Published online Apr 14, 2013. doi: 10.3748/wjg.v19.i14.2187
Revised: January 25, 2013
Accepted: February 5, 2013
Published online: April 14, 2013
Processing time: 115 Days and 4.3 Hours
AIM: To assess the diagnostic value of a combination of intragastric bile acids and hepatobiliary scintigraphy in the detection of duodenogastric reflux (DGR).
METHODS: The study contained 99 patients with DGR and 70 healthy volunteers who made up the control group. The diagnosis was based on the combination of several objective arguments: a long history of gastric symptoms (i.e., nausea, epigastric pain, and/or bilious vomiting) poorly responsive to medical treatment, gastroesophageal reflux symptoms unresponsive to proton-pump inhibitors, gastritis on upper gastrointestinal (GI) endoscopy and/or at histology, presence of a bilious gastric lake at > 1 upper GI endoscopy, pathologic 24-h intragastric bile monitoring with the Bilitec device. Gastric juice was aspirated in the GI endoscopy and total bile acid (TBA), total bilirubin (TBIL) and direct bilirubin (DBIL) were tested in the clinical laboratory. Continuous data of gastric juice were compared between each group using the independent-samples Mann-Whitney U-test and their relationship was analysed by Spearman’s rank correlation test and Fisher’s linear discriminant analysis. Histopathology of DGR patients and 23 patients with chronic atrophic gastritis was compared by clinical pathologists. Using the Independent-samples Mann-Whitney U-test, DGR index (DGRi) was calculated in 28 patients of DGR group and 19 persons of control group who were subjected to hepatobiliary scintigraphy. Receiver operating characteristic curve was made to determine the sensitivity and specificity of these two methods in the diagnosis of DGR.
RESULTS: The group of patients with DGR showed a statistically higher prevalence of epigastric pain in comparison with control group. There was no significant difference between the histology of gastric mucosa with atrophic gastritis and duodenogastric reflux. The bile acid levels of DGR patients were significantly higher than the control values (Z: TBA: -8.916, DBIL: -3.914, TBIL: -6.197, all P < 0.001). Two of three in the DGR group have a significantly associated with each other (r: TBA/DBIL: 0.362, TBA/TBIL: 0.470, DBIL/TBIL: 0.737, all P < 0.001). The Fisher’s discriminant function is followed: Con: Y = 0.002TBA + 0.048DBIL + 0.032TBIL - 0.986; Reflux: Y = 0.012TBA + 0.076DBIL + 0.089TBIL - 2.614. Eighty-four point zero five percent of original grouped cases were correctly classified by this method. With respect to the DGR group, DGRi were higher than those in the control group with statistically significant differences (Z = -5.224, P < 0.001). Twenty eight patients (59.6%) were deemed to be duodenogastric reflux positive by endoscopy, as compared to 37 patients (78.7%) by hepatobiliary scintigraphy.
CONCLUSION: The integrated use of intragastric bile acid examination and scintigraphy can greatly improve the sensitivity and specificity of the diagnosis of DGR.
Core tip: The study results suggest that total bile acid is the most important factor of the bile acids to determine duodenogastric reflux (DGR) by using a variety of statistical methods. Using the receiver operator curve, we found the hepatobiliary scintigraphy is better than the examination of gastric juice in the diagnosis of DGR. From this study, the biggest revelation is that we can research other medical problems particularly using many statistical methods.
- Citation: Chen TF, Yadav PK, Wu RJ, Yu WH, Liu CQ, Lin H, Liu ZJ. Comparative evaluation of intragastric bile acids and hepatobiliary scintigraphy in the diagnosis of duodenogastric reflux. World J Gastroenterol 2013; 19(14): 2187-2196
- URL: https://www.wjgnet.com/1007-9327/full/v19/i14/2187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i14.2187
Duodenogastric reflux (DGR) is a natural physiological phenomenon which is commonly defined as the transport of duodenal contents from the duodenum to the stomach[1]. Chernov et al[2] concluded that DGR was involved in the formation of the internal gastric environment, which played a significant role in gastric digestion and that its regulation was affected by the coordinated motor and evacuated performance of the gastroduodenal junction and duodenum. Duodenal fluid causes an increase in inflammatory cells in the gastric mucosa, decrease in parietal cells, hyperplasia of mucous cells and changes in glandular morphology. Patients with DGR will feel heartburn, nocturnal cough and chest pain, nausea, epigastric pain, gassy or bloating feelings, vomiting and so on. DGR has been implicated in the pathogenesis of a variety of upper gastrointestinal disorders including esophagitis, gastritis, duodenal and gastric ulcers[3].
With the increasing number of research in this field, reliable, repeatable and simple methods of assessment of DGR are required, especially in the early stage. Earlier used techniques included radiology, endoscopy and intubation methods such as nasogastric aspiration of bile marker or the measurement of bile acids in fasting gastric aspirates. At present, several methods are available to detect duodenogastric reflux in general hospital. For example, intubation of the upper gastrointestinal tract is the essential method to assess the extent and severity of tissue damage of duodenogastric reflux disease in our daily work. This intubation should be gentle because it may causes disturbances in gastric and duodenal motility. The conventional and most widely accepted method of diagnosing DGR is the measurement of intragastric bile acid in the gastric juice aspirated through nasogastric tube and hepatobiliary scintigraphy. In the last few years, scintigraphic radiological techniques, such as imaging with hepatobiliary scintigraphy, has become available to study dynamic duodenogastric reflux[4-6], but they also have limitations[7,8].
The aim of this study was to represent the visualization of endoscopy, to measure intragastric bile acids aspirated at endoscopy and to compare them with DGR index (DGRi) assessed by hepatobiliary scintigraphy to assess the sensitivity and specificity of these two techniques in the diagnosis of DGR.
A total of 99 patients (41 male and 58 female) with DGR were undergoing esophagogastroduodenoscopy (EGD) from September 2011 to March 2012 at Shanghai Tenth People’s Hospital, Tongji University. The diagnosis of DGR was based on the combination of the following arguments: a long history of gastric symptoms poorly responsive to prokinetics, mucosa-protective medicines, H2-blockers and/or proton-pump inhibitors (PPI), gastroesophageal reflux symptoms unresponsive to PPI, gastritis on upper GI endoscopy, and/or at histology, presence of a large amount of bile in the gastric cavity at > 1 endoscopic examination, pathologic at 24-h intragastric bile monitoring with the Bilitec device. The gastric juice was often lucidity or light yellow-green and/or associated mucosal change in these patients’ endoscopic images. Before investigation, all patients were interviewed by the senior author for the presence of both upper abdominal symptoms (heartburn, regurgitation, nocturnal cough and chest pain) and dyspeptic symptoms (nausea, epigastric pain, gassy or bloating feelings, vomiting). None of the patients had diabetes mellitus, neurological disorders, vascular diseases, collagen diseases, neoplastic diseases or inflammatory bowel disease. Acute cases and patients who had previously undergone gastrectomy or esophagotomy were excluded.
As a control group, 70 consecutive patients (35 male and 35 female) who needed EGD for an annual medical check-up were enrolled. None had undergone earlier esophageal, gastric or biliary surgery; and none had earlier gastrointestinal diseases or was on medication which would influence gastric acidity or motility. After this, all patients underwent upper gastrointestinal endoscopy and found gastric juice was normal and the gastric mucosa was not damaged obviously under the macroscopic observation.
The protocol of this study was approved by the ethics committee of the Shanghai Tenth People’s Hospital. Written informed consent was obtained from all participants.
Endoscopic examination was performed to find the evidence of DGR in all patients using fiber optic gastroduodenoscopy (The GIF-H260 and Q260 endoscopes, Olympus Medical Systems Co., Tokyo, Japan). To ensure the most accurate results possibly, every patient was not taken any food or drink for 8-10 h before examination to allow a valid examination of the upper gastric intestinal (GI) tract and to lower the risk of vomiting.
The doctor explained the test to everyone, including the possibility of biopsy and risks such as the need to remove polyps or other surgical procedures and asked to sign a consent form agreeing to the procedure. At the same time, all the participants informed the endoscopy team about any medications he/she was taking and any allergy or bad reactions in previous tests. People who have had cardiac valve replacement or blood vessel graft suggested to continue medications to prevent infection. All dentures and eyeglasses prior to begin an upper endoscopy were removed. Each of the subjects was given a topical anesthetic before the test to numb his/her throat to prevent gagging. The patient was placed on his/her left side and had a plastic mouthpiece placed between his teeth to keep his mouth opening that makes easier to pass the tube. The doctor lubricated the endoscope, passed it through the mouthpiece, and then asked him to swallow it. The doctor guided the endoscope under direct visualization through his esophagus to the first part of small intestine (duodenum). Any saliva was cleared using a small suction tube that was removed quickly and easily after the test.
The doctor inspected portions of the linings of everyone’s esophagus, stomach, and the first part of small intestine and then re-inspects them as the instrument is withdrawn. To determine the presence and severity of DGR, biopsies of gastric inflammation was necessary to be performed in the antrum of the stomach. All endoscopic examinations were done by well-trained endoscopists, and three expert endoscopists examined the endoscopy photographs to determine whether the attending endoscopists had diagnosed accurately. The endoscopic diagnosis was established by consensus of two or three expert endoscopists and the attending endoscopist.
Biopsy samples, no less than four sequential sections, were taken from the inflammatory mucosa for each enrolled patient. Mucosal erythema, erosion or ulcerations of the gastric wall were usually considered signs of gastric inflammation. Biopsy specimens were immediately placed in a 10% buffered formalin solution, routinely processed, and embedded in paraffin in the department of Pathology. Two sections were stained with hematoxylin and eosin (HE). At the same time, 23 patients with chronic atrophic gastritis were reviewed for comparison. The estimation of inflammatory was made only when the biopsy specimen consisted of intestinal columnar epithelial cells with goblet cells. All biopsy examinations were done by well-trained clinical pathologists and the pathological diagnosis was established by consensus of two or three expert pathologists.
For all patients, resting gastric juice was aspirated through a sterile wash tube inserted down the biopsy channel of the gastroscope. The gastric aspirate was stored at -20˚C until batch analysis. The concentration of free and total bile acid was made by the steroid dehydrogenase method (Modular P800, Hoffmann-La Roche Ltd, Basel, Switzerland), performed in duplicate with a mean coefficient of variation of 5% for each patient. The mean overall percentage recovery was 89 percent and the variance was less than 10 percent in duplicate analyses. In the present study, three bile acids were analyzed in accordance with the clinical processes: total bile acid (TBA), total bilirubin (TBIL) and direct bilirubin (DBIL).
Twenty eight patients of DGR group and 19 persons of control group were subjected to hepatobiliary scintigraphy for the diagnosis of DGR. 99mTc-ethyl hepatic iminodiacetic acid (EHIDA) imaging was performed using single-photon emission-computed tomography (SPE-CT)/CT (PHILIPS Precedence 16 SPE-CT/CT, Koninklijke Philips Electronics NV, The Netherlands) in accordance with our institution’s standard protocol. Stress and rest images were acquired 1 h after injecting 111-185 MBq (3-5mCi) of technetium 99m ethyl hepatic iminodiacetic acid, [99mTc(CO)3(EHIDA)]-.
Patients were in fasting, non-smoking for 4-12 h and oral potassium perchlorate 400 mg was taken to close the thyroid function before examination. DGR was studied scintigraphically using a modified and extended version of the conventional hepatobiliary scintigraphy. The study was conducted with the patient in the supine position and the gamma camera detector placed above the patients’ abdomen. About 111-185 MBq [99mTc(CO)3(EHIDA)]- (99mTc-EHIDA) was injected intravenously. Gallbladder contraction was then stimulated by a fatty meal and/or intravenous cholecystokinin (1-5 units/kg). SPECT was performed by acquiring 32 projections over 180° (from 45°RAO to 45°LPO) on a circular, 400-mm field of view gamma camera. Serial images of the liver and hepatobiliary system were obtained at every 5 min up to one hour, followed by imaging at every 10 min for the next two hours. At the end of the study, 20-40 MBq 99mTc-EHIDA was given orally to confirm the location of the stomach if necessary.
In this research, the films of all participants, showing both SPECT and planar projection image, were evaluated retrospectively by two nuclear consultant radiologists working together. Scans were scored as positive for DGR only if the two physicians agreed on the presence of DGR. Retrograde movement of radioactivity from the duodenum into the stomach was considered abnormal and diagnostic of DGR. DGRi was calculated to estimate the severity of DGR, following the formula:
DGRi (%) =
All statistical analyses were performed using Statistical Analysis Software IBM SPSS Statistics 20 (Chicago, IL, United States). The significance level was set at 0.05 for all statistical tests. Values are expressed as mean ± SD or stand error of mean. Continuous data of gastric juice and DGRi were using the Independent-samples Mann-Whitney U-test between DGR and control group. The relationship among the TBA, DBIL and TBIL of DGR group was analysed by Spearman’s rank correlation test and Fisher’s linear discriminant analysis. The comparison between intragastric bile acids and hepatobiliary scintigraphy in the diagnosis of DGR was demonstrated by receiver operating characteristic (ROC) curve.
Characteristics of the enrolled patients are shown in Table 1. The group of patients with DGR was 41 males and 58 females, with a mean ± SD age of 48.62 ± 16.20 years (95%CI: 45.39-51.85). The group of patients without DGR was 35 males and 35 females, with a mean ± SD age of 50.16 ± 13.23 years (95%CI: 47.00-53.31). The group of patients with DGR showed a statistically higher prevalence of epigastric pain in comparison with that without DGR.
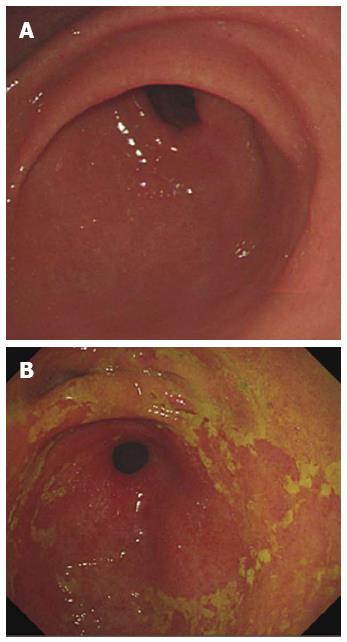
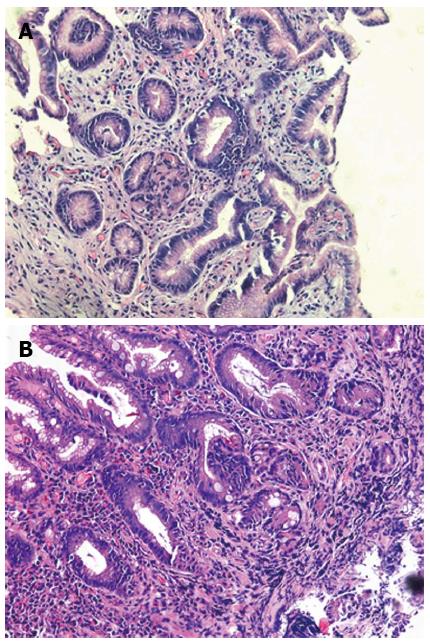
The images of patients which were got in the endoscopic examination were revealed in Figure 1. The gastric juice of DGR patients was lucidity or light yellow-green and/or associated mucosal changes. Pathologically the reflux was associated with infiltration of mononuclear leukocytes, neutrophilic granulocytes, and eosinophilic granulocytes and with foveolar hyperplasia in the gastric mucosa. Our results suggest that postprandial duodenogastric bile reflux is characterized by superficial inflammatory changes in the gastric mucosa. Reviewed with past recording, there is no significant difference between atrophic gastritis and duodenogastric reflux (Figure 2).
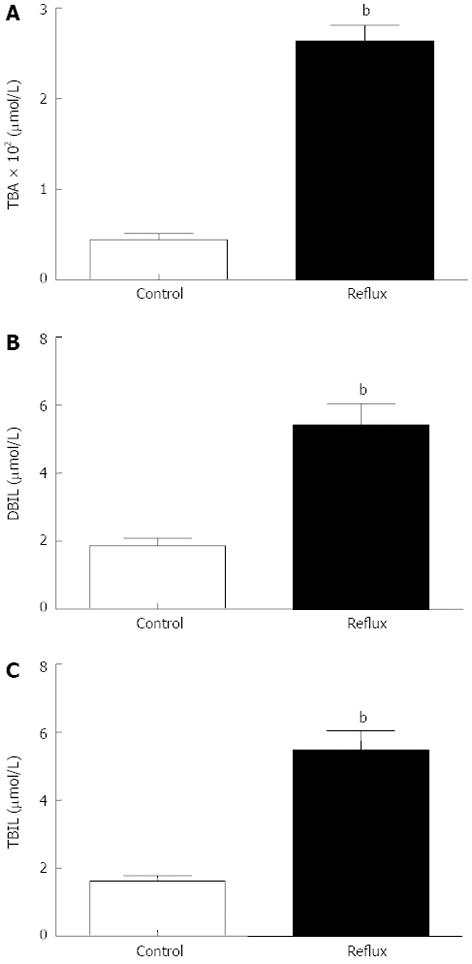
Gastric juice was successfully collected from all enrolled patients, and the concentration of bile acids in gastric juice was measured in the clinical laboratory. Analysis of the gastric aspirates was described in the Table 2, Figure 3. The bile acids levels of DGR patients were significantly higher than the control values (Z: TBA: -8.916, DBIL: -3.914, TBIL: -6.197, all P < 0.001). Using Nonparametric correlations, two of three in the DGR group have a significantly associated with each other (r: TBA/DBIL: 0.362, TBA/TBIL: 0.470, DBIL/TBIL: 0.737, all P < 0.001). Using the Fisher’s linear discriminant analysis, we found the canonical correlation is 0.631 (P < 0.001). The standardized canonical discriminant function coefficient of TBA, DBIL and TBIL is individually 0.899, 0.084 and 0.152, from which we found TBA is the most important factor in the diagnosis of DGR in the examination of gastric juice. The Fisher’s discriminant function is followed: Con: Y = 0.002TBA + 0.048DBIL + 0.032TBIL - 0.986; Reflux: Y = 0.012TBA + 0.076DBIL + 0.089TBIL - 2.614. Eighty-four point zero five percent of original grouped cases were correctly classified by this method. In other words, the result of endoscopy and gastric juice biochemistry detection were consistent more than 80% by this method. The sensitivity and the specificity is separately 83.8% and 84.3%.
| Type | mean ± SD | Range | 95%CI | Z | Sig. | |
| TBA | Con | 44.51 ± 56.53 | 0.80-235.80 | 31.05-58.01 | -8.916 | 0.000 |
| Reflux | 263.64 ± 171.61 | 0.70-660.50 | 229.41-297.87 | |||
| DBIL | Con | 1.87 ± 1.85 | 0.00-8.90 | 1.43-2.31 | -3.914 | 0.000 |
| Reflux | 5.43 ± 6.12 | 0.00-23.70 | 4.20-6.65 | |||
| TBIL | Con | 1.63 ± 1.34 | 0.10-7.40 | 1.31-1.94 | -6.197 | 0.000 |
| Reflux | 5.49 ± 5.51 | 0.30-21.90 | 4.39-6.59 |
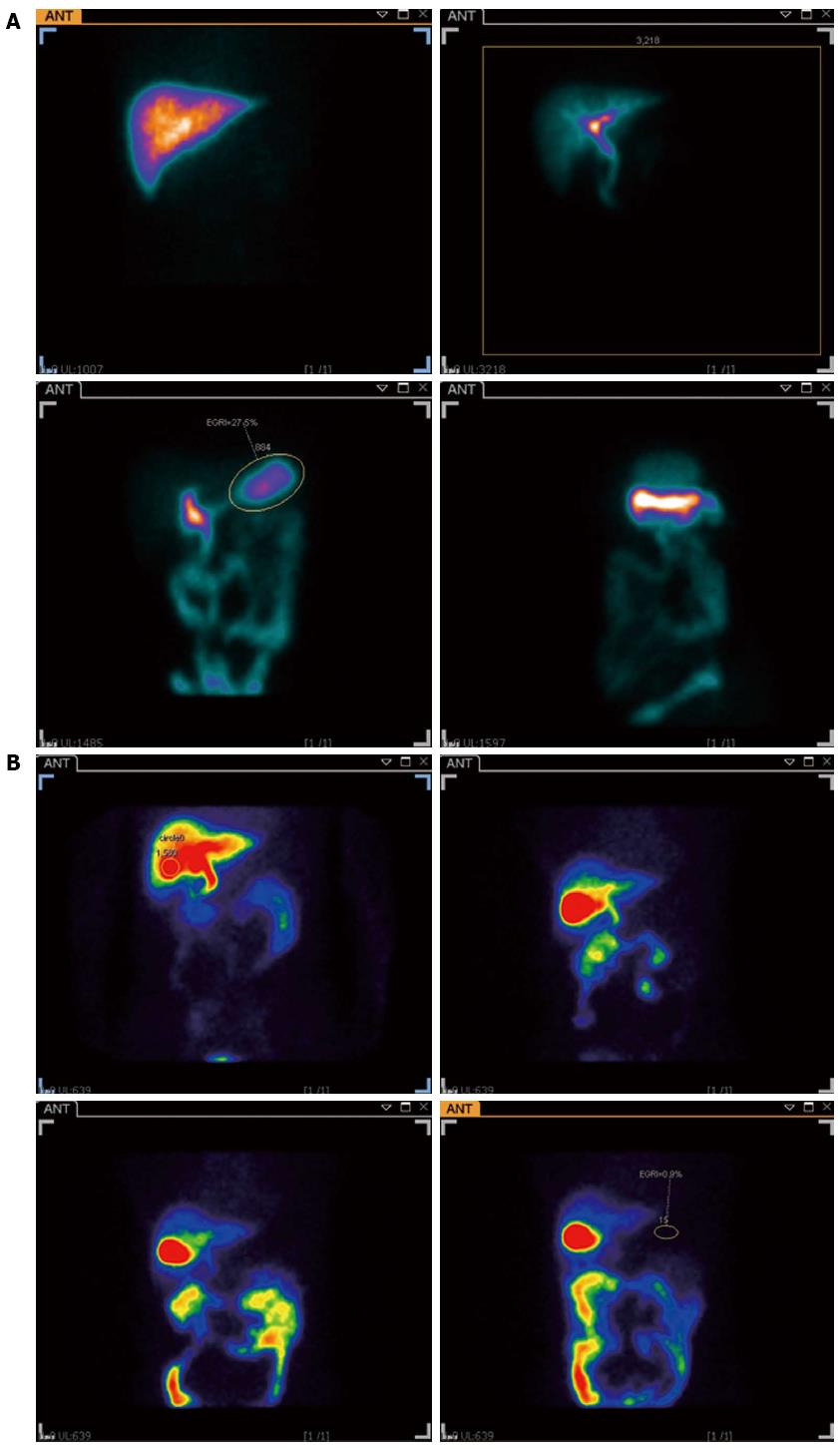
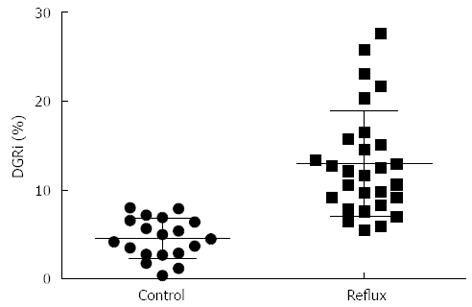
When hepatobiliary scintigraphy was administered by constant intravenous infusion it resulted in an increased elimination in bile for the first 80-100 min, and the concentration in bile then remained relatively constant for the rest of the test. Normally no increase in radioactivity in the stomach can be recorded, while the local radioactivity of the stomach increased during the investigation in DGR patients (Figure 4). The DGRi of DGR group were higher than those of the control group significantly (Z = -5.224, P < 0.001) (Figure 5). Twenty eight patients (59.6%) were deemed to be duodenogastric reflux positive by endoscopy, as compared to 37 patients (78.7%) by hepatobiliary scintigraphy. In this study, we also found some patients who were not determined with DGR by endoscopy were found the clue of duodenogastric reflux in the hepatobiliary scintigraphy. Furthermore, 11 patients were evaluated twice by the hepatobiliary scintigraphy at intervals ranging from 3-14 d. The result was identical in 8 patients, from which it indicates the good reproducibility of the test.
DGR is a natural physiological phenomenon often occurring during the early hours of the morning and postprandial period[9]. It is commonly understood to mean the passing into the stomach of duodenal fluid containing secretions from the intestinal mucosa, bile and pancreatic fluid[10]. The prevalence of upper gastrointestinal symptoms and frequency of established diagnosis of upper gastrointestinal disease is greatest for the patients with marked DGR, being approximately twice that of patients without evidence of DGR[11]. For over a century DGR has been considered the main cause of the primary or secondary alkaline gastritis and plays the basic role in the pathogenesis of gastritis and other GI tract diseases (reflux oesophagitis, gastric ulcer, progressing metaplasia or oesophageal and gastric cancer). In the previous researches, DGR occurred in 30% to 40% of adult patients presenting with acid reflux esophagitis or gastroesophageal reflux disease[12,13]. It is common even in asymptomatic subjects, especially in gastric and duodenal ulcer patients, gastric surgery, gallstone patients, patients undergoing gallbladder operations and cases of chronic pulmonary disease. DGR is a physiologic event, but also that the pathologic presence of duodenal juice in the foregut lumen may account for the development of Barrett’s metaplasia and dysplasia[14,15], and for that of gastric polyps[16], as well. Excessive DGR has been associated with the development of antral gastritis, gastric ulcers, alkaline esophagitis, esophageal or gastric adenocarcinoma, and intestinal metaplasia of the gastric mucosa[17-20]. Gastric mucosal damage induces mast cell degranulation and a release of vasoactive mediators, such as histamine, leading to vascular congestion and lamina propria edema[21]. Accurate detection of DGR has been a major problem for many years. The exact pathogenic features of bile reflux in unoperated stomach as well as its contributions to gastric mucosal lesions in chronic gastritis are still remaining unrevealed[22]. The clinical diagnosis of excessive DGR is usually based on endoscopic observation of bile reflux found in the stomach, antral gastritis or ulceration, or the histologic documentation of foveolar hyperplasia, vascular congestion, lamina propria edema or chemical gastritis[23-25].
The various techniques employed to detect DGR are endoscopy gastroduodenal intubation and direct sampling, gastric pH monitoring, ambulatory gastric bilirubin monitoring and hepatobiliary scintigraphy. Among them, the use of the intubation technique is considered non-physiologic since it is invasive and thereby may spuriously provoke reflux. Gastric pH monitoring is cumbersome, entails the use of sophisticated instruments and is uncomfortable for the patients. Scintigraphic documentation of DGR is technically easy, simple and physiologic as it is noninvasive[3]. Bilitec method reliably identified the presence of bilirubin and it has made feasible to quantitatively detect duodenogastroesophageal reflux of bile[26]. Just et al[27] showed that there was no correlation between an alkaline pH and the presence of bilirubin. Due to methodological discrepancies, research into the significance of duodenogastric reflux in the diagnosis of DGR has yielded varying results. Combined with past researches and practice, we think the diagnosis of DGR is still based on the systematic analysis of endoscopy, gastric fluid samples obtained by intubation and hepatobiliary scintigraphy, a more physiological, non-invasive method.
Endoscopy is one of the principal means of studying upper abdominal complaints for routine clinical purposes and is considered as a minimally invasive procedure, since it does not require an incision into one of the major body cavities and does not require any significant recovery after the procedure (unless sedation or anesthesia has been used). Stein et al[28] reported that upper gastrointestinal endoscopy had lower accuracy and predictive value than scintigraphy or gastric pH monitoring in the assessment of DGR. We can find duodenogastric reflux under direct visualization in our daily clinical work. But this is only a temporary phenomenon for the most part, and not on behalf of the patient’s disease status. The chief source of error in this technique is the possible effect of the intubation in either promoting or hindering reflux. Therefore, endoscopic findings only give us an intuitive, subjectivity evidence of the bile reflux and the test is largely a qualitative one.
In addition to the observation of DGR situation, we did pathological examinations during the routine gastroscopy examination. It has been demonstrated in animal experiments that duodenal fluid caused an increase in inflammatory cells in the gastric mucosa, a decrease in parietal cells, a hyperplasia of mucous cells and changes in glandular morphology. The important factor is the antrum which serves to protect the mucosa of the gastric body from the toxic effects of DGR. In our research, we found the atrophic gastritis was very common in patients with severe reflux in endoscopy, and we didn’t found the histopathology significantly different between DGR and atrophic gastritis, which was consistent with the previously research[29].
The assessment of gastric fluid, an important work in the endoscopic progress, is another important impact in the diagnosis of DGR. The surfactant effect of bile acids is closely related to their hydrophobic-hydrophilic balance. Bile acids have a surfactant effect for lipid absorption[30], and they may have a cytotoxic action if the surfactant effect is too strong[31,32]. Indeed, Heuman reported that the hydrophilic-hydrophobic balance of bile acids correlates with their toxicity, and increasing hydrophobicity was associated with increasing cytotoxicity towards the gastrointestinal epithelium[33]. Therefore, the bile acids may also have some roles in the formation of duodenogastric gastritis and in the diagnosis of DGR. In our study, we found there was a good correlation between TBA and DBIL, TBA and TBIL, DBIL and TBIL in DGR group. When we used Fisher’s linear discriminant analysis to analyze the three indexes in the determination of DGR, we found TBA was the most important factor in the diagnosis and created two formats to discriminant the diagnosis of DGR. The consistency between the direct vision of endoscopy and gastric juice examination was nearly 84%. By this method, the sensitivity and the specificity was separately 83.8% and 84.3% and this is the first time that we used this method to determine DGR.
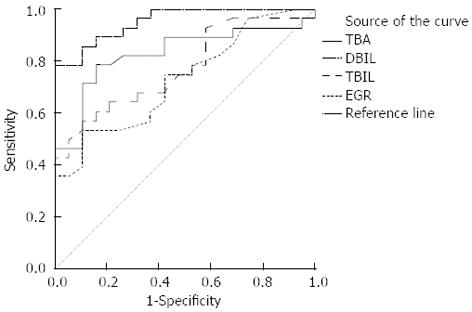
Hepatobiliary scintigraphy, using 99mTc-EHIDA derivatives, is superior to upper gastrointestinal endoscopy in the detection of DGR and also has the advantage of being non-invasive and physiological. A hepatobiliary tracer is injected intravenously and 99mTc-EHIDA excreted through the liver into the biliary tract and further into the duodenum in cholescintigraphy. When DGR happened, 99mTc-EHIDA passes into the duodenum and via reflux into the stomach. About 60% (28/47) of the isotope dose was secreted into the bile in 1.5 h. In the past researches, a good correlation was shown between the severity of mucosal changes on histology and the presence of DGR on scintigraphy[34,35]. The present study not only confirms this sensitive method for the diagnosis of DGR, but also proves its superiority over intragastric bile acids estimation (Figure 6). When we used ROC curve, we found the hepatobiliary scintigraphy was better than the examination of gastric juice (Figure 6). This means the hepatobiliary scintigraphy has better sensitivity and specificity. From the statistical analysis, we also found TBA was the most important factor of bile acids to determinate the diagnosis of DGR, which was in accordance with the result of the standardized canonical discriminant function coefficient of TBA. But the method gave no information on the nature of the reflux fluid, i.e., the substances neither contained in bile, nor did it measure anything more than bile reflux. It is well accepted that hepatobiliary scintigraphy recorded only a relatively short period. Being noninvasive, physiological and good repeatability, hepatobiliary scintigraphy appears suitable for routine clinical use in the diagnosis of DGR[36,37].
All in all, the results of endoscopy, discriminant function of intragastric bile acid examination and scintigraphy were correlated with the final diagnosis of DGR. Integrated use of these three methods will help improve the accuracy of diagnosis of DGR.
The authors are grateful to Dr. Xiao-Rong Xu, Dr. Chun-Hua Tao and Dr. Yan-Hong Shi for their invaluable help with the endoscopic procedures and above all to the following registered nurses, whose support during the study was indispensable: Yi Jin, Ya-Juan Lu, Jin Wang.
Duodenogastric reflux (DGR) is a natural physiological phenomenon often occurring during the early hours of the morning and postprandial period, which is commonly understood to mean the passing into the stomach of duodenal fluid containing secretions from the intestinal mucosa, bile and pancreatic fluid. Earlier used techniques employed to detect DGR included gastroduodenal intubation and direct sampling, gastric pH monitoring, endoscopy, gastric mucosal biopsy and hepatobiliary scintigraphy, but every method has its limit in the diagnosis of DGR.
Gastric pH monitoring is cumbersome, entails the use of sophisticated instruments and is uncomfortable for the patients. Bilitec method reliably identified the presence of bilirubin and it has made feasible to quantitatively detect duodenogastroesophageal reflux of bile. Due to methodological discrepancies, research into the significance of duodenogastric reflux in the diagnosis of DGR has yielded varying results.
This is the first time that we used the Fisher’s linear discriminant analysis to determine the bile acids in gastric juice and found total bile acid is the most important factor in the diagnosis of DGR. Using the Receiver operator curve, authors found the hepatobiliary scintigraphy is better than the examination of gastric juice.
By understanding the advantages and disadvantages of intragastric bile acids and scintigraphy, this study demonstrates the hepatobiliary scintigraphy have better sensitivity and specificity than intragastric bile acids in the diagnosis of DGR and the integrated use of these two methods can greatly improve the accuracy and sensitivity of the diagnosis of DGR.
Hepatobiliary scintigraphy is a radionuclide diagnostic imaging study that evaluates hepatocellular function and patency of the biliary system by tracing the production and flow of bile from the liver through the biliary system into the small intestine. Sequential images of the liver, biliary tree and gut are obtained. Computer acquisition and analysis as well as pharmacological interventions are frequently employed.
Many reports evaluate duodenogastric reflux with endoscopic examination or gastric juice examination. Hepatobiliary scintigraphy can check objectively dynamic duodenogastric reflux and is no invasive method. This report results hepatobiliary scintigraphy is a useful method for evaluating duodenogastric reflux and help improve the accuracy of diagnosis of duodenogastric reflux with integrated use of endoscopy and intragastric bile examination.
P- Reviewers Liu QD, Fukuhara K S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Fein M, Fuchs KH, Bohrer T, Freys SM, Thiede A. Fiberoptic technique for 24-hour bile reflux monitoring. Standards and normal values for gastric monitoring. Dig Dis Sci. 1996;41:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Chernov VF, Kuznetsov AP, Danilova AV, Beriashvili ZA, Chernov AV. [Duodenogastric reflux in humans]. Vestn Ross Akad Med Nauk. 2000;37-41. [PubMed] |
| 3. | Mittal BR, Ibrarullah M, Agarwal DK, Maini A, Ali W, Sikora SS, Das BK. Comparative evaluation of scintigraphy and upper gastrointestinal tract endoscopy for detection of duodenogastric reflux. Ann Nucl Med. 1994;8:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Shaffer EA, McOrmond P, Duggan H. Quantitative cholescintigraphy: assessment of gallbladder filling and emptying and duodenogastric reflux. Gastroenterology. 1980;79:899-906. [PubMed] |
| 5. | Muhammed I, McLoughlin GP, Holt S, Taylor TV. Non-invasive estimation of duodenogastric reflux using technetium-99m p-butyl-iminodiacetic acid. Lancet. 1980;2:1162-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mackie CR, Wisbey ML, Cuschieri A. Milk 99Tcm-EHIDA test for enterogastric bile reflux. Br J Surg. 1982;69:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Müller-Lissner SA, Fimmel CJ, Sonnenberg A, Will N, Müller-Duysing W, Heinzel F, Müller R, Blum AL. Novel approach to quantify duodenogastric reflux in healthy volunteers and in patients with type I gastric ulcer. Gut. 1983;24:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Thomas WE, Jackson PC, Cooper MJ, Davies ER. The problems associated with scintigraphic assessment of duodenogastric reflux. Scand J Gastroenterol Suppl. 1984;92:36-40. [PubMed] |
| 9. | Tzaneva M. Effects of duodenogastric reflux on gastrin cells, somatostatin cells and serotonin cells in human antral gastric mucosa. Pathol Res Pract. 2004;200:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kawiorski W, Herman RM, Legutko J. [Pathogenesis and significance of gastroduodenal reflux]. Przegl Lek. 2001;58:38-44. [PubMed] |
| 11. | Dai F, Gong J, Zhang R, Luo JY, Zhu YL, Wang XQ. Assessment of duodenogastric reflux by combined continuous intragastric pH and bilirubin monitoring. World J Gastroenterol. 2002;8:382-384. [PubMed] |
| 12. | Attwood SE, Ball CS, Barlow AP, Jenkinson L, Norris TL, Watson A. Role of intragastric and intraoesophageal alkalinisation in the genesis of complications in Barrett’s columnar lined lower oesophagus. Gut. 1993;34:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lirón R, Parrilla P, Martinez de Haro LF, Ortiz A, Robles R, Luján JA, Fuente T, Andrés B. Quantification of duodenogastric reflux in Barrett’s esophagus. Am J Gastroenterol. 1997;92:32-36. [PubMed] |
| 14. | Byrne JP, Romagnoli R, Bechi P, Attwood SE, Fuchs KH, Collard JM. Duodenogastric reflux of bile in health: the normal range. Physiol Meas. 1999;20:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Romagnoli R, Collard JM, Serra AM. Is the DGR Bilitec profile different in GERD patients with and without Barrett’s esophagus? The Duodenogastroesophageal reflux. Paris: John Libbey 2006; 445-449. |
| 16. | Mabrut JY, Romagnoli R, Collard JM, Saurin JC, Detry R, Mion F, Baulieux J, Kartheuser A. Familial adenomatous polyposis predisposes to pathologic exposure of the stomach to bilirubin. Surgery. 2006;140:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Malagelada JR, Phillips SF, Shorter RG, Higgins JA, Magrina C, van Heerden JA, Adson MA. Postoperative reflux gastritis: pathophysiology and long-term outcome after Roux-en-Y diversion. Ann Intern Med. 1985;103:178-183. [PubMed] |
| 18. | Stein HJ, Barlow AP, DeMeester TR, Hinder RA. Complications of gastroesophageal reflux disease. Role of the lower esophageal sphincter, esophageal acid and acid/alkaline exposure, and duodenogastric reflux. Ann Surg. 1992;216:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 165] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Attwood SE, Smyrk TC, DeMeester TR, Mirvish SS, Stein HJ, Hinder RA. Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery. 1992;111:503-510. [PubMed] |
| 20. | Gowen GF. Spontaneous enterogastric reflux gastritis and esophagitis. Ann Surg. 1985;201:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Bechi P, Amorosi A, Mazzanti R, Dei R, Bianchi S, Mugnai L, Masini E. Reflux-related gastric mucosal injury is associated with increased mucosal histamine content in humans. Gastroenterology. 1993;104:1057-1063. [PubMed] |
| 22. | Chen SL, Mo JZ, Cao ZJ, Chen XY, Xiao SD. Effects of bile reflux on gastric mucosal lesions in patients with dyspepsia or chronic gastritis. World J Gastroenterol. 2005;11:2834-2837. [PubMed] |
| 23. | Vaezi MF, Richter JE. Importance of duodeno-gastro-esophageal reflux in the medical outpatient practice. Hepatogastroenterology. 1999;46:40-47. [PubMed] |
| 24. | Rutledge PL, Warshaw AL. Diagnosis of symptomatic alkaline reflux gastritis and prediction of response to bile diversion operation by intragastric alkali provocation. Am J Surg. 1988;155:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Ritchie WP. Alkaline reflux gastritis: a critical reappraisal. Gut. 1984;25:975-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Cuomo R, Koek G, Sifrim D, Janssens J, Tack J. Analysis of ambulatory duodenogastroesophageal reflux monitoring. Dig Dis Sci. 2000;45:2463-2469. [PubMed] |
| 27. | Just RJ, Leite LP, Castell DO. Changes in overnight fasting intragastric pH show poor correlation with duodenogastric bile reflux in normal subjects. Am J Gastroenterol. 1996;91:1567-1570. [PubMed] |
| 28. | Stein HJ, Smyrk TC, DeMeester TR, Rouse J, Hinder RA. Clinical value of endoscopy and histology in the diagnosis of duodenogastric reflux disease. Surgery. 1992;112:796-803; discussion 803-4. [PubMed] |
| 29. | Niemelä S, Karttunen T, Heikkilä J, Lehtola J. Characteristics of reflux gastritis. Scand J Gastroenterol. 1987;22:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Carey MC, Small DM. The characteristics of mixed micellar solutions with particular reference to bile. Am J Med. 1970;49:590-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 277] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Sagawa H, Tazuma S, Kajiyama G. Protection against hydrophobic bile salt-induced cell membrane damage by liposomes and hydrophilic bile salts. Am J Physiol. 1993;264:G835-G839. [PubMed] |
| 32. | O’Connor CJ, Wallace RG, Iwamoto K, Taguchi T, Sunamoto J. Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta. 1985;817:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719-730. [PubMed] |
| 34. | Thomas WE, Cooper MJ, Mortensen NJ, Burton PA, Davies ER. The clinical assessment of duodenogastric reflux by scintigraphy and its relation to histological changes in gastric mucosa. Scand J Gastroenterol Suppl. 1984;92:195-199. [PubMed] |
| 35. | Robles-Campos R, Lujan-Mompean JA, Parrilla-Paricio P, Bermejo-Lopez J, Liron-Ruiz R, Torralba-Martinez JA, Morales-Cuenca G, Molina-Martinzez JA. Role of Helicobacter pylori infection and duodenogastric reflux in the pathogenesis of alkaline reflux gastritis after gastric operations. Surg Gynecol Obstet. 1993;176:594-598. [PubMed] |
| 36. | Padhy AK, Losu V, Shukla NK, Chattopadhyaya TK, Tandon RK, Gupta K, Gopinath PG. Thoracic stomach: comparative evaluation of endoscopy, gastric aspirate analysis and hepatobiliary scintigraphy in the diagnosis of duodeno-gastric reflux. Indian J Gastroenterol. 1990;9:277-279. [PubMed] |
| 37. | Sorgi M, Wolverson RL, Mosimann F, Donovan IA, Alexander-Williams J, Harding LK. Sensitivity and reproducibility of a bile reflux test using 99mTc HIDA. Scand J Gastroenterol Suppl. 1984;92:30-32. [PubMed] |