Published online Apr 21, 2013. doi: 10.3748/wjg.v19.i15.2340
Revised: February 14, 2013
Accepted: March 22, 2013
Published online: April 21, 2013
Processing time: 116 Days and 1 Hours
AIM: To explore effects of telomerase RNA-targeting phosphorothioate antisense oligodeoxynucleotides (PS-ASODN) on growth of human gastrointestinal stromal tumors transplanted in mice.
METHODS: A SCID mouse model for transplantation of human gastrointestinal stromal tumors (GISTs) was established using tumor cells from a patient who was diagnosed with GIST and consequently had been treated with imatinib. GIST cells cultured for 10 passages were used for inoculation into mice. Transfection of PS-ASODN was carried out with Lipotap Liposomal Transfection Reagent. GISTs that subsequently developed in SCID mice were subjected to intra-tumoral injection once daily from day 7 to day 28 post-inoculation, and mice were divided into the following four groups according to treatment: PS-ASODN group (5.00 μmoL/L of oligonucleotide, each mouse received 0.2 mL once daily); imatinib group (0.1 mg/g body weight); liposome negative control group (0.01 mL/g); and saline group (0.01 mL/g). On day 28, the mice were sacrificed, and tumor attributes including weight and longest and shortest diameters were measured. Tumor growth was compared between treatment groups, and telomerase activity was measured by enzyme-linked immunosorbent assay. Apoptosis was examined by flow cytometry. Real-time polymerase chain reaction was used to detect expression of the mRNA encoding the apoptosis inhibition B-cell leukemia/lymphoma 2 (bcl-2) gene.
RESULTS: In the PS-ASODN group, tumor growth was inhibited by 59.437%, which was markedly higher than in the imatinib group (11.071%) and liposome negative control group (2.759%) [tumor inhibition = (mean tumor weight of control group - mean tumor weight of treatment group)/(mean tumor weight of control group) × 100%]. Telomerase activity was significantly lower (P < 0.01) in the PS-ASODN group (0.689 ± 0.158) compared with the imatinib group (1.838 ± 0.241), liposome negative control group (2.013 ± 0.273), and saline group (2.004 ± 0.163). Flow cytometry revealed that the apoptosis rate of tumor cells treated with PS-ASODN was 20.751% ± 0.789%, which was higher (P < 0.01) than that of the imatinib group (1.163% ± 0.469%), liposome negative control group (1.212% ± 0.310%), and saline group (1.172% ± 0.403%). Expression of bcl-2 mRNA in the transplanted GISTs was markedly downregulated (P < 0.01) in the PS-ASODN group (7.245 ± 0.739) compared with the imatinib group (14.153 ± 1.618) and liposome negative control group (16.396 ± 1.351).
CONCLUSION: PS-ASODN can repress GIST growth, mediated perhaps by inhibition of telomerase activity and downregulation of bcl-2 expression.
Core tip: Gastrointestinal stromal tumors (GISTs) are low-grade malignant mesenchymal tumors of the gastrointestinal tract. In our study, telomerase activity was repressed and the level of B-cell leukemia/lymphoma 2 mRNA markedly downregulated in SCID mice carrying transplanted human GISTs and treated with telomerase RNA-targeting phosphorothioate antisense oligodeoxynucleotides (PS-ASODN). Therefore, the therapeutic effect of PS-ASODN on GISTs is remarkable.
- Citation: Sun XC, Yan JY, Chen XL, Huang YP, Shen X, Ye XH. Depletion of telomerase RNA inhibits growth of gastrointestinal tumors transplanted in mice. World J Gastroenterol 2013; 19(15): 2340-2347
- URL: https://www.wjgnet.com/1007-9327/full/v19/i15/2340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i15.2340
Gastrointestinal stromal tumor (GIST) is a recently recognized tumor entity. It is now evident that GIST is a distinct tumor type and the most common sarcoma of the gastrointestinal tract in humans[1]. GISTs account for 2.2% of morbidity associated with malignant tumors of the gastrointestinal tract[2]. GISTs occur at a median age of 60 years, with a slight male predominance. Approximately 60% and 30% of GISTs arise in the stomach and small intestine, respectively. GISTs have a high propensity for metastatic relapse, specifically in the liver and peritoneum, after initial surgery for localized disease[3,4]. GISTs are currently categorized based on cell morphology, namely spindle cell, epithelioid, or occasionally pleomorphic; the tumors generally arise in the gastrointestinal tract and usually express the protein KIT. GISTs are generally resistant to conventional cancer chemotherapy and are associated with poor outcome; in 2001, however, an adjuvant therapy with the tyrosine kinase inhibitor imatinib was found to be highly effective against GIST[5]. Although imatinib has revolutionized the treatment of advanced GISTs, clinical resistance to this drug has proved to be a substantial problem requiring prolonged treatment[6,7].
Zamecnik et al[8] originally proposed the concept and therapeutic application of antisense nucleic acids. Antisense oligodeoxyribonucleotides are short DNA sequences that hybridize to complementary mRNA sequences by Watson-Crick base pairing. Antisense oligodeoxyribonucleotides do not form hybrids with noncomplementary RNAs encoded by other genes, and thus each individual oligodeoxyribonucleotide targets a unique RNA sequence, thereby effectively blocking the expression of the associated gene while transcription from other genes remains unaffected[9]. The antisense approach has been extensively applied in oncology research. Indeed, research has suggested that antisense oligodeoxyribonucleotides, which typically are approximately 20 nucleotides long, can preferentially penetrate tumor vessels because tumor vessels are leakier than normal vessels[10,11]. Thus, antisense oligodeoxyribonucleotides show tremendous potential as drug candidates that can selectively downregulate and effectively block oncogene expression[9]. In our present study, we used liposome-assisted transfection to investigate the therapeutic efficacy of delivering telomerase RNA-targeting phosphorothioate antisense oligodeoxynucleotides (PS-ASODN) to human GISTs transplanted into mice, with the goal of inhibiting tumor growth and enhancing tumor-cell apoptosis. Our results suggest a potential new therapeutic intervention for GISTs.
GIST samples were obtained from a 51-year-old female patient upon admission to the First Affiliated Hospital of Wenzhou Medical College. Standard resection of GISTs in the small intestine was performed on the patient in June 2004, and treatment with a 400-mg daily dose of imatinib was applied for the postoperative period. The patient underwent surgery again in 2006 owing to GIST recurrence, and the daily dose of imatinib was increased to 800 mg postoperatively. A third surgical resection was carried out in 2009 owing again to GIST recurrence, and tumor tissues were resected and used to establish cell lines after obtaining informed consent.
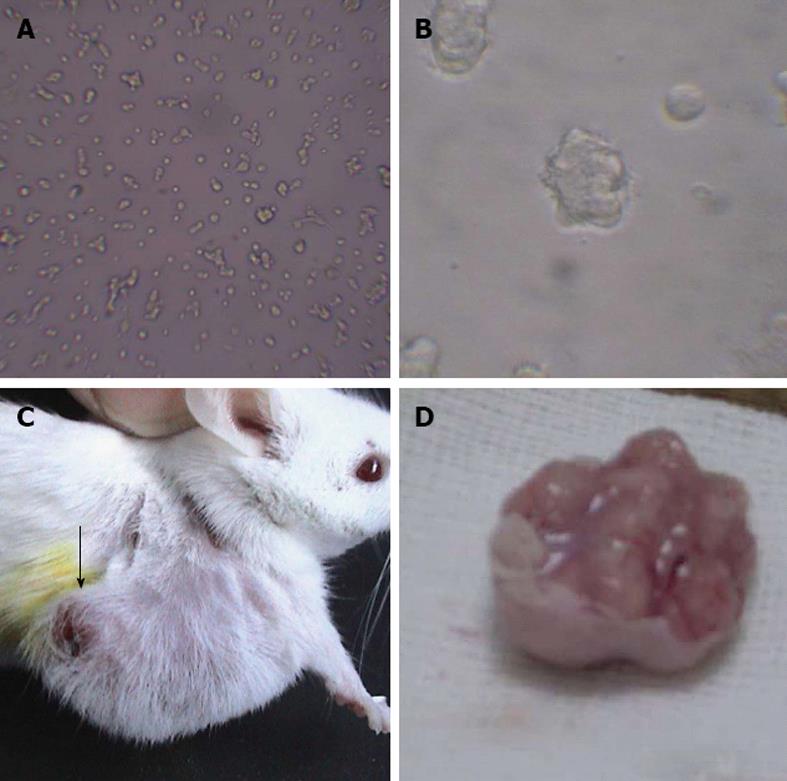
GIST samples were washed twice with Hanks Balanced Salt Solution and cut into cubes of about 1-2 mm3 before 1 mL 0.1% collagenase type I (Gibco, Carlsbad, CA, United States) was added. Each sample was again incubated with another 5 mL of 0.1% collagenase type I in RPMI-1640 culture medium at 37 °C under sterile conditions. The tissues were pipetted 50 times with a slender-tip pipette, and then specimens were incubated for 1 h at 37 °C with gentle shaking every 20 min. The resultant cell suspensions were pipetted 20 times and centrifuged at 1000 ×g for 5 min at room temperature, and the supernatant was discarded. Each pellet was resuspended in 5 mL RPMI-1640, and larger cell clumps were removed by filtration through a 200-μm mesh nylon gauze. Cells in the filtrate were placed in 4 mL RPMI-l640 complete medium containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 U/L streptomycin and incubated at 37 °C in an atmosphere of 5% CO2 in air. The medium was renewed after 24 h and thereafter renewed every 2 d. After 10 d of culture, trypsin (Sigma, St. Louis, United States) at 0.25% was applied to partially digest the cells, and the cells were purified by differential adhesion. The cells that were most adherent were then subcultured twice a week. GIST cells of different generations were preserved in liquid nitrogen for subsequent experimentation. This cell line was named GIST867 (Figure 1A and B).
Female SCID mice (7-wk old, 22 ± 2 g) were purchased from Shanghai Experimental Animal Center of the National Academy of Sciences, Shanghai, China. All mice were fed standard laboratory chow and water ad libitum. All procedures were performed in accordance with the Guidelines for Animal Experiments of Wenzhou Medical College, Wenzhou, China.
PS-ASODN with the sequence 5’-CTCAGTTAGGGTTAGACA-3’, which is a complementary region of the templating RNA of telomerase, was synthesized by Shanghai Biotechnology Engineering Company (Shanghai, China). Transfection of PS-ASODN was carried out with Lipotap Liposomal Transfection Reagent (Beyotime, Shanghai, China). This oligonucleotide was used directly without further purification, and all pipettes and tubes were autoclaved prior to use. The oligonucleotide was first diluted to a final concentration of 100 μmol/L with 550 μL deionized H2O and stored at -20 °C. The Lipotap reagent was diluted with serum-free RPMI-1640 before transfection. PS-ASODN at a final concentration of 5.00 μmol/L was then added and incubated for 15 min with diluted Lipotap reagent in Dulbecco’s modified Eagle’s medium without antibiotics or glutamine at various temperatures ranging from 15 °C to 25 °C.
For inoculation into SCID mice, GIST cells of the tenth generation were digested with 0.25% trypsin and subcultured in RPMI-1640. Centrifugation yielded a single cell suspension having a density of 1.0 × 107 viable cells per 1 mL serum-free medium. A dose of 0.25 mL of single cell suspension was injected subcutaneously into the flank skin of each of two female SCID mice. The two mice were fed under sterile conditions, and at 28-d post-inoculation the diameter of the resultant tumor was 1-2 cm in each mouse. These two mice were anaesthetized and decapitated to obtain the tumors, which subsequently were cut into cubes of 1 mm3 in 10% fetal bovine serum. The tumor cubes were placed subcutaneously into the left flank skin of 40 female SCID mice, and after 1 wk tumors were successfully induced in all mice (Figure 1C and D). The 40 tumor-bearing SCID mice were randomly divided into four groups (10 mice per group): the PS-ASODN group (5.00 μmol/L, each mouse received 0.2 mL by intra-tumor injection once daily); imatinib group (0.1 mg/g body weight, imatinib obtained from Novartis Pharma, Basel, Switzerland); liposome negative control group (0.01 mL/g); and saline group (0.01 mL/g). The mice in each group received the relevant treatment by intra-tumor injection once daily from day 7 to day 28 after implantation. After 28 d, the mice were sacrificed, and tumor weight and longest and shortest diameters were measured by electronic scale and vernier caliper, respectively. Inhibition of tumor growth was calculated as follows: inhibition of growth = (mean tumor weight of control group - mean tumor weight of treatment group)/(mean tumor weight of control group) × 100%. Aliquots of the tumor specimens were frozen in liquid nitrogen for further use.
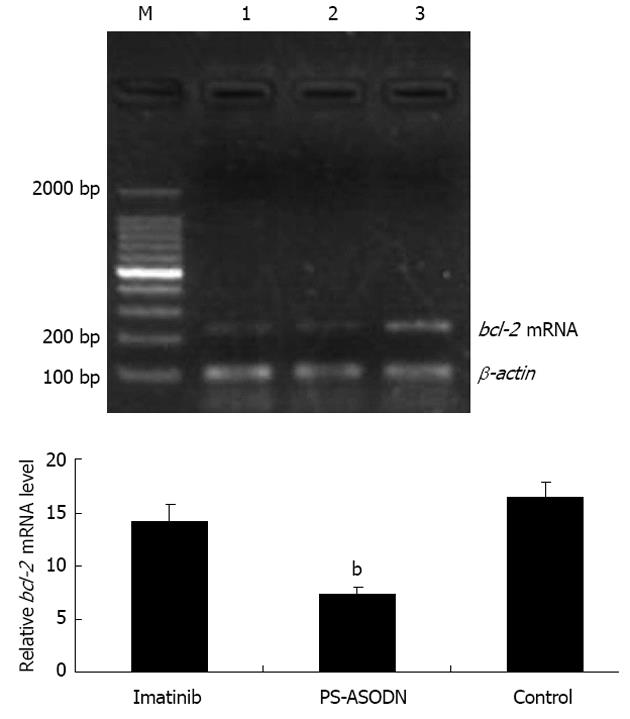
Total RNA was extracted using Trizol reagent (Beyotime), and the concentration and purity of RNA were determined by measuring the absorbance at 260 nm and 280 nm (2.0 > A260, and A280 > 1.7). Real-time polymerase chain reaction (PCR) analysis was performed using an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems Inc., Carlsbad, CA, United States). Each well (20 μL volume) contained 10 μL Power SYBR Green PCR master mix (Applied Biosystems), 1 μL of each primer (5 μmol/L) and 1 μL template. Primer sequences were designed by PrimerExpress 5.0 and synthesized by the Shanghai Biotechnology Corporation (Shanghai, China); the sequences were (5’-3’): B-cell leukemia/lymphoma 2 (bcl-2) gene (235 bp): forward CAGCTGCACCTGACGCCCTT and reverse CCCAGCCTCCGTTATCCTGGA; β-actin (99 bp): forward CCACACTGTGCCCATCTACG and reverse AGGATCTTCATGAGGTAGTCAGTCAG.
Telomerase activity was assayed by enzyme-linked immunosorbent assay following the procedure recommended by the manufacturer (Boehringer, Mannheim, Germany). The absorbance value in each well was read at 490 nm with a microtiter plate reader (BIO-TEK ELX800, Winooski, Vermont, CA, United States).
Apoptosis was assessed by flow cytometry. Tumor specimens were cut into cubes of 1 mm3, homogenized in 2 mL PBS, and filtered through 200-μm mesh nylon gauze. The filtrate was left for 10 min in the dark and was then centrifuged at 2500 ×g for 7 min at room temperature. The pellet was resuspended in 200 μL Binding Buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 2.5 mmol/L CaCl2, pH 7.4) and labeled with 10 μL annexin V-FITC and 5 μL propidium iodide from the annexin V-FITC Apoptosis Detection kit (eBioscience, San Diego, CA, United States). Apoptosis was assayed by flow cytometry (BD FACSCalibur CellSorting System, Becton Dickinson, Franklin Lakes, NJ, United States). The samples were tested in sextuplicate, and mean values were calculated.
All data are presented as the mean ± SD deviation. Statistical analysis was carried out with SPSS 13.0 software (SPSS, Chicago, IL, United States). Statistically significant differences between groups were established using Fisher’s least significant difference test. P < 0.05 was considered to be statistically significant.
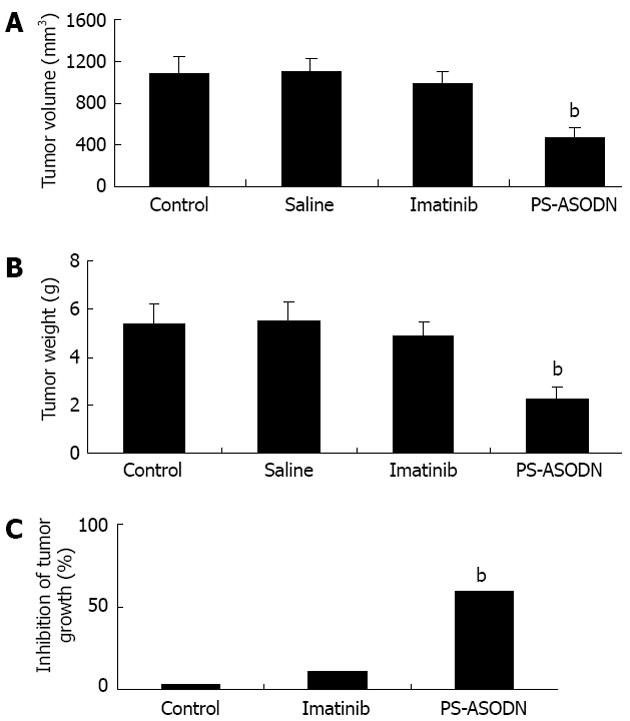
Tumor volume and weight were significantly lower in the PS-ASODN group compared with the liposome negative control and saline groups (P < 0.01, Figure 2A and B). Tumor volume and weight in the imatinib group were slightly lower than in the liposome negative control and saline groups, but the difference was not significant (P > 0.05). Inhibition of tumor growth in the PS-ASODN group (59.437%) was significantly greater than in the imatinib (11.071%) and liposome negative control groups (2.759%) (all relative to tumor growth observed in the saline control group, Figure 2C).
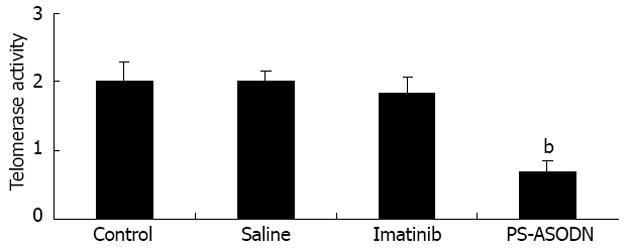
Telomerase activity was significantly repressed in the PS-ASODN group compared with the imatinib and liposome negative control groups (P < 0.01, Figure 3). As expected, administration of imatinib did not significantly affect telomerase activity compared with the liposome negative control group (P > 0.05).
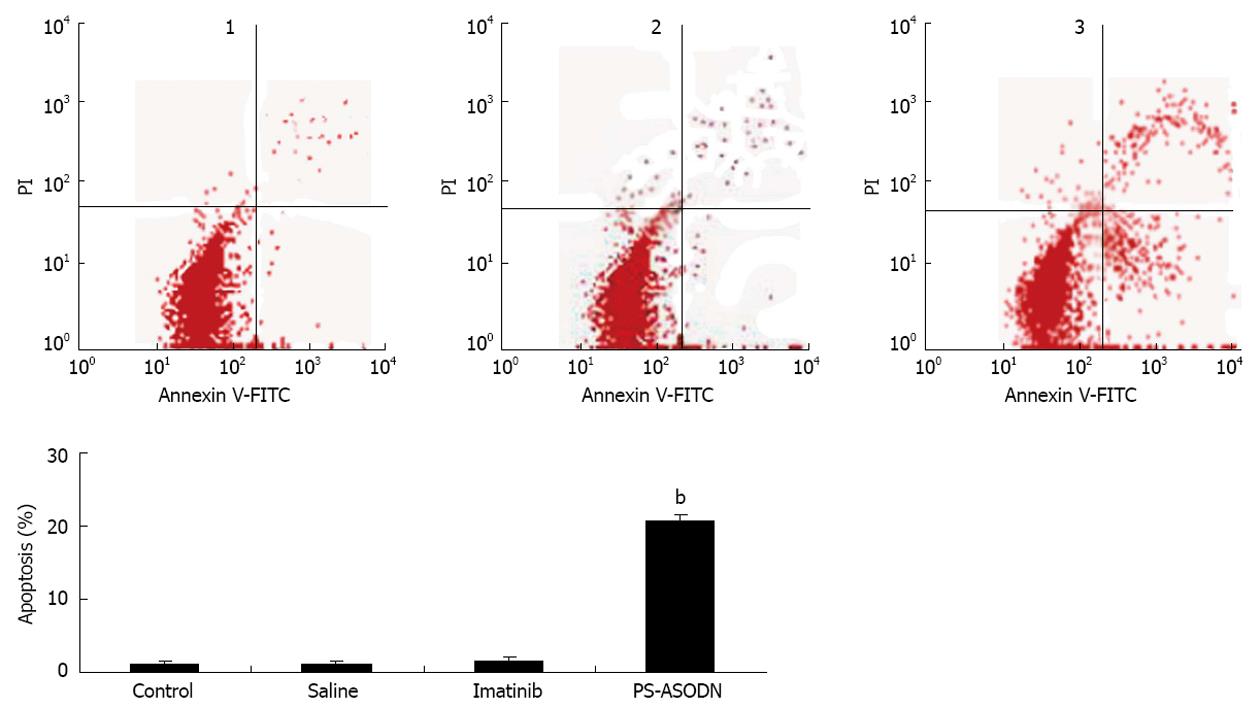
The percentage of apoptotic cells in tumors was determined by flow cytometry on day 28 after tumor implantation. Apoptosis was significantly higher in the PS-ASODN group (20.751% ± 0.789%) compared with the imatinib (1.637% ± 0.469%), liposome negative control, and saline groups (P < 0.01, Figure 4). There was no significant difference (P > 0.05) between the imatinib group and the liposome negative control and saline groups.
Agarose gel electrophoresis was used to verify the lengths of the PCR amplification fragments, namely 235 bp for bcl-2 (encoding B-cell lymphoma protein 2) and 99 bp for β-actin (Figure 5). The level of bcl-2 mRNA was significantly downregulated (P < 0.01) in the PS-ASODN group compared with the liposome negative control group (Figure 5).
GISTs are the most common mesenchymal neoplasms of the gastrointestinal tract, and the worldwide incidence of GISTs has been estimated to be 14-20 per million people. GISTs are low-grade malignant tumors that are believed to originate from neoplastic transformation of the interstitial cells of Cajal[12-14]. The overall 5-year survival rate for GIST patients is about 45% in the United States[15]. Nearly 50% of GISTs treated with imatinib ultimately demonstrate resistance in the first 2 years post-treatment, and thus a new treatment strategy and/or more effective drug is needed.
There are at least two different mechanisms for the immortalization of tumor cells: reactivation of telomerase, and the inactivation of tumor suppressor genes such as p53 and pRB that control cellular senescence[16]. Human telomerase, which contains an RNA component, telomerase-associated protein and a catalytic subunit[17-20], is activated in 80%-90% of carcinomas derived from various organs such as stomach, colon, lung and breast[21-23]. The rate of telomere DNA shortening is regulated by telomerase expression and activity[24-26]. In our study, we evaluated telomerase activity in GISTs and found that telomerase activity was markedly elevated, consistent with findings for other tumor types.
Controlling the levels of the anti-apoptotic bcl-2 family proteins is critical for regulating cell growth and apoptosis. bcl-2 localizes to cellular membranes, particularly in mitochondria, and can inhibit mitochondrial release of substances involved in signaling either the onset or execution of apoptosis[27], and higher levels of bcl-2 promote the development and progression of many tumors[28]. bcl-2 promotes cell survival by inhibiting adapters needed for activation of the proteases (caspases) that dismantle the cell. Therefore, bcl-2 and related cytoplasmic proteins are key inhibitory factors of apoptosis, which indeed is critical for development, tissue homeostasis, and protection against pathogens[29-31]. Here, we found that the level of bcl-2 mRNA was significantly upregulated in GISTs, consistent with its established role in promoting tumorigenesis.
The drug resistance of a malignant tumor is an important issue for conventional clinical therapies such as chemotherapy, radiotherapy, and immunotherapy. If telomerase activity and/or expression is inhibited in cancer cells, the cells may become relatively more vulnerable to these conventional therapies[32].
In this study, transfection with PS-ASODN significantly inhibited telomerase activity and induced apoptosis compared with the imatinib and control groups. Recently, research has shown that cells with long telomeres possess the ability to proliferate in the absence of telomerase, which demonstrates that telomerase activity does not require basic replicative functions of these cells; rather, maintaining a minimum telomere length seemingly requires telomerase activity[33]. Other studies have shown that cells with high telomerase activity were more resistant to apoptosis than those with low telomerase activity[34,35]. Kondo et al[32] found that inhibition of telomerase with an antisense telomerase expression vector not only decreased telomerase activity but also increased the susceptibility to cisplatin-induced apoptotic cell death in cisplatin-resistant U251-MG cells. Research suggests that bcl-2 is a regulator of programmed cell death, and its overexpression has been implicated in pathogenesis of some lymphomas. In our study, the SCID mice treated with PS-ASODN had significantly downregulated expression of bcl-2 mRNA compared with the liposome negative control and saline groups.
In conclusion, our study demonstrates that a synthetic antisense oligonucleotide can reduce both telomerase activity and bcl-2 mRNA expression and increase apoptosis of human GIST cells in vivo. The therapeutic effect of PS-ASODN on GISTs is remarkable, and the use of synthetic antisense oligonucleotides has the advantage of therapeutic convenience and flexibility. Our data clearly show the potential efficacy of antisense oligonucleotides for the treatment of human GISTs.
Gastrointestinal stromal tumor (GIST) is a distinct tumor type and the most common sarcoma of the gastrointestinal tract in humans, and it has a high propensity for metastatic relapse, specifically in the liver and peritoneum, after initial surgery for localized disease. Previous research has shown that antisense oligodeoxyribonucleotides, which can target a unique sequence of a single gene and block its expression while other genes are transcribed without interruption, have tremendous potential as promising drugs that can selectively downregulate oncogene expression.
GISTs are low-grade malignant mesenchymal tumors of the gastrointestinal tract, and the overall 5-year survival rate for GIST patients is about 45% in the United States. The authors found telomerase activity was markedly elevated in GISTs. Therefore, telomerase may be reactivated at a certain stage in GIST progression, enabling cancer cells to escape telomere shortening and continue proliferating. B-cell leukemia/lymphoma 2 (bcl-2) is a key regulator of apoptosis and the level of bcl-2 mRNA is significantly upregulated in GISTs.
Drug resistance of a malignant tumor is an important challenge for conventional clinical therapies such as chemotherapy, radiotherapy, and immunotherapy. This study is the first to report a new viewpoint on GIST pathogenesis and the potential therapeutic effect of telomerase RNA-targeting phosphorothioate antisense oligodeoxynucleotides (PS-ASODN) on GIST.
The authors measured telomerase activity and the level of bcl-2 mRNA in mice carrying transplanted human GISTs. The results provide new insight into the pathogenesis of GIST and suggest an efficacious therapy for GIST.
GISTs are low-grade malignant mesenchymal tumors of the gastrointestinal tract and are believed to originate from neoplastic transformation of the interstitial cells of Cajal. Antisense oligodeoxyribonucleotides are short DNA sequences that do not form hybrids with noncomplementary RNAs encoded by other genes, and thus each individual oligodeoxyribonucleotide targets a unique RNA sequence, thereby effectively blocking the expression of the associated gene while transcription from other genes remains unaffected.
The authors of this study investigated the pathogenesis of GISTs. The results are interesting and suggest that telomerase activity was repressed and the level of bcl-2 mRNA significantly downregulated in SCID mice treated with PS-ASODN. They investigated the effect of PS-ASODN on proliferation, apoptosis, and telomerase activity of tumor cells in mouse transplanted GISTs, with the goal of attaining a new viewpoint on GIST pathogenesis and providing a new therapeutic intervention.
P- Reviewers Andrei S, Kanda T S- Editor Zhai HH L- Editor Logan S E- Editor Li JY
| 1. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 2. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 3. | Rubin BP, Fletcher JA, Fletcher CD. Molecular Insights into the Histogenesis and Pathogenesis of Gastrointestinal Stromal Tumors. Int J Surg Pathol. 2000;8:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 5. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1326] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 6. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 7. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1646] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 8. | Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA. 1978;75:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1162] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Kausch I, Böhle A. Antisense oligonucleotide therapy for urologic tumors. Curr Urol Rep. 2003;4:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 373] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Plenat F, Klein-Monhoven N, Marie B, Vignaud JM, Duprez A. Cell and tissue distribution of synthetic oligonucleotides in healthy and tumor-bearing nude mice. An autoradiographic, immunohistological, and direct fluorescence microscopy study. Am J Pathol. 1995;147:124-135. [PubMed] |
| 12. | Goh BK, Chow PK, Ong HS, Wong WK. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006;391:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Kwon SH, Cha HJ, Jung SW, Kim BC, Park JS, Jeong ID, Lee JH, Nah YW, Bang SJ, Shin JW. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol. 2007;13:3396-3399. [PubMed] |
| 15. | Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 437] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 16. | Shay JW. Telomerase in human development and cancer. J Cell Physiol. 1997;173:266-270. [PubMed] |
| 17. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1606] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 18. | Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 476] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1660] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 20. | Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1318] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 21. | Nouso K, Urabe Y, Higashi T, Nakatsukasa H, Hino N, Ashida K, Kinugasa N, Yoshida K, Uematsu S, Tsuji T. Telomerase as a tool for the differential diagnosis of human hepatocellular carcinoma. Cancer. 1996;78:232-236. [PubMed] |
| 22. | Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218-222. [PubMed] |
| 24. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5233] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 25. | Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921-1929. [PubMed] |
| 26. | Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 4040] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 27. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 536] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Gautschi O, Tschopp S, Olie RA, Leech SH, Simões-Wüst AP, Ziegler A, Baumann B, Odermatt B, Hall J, Stahel RA. Activity of a novel bcl-2/bcl-xL-bispecific antisense oligonucleotide against tumors of diverse histologic origins. J Natl Cancer Inst. 2001;93:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3937] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 30. | Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052-1061. [PubMed] |
| 31. | He ZG, Zhang Y. Effect of antisense oligodeoxynucleotide of bcl-2 on drug sensitivity of leukemic cells. Zhongguo Bingli Shengli Zazhi. 1998;14:591-595. |
| 32. | Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998;16:3323-3330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Zhang X, Mar V, Zhou W, Harrington L, Robinson MO. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 477] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264-7271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Yashima K, Milchgrub S, Gollahon LS, Maitra A, Saboorian MH, Shay JW, Gazdar AF. Telomerase enzyme activity and RNA expression during the multistage pathogenesis of breast carcinoma. Clin Cancer Res. 1998;4:229-234. [PubMed] |