypsinogens could not be explained by the upregulation of TATI. The low expression of TATI in pediatric UC patients is in line with earlier findings showing reduced intestinal TATI in IBD and other GI conditions associated with abnormalities in the internal mucous layer[30]. However, as TATI and trypsinogens were also expressed in healthy colon, their low expression might be physiological.
This study reports for the first time together the expression of MMP-9 and trypsinogens in pediatric UC patients. However, the immunopositivity of MMP-9 had no association with that of Tryp-1 or Tryp-2. However, the small sample size in the present study has to be considered, and this finding does not either contradict or support the earlier report that trypsins associate with proMMP-9[16-19]. MMP-9 is expressed in the neutrophils but not in the colon epithelium in pediatric IBD[31]. In the present study we showed, in addition to the upregulated MMP-9 expression in the inflammatory cells, epithelial immunopositivity for MMP-9 as well. This may be a novel finding as we validated our staining with care to avoid any unspecific staining reactivity. Of the MMP inhibitors (TIMPs), TIMP-3 is expressed in pediatric IBD[14,30,32,33], and it correlates with the degree of inflammation[30]. Unfortunately, the TIMP levels could not be assessed in this study.
The aim of the study was to compare two pediatric UC groups: those who would undergo surgery during childhood or adolescence and patients whose disease course is more benign. Although the patient population in this study is quite small, one strength of the study was that the patient age and disease extension at diagnosis were comparable. Also, the duration of disease of patients who had undergone surgery and the disease controls was matched. Restorative proctocolectomy was performed at a median of 2 years after diagnosis (median age 15 years), and all the patients were followed-up at least until that time. In total, the disease controls were followed up for a median of 6 years and none of them had undergone surgery during this time period. We also had control samples from non-IBD children, which demonstrated the natural expression of studied markers in non-inflammatory bowel disease.
In terms of the limitations of the results, we could only use one method for assessing trypsinogens, TATI, and MMP-9 profiles because the biopsy samples were very small and did not allow further analysis. Using molecular biological methods such as reverse transcription-polymerase chain reaction or Western blotting to analyze the expression of the studied markers would have given more strength to the study. However, we had the unique possibility to study the diagnostic samples and relate the data to the disease course of the patients. Furthermore, two investigators blinded to the patient data assessed the samples. The discovery of a low Tryp-1 level related to an aggressive disease course is preliminary and was not related to the altered expression of its major inhibitor, TATI. Thus, the physiological mechanisms underlying these findings require further studies.
In conclusion, significant proportions of pediatric UC patients suffer from aggressive disease and require surgery. It is estimated that approximately one in four pediatric patients undergo colectomy during the first decade after the diagnosis[2,3,34]. No change in the surgery rate has been seen between the years 1994 and 2007[35]. By current diagnostic modalities, it is impossible to foresee the disease outcome at the time of the diagnosis of UC, although an initial aggressive disease increases the risk of colectomy[6]. We found the expression of Tryp-1 to be weaker in the epithelial cells of patients with aggressive disease but the mechanism and significance of that finding remained unclear. Therefore, there is a need for further investigations on Tryp-1 and other biomarkers to identify patients with aggressively proceeding UC.
The authors thank Ms Anne Nikkonen for her excellent help in collecting the patient data and Ms Päivi Peltokangas for her skilful technical assistance.
Significant proportions of pediatric patients with ulcerative colitis (UC) suffer from aggressive disease and require surgery. It is estimated that approximately one out of four pediatric patients undergo colectomy during the first decade after the diagnosis. No change in the surgery rate was observed between the years 1994 and 2007.
By current diagnostic modalities, it is impossible to foresee the disease outcome at the time of the diagnosis of UC, even though an aggressive initial disease presentation increases the risk of colectomy. If it was possible to recognize an aggressive disease course by biomarkers in the early disease phase, such patients could be introduced to early immunosuppressive treatment aimed at improving the disease outcome. The role of matrix metalloproteinase-9 (MMP-9) is scarcely reported in pediatric inflammatory bowel disease (IBD). Trypsinogens and a trypsin inhibitor (TATI) have not been studied at all in pediatric UC patients. Accordingly, the authors hypothesized that the expression of such proteases would be associated with the severity of the course of the UC. The hypothesis was tested by comparing the results of immunohistochemical staining on the diagnostic biopsy material of pediatric patients who underwent surgery vs conservatively treated patients and subjects without inflammatory bowel disease.
For the first time, the presence of the serine proteases trypsinogen-1 (Tryp-1) and Tryp-2 was demonstrated in the epithelium and in the inflammatory cells of the colon and ileum in pediatric UC. Accordingly, this is the first study to describe the expression of their inhibitor, TATI, in the epithelium of the colon and in the ileum in pediatric UC patients. The authors found that at the time of the diagnosis, the immunopositivity of Tryp-1 in the colon epithelium is lower when comparing pediatric UC patients who have an aggressive disease course requiring surgery to those managed by non-operative treatment. The authors could also confirm that the expression of MMP-9 correlated with the grade of inflammation of the colon similarly, as reported earlier in adult and pediatric IBD patients.
By demonstrating the protease profile of the colon mucosa of pediatric patients who will suffer from aggressive UC, this study may aid experts in characterizing the molecular environment of aggressive UC. The final goal is to find clinically applicable biomarkers for an aggressive disease.
UC is a chronic inflammatory disease of the colon and the rectum that is of unknown origin; it is characterized by bloody diarrhea, abdominal pain, and weight loss; MMP, a family of 24 zinc-dependent enzymes, comprise a group of proteinases that degrade the extracellular matrix and basement membrane proteins in tissue remodeling processes both in normal and in pathological conditions; Tryps are serine proteases that are capable of degrading extracellular matrix proteins and the pro-forms of acute phase reaction proteins, such as tumor necrosis factor, which cause damage to the mucosal barrier and UC-like inflammation. Tumor associated TATI inhibits trypsin in a 1:1 molar ratio.
This is an interesting study delineating the colonic expression of key proteases in the setting of paediatric UC.
P- Reviewer Day AS S- Editor Huang XZ L- Editor O’Neill M E- Editor Li JY
| 1. | Beattie RM, Croft NM, Fell JM, Afzal NA, Heuschkel RB. Inflammatory bowel disease. Arch Dis Child. 2006;91:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Turunen P, Ashorn M, Auvinen A, Iltanen S, Huhtala H, Kolho KL. Long-term health outcomes in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2009;15:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Coran AG. New surgical approaches to ulcerative colitis in children and adults. World J Surg. 1985;9:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Falcone RA Jr, Lewis LG, Warner BW. Predicting the need for colectomy in pediatric patients with ulcerative colitis. J Gastrointest Surg. 2000;4:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Gower-Rousseau C, Dauchet L, Vernier-Massouille G, Tilloy E, Brazier F, Merle V, Dupas JL, Savoye G, Baldé M, Marti R. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Hyams JS, Davis P, Grancher K, Lerer T, Justinich CJ, Markowitz J. Clinical outcome of ulcerative colitis in children. J Pediatr. 1996;129:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Jakobsen C, Paerregaard A, Munkholm P, Faerk J, Lange A, Andersen J, Jakobsen M, Kramer I, Czernia-Mazurkiewicz J, Wewer V. Pediatric inflammatory bowel disease: increasing incidence, decreasing surgery rate, and compromised nutritional status: A prospective population-based cohort study 2007-2009. Inflamm Bowel Dis. 2011;17:2541-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, Merle V, Salomez JL, Branche J, Marti R. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 467] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 9. | Gupta N, Cohen SA, Bostrom AG, Kirschner BS, Baldassano RN, Winter HS, Ferry GD, Smith T, Abramson O, Gold BD. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology. 2006;130:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | de Bie CI, Buderus S, Sandhu BK, de Ridder L, Paerregaard A, Veres G, Dias JA, Escher JC. Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: results of a 5-year audit of the EUROKIDS registry. J Pediatr Gastroenterol Nutr. 2012;54:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis. 2007;13:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Mäkitalo L, Sipponen T, Kärkkäinen P, Kolho KL, Saarialho-Kere U. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn’s disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int J Colorectal Dis. 2009;24:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Biancheri P, Di Sabatino A, Corazza GR, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res. 2013;351:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533-539. [PubMed] |
| 16. | Lauhio A, Sorsa T, Srinivas R, Stenman M, Tervahartiala T, Stenman UH, Grönhagen-Riska C, Honkanen E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann Med. 2008;40:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Paju A, Sorsa T, Tervahartiala T, Koivunen E, Haglund C, Leminen A, Wahlström T, Salo T, Stenman UH. The levels of trypsinogen isoenzymes in ovarian tumour cyst fluids are associated with promatrix metalloproteinase-9 but not promatrix metalloproteinase-2 activation. Br J Cancer. 2001;84:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sorsa T, Salo T, Koivunen E, Tyynelä J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkilä P. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997;272:21067-21074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Lukkonen A, Sorsa T, Salo T, Tervahartiala T, Koivunen E, Golub L, Simon S, Stenman UH. Down-regulation of trypsinogen-2 expression by chemically modified tetracyclines: association with reduced cancer cell migration. Int J Cancer. 2000;86:577-581. [PubMed] |
| 20. | Huhtala ML, Pesonen K, Kalkkinen N, Stenman UH. Purification and characterization of a tumor-associated trypsin inhibitor from the urine of a patient with ovarian cancer. J Biol Chem. 1982;257:13713-13716. [PubMed] |
| 21. | Marchbank T, Freeman TC, Playford RJ. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59:167-174. [PubMed] |
| 22. | Marchbank T, Chinery R, Hanby AM, Poulsom R, Elia G, Playford RJ. Distribution and expression of pancreatic secretory trypsin inhibitor and its possible role in epithelial restitution. Am J Pathol. 1996;148:715-722. [PubMed] |
| 23. | Playford RJ, Batten JJ, Freeman TC, Beardshall K, Vesey DA, Fenn GC, Baron JH, Calam J. Gastric output of pancreatic secretory trypsin inhibitor is increased by misoprostol. Gut. 1991;32:1396-1400. [PubMed] |
| 24. | Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 820] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 25. | Beattie RM, Nicholls SW, Domizio P, Williams CB, Walker-Smith JA. Endoscopic assessment of the colonic response to corticosteroids in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. 1996;22:373-379. [PubMed] |
| 26. | Itkonen O, Kylänpää L, Zhang WM, Stenman UH. Reference intervals for and validation of recalibrated immunoassays for trypsinogen-1 and trypsinogen-2. Clin Chem. 2012;58:1494-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Osman S, Turpeinen U, Itkonen O, Stenman UH. Optimization of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J Immunol Methods. 1993;161:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Böckelman C, Hagström J, Mäkinen LK, Keski-Säntti H, Häyry V, Lundin J, Atula T, Ristimäki A, Haglund C. High CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancer. Br J Cancer. 2011;104:1890-1895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Lohman RJ, Cotterell AJ, Suen J, Liu L, Do AT, Vesey DA, Fairlie DP. Antagonism of protease-activated receptor 2 protects against experimental colitis. J Pharmacol Exp Ther. 2012;340:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Playford RJ, Hanby AM, Patel K, Calam J. Influence of inflammatory bowel disease on the distribution and concentration of pancreatic secretory trypsin inhibitor within the colon. Am J Pathol. 1995;146:310-316. [PubMed] |
| 31. | Mäkitalo L, Kolho KL, Karikoski R, Anthoni H, Saarialho-Kere U. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand J Gastroenterol. 2010;45:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005-1014. [PubMed] |
| 34. | von Allmen D, Goretsky MJ, Ziegler MM. Inflammatory bowel disease in children. Curr Opin Pediatr. 1995;7:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Benchimol EI, Guttmann A, To T, Rabeneck L, Griffiths AM. Changes to surgical and hospitalization rates of pediatric inflammatory bowel disease in Ontario, Canada (1994-2007). Inflamm Bowel Dis. 2011;17:2153-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |