Published online Feb 28, 2012. doi: 10.3748/wjg.v18.i8.778
Revised: March 24, 2011
Accepted: March 31, 2011
Published online: February 28, 2012
AIM: To evaluate immunoexpression of cyclooxygenase-2 (COX-2) in primary gastric carcinomas and respective lymph node metastases.
METHODS: Immunohistochemistry to analyze COX-2 expression was performed on tissue microarray slices obtained from 36 specimens of gastrectomy and satellite lymph nodes from patients with gastric carcinoma.
RESULTS: Immunostaining was seen in most cases, and COX-2 expression was higher in lymph node metastases than in corresponding primary gastric tumors of intestinal, diffuse and mixed carcinomas, with a statistically significant difference in the diffuse histotype (P = 0.0108).
CONCLUSION: COX-2 immunoexpression occurs frequently in primary gastric carcinomas, but higher expression of this enzyme is observed in lymph node metastases of the diffuse histotype.
- Citation: Almeida PR, Ferreira FV, Santos CC, Rocha-Filho FD, Feitosa RR, Falcão EA, Cavada BK, Lima-Júnior RC, Ribeiro RA. Immunoexpression of cyclooxygenase-2 in primary gastric carcinomas and lymph node metastases. World J Gastroenterol 2012; 18(8): 778-784
- URL: https://www.wjgnet.com/1007-9327/full/v18/i8/778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i8.778
Gastric carcinoma is one of the most common malignancies worldwide[1]. Despite improvements in the detection of early gastric cancer and more efficient surgical procedures, mortality remains high. Current challenges include studies on gastric carcinogenesis, tumor progression, and the investigation of possible molecular targets that could be utilized in diagnosis, prevention and therapeutic approaches.
Several recent studies have focused on gastric carcinogenesis. According to a known model, it is a multistep process beginning with chronic gastritis and proceeding to gastric atrophy, intestinal metaplasia, dysplasia and cancer[2]. Helicobacter pylori (H. pylori), a cause of chronic gastritis, is considered a carcinogenic agent and a link between inflammatory and neoplastic processes in the stomach[3,4].
Cyclooxygenase-2 (COX-2) is an important enzyme that catalyzes arachidonic acid to prostaglandins, which participate in inflammatory and neoplastic processes. H. pylori-associated with chronic gastritis induces COX-2 expression in gastric mucosa[5]. Following eradication of bacteria in gastritis, there is a tendency for COX-2 expression to reduce or disappear[5]. COX-2 expression is seen more frequently in intestinal metaplasia than in normal gastric mucosa[6] and is prominent in dysplasias[7].
COX-2 immunostaining levels correlate with the degree of dysplasia in the epithelia and stroma[8]. It is widely accepted that COX-2 immunoexpression occurs in the lamina propria and epithelia in gastritis, in intestinal metaplasia, in dysplasia, and more strongly in adenocarcinomas in a progressive manner according to the degree of the lesion[6,9-11].
In contrast to the many published reports on gastric carcinogenesis, we found few studies on the involvement of COX-2 in gastric cancer progression, all of them concerning local invasion. Results are controversial, with some authors finding a relationship between a higher degree of COX-2 expression and advanced local invasion[12-14] and others finding no correlation[15-17].
Several reports evaluated a possible association between COX-2 expression in primary gastric tumors and the presence or absence of lymph node metastasis, with mixed results. In some reports, COX-2 positivity[14,16,17] and COX-2 overexpression in primary carcinomas[18,19] were associated with the presence of lymph node metastases. Others found no correlation[13,15,20]. None of these studies evaluated COX-2 expression in both primary and metastatic gastric carcinomas. Here, we evaluated the immunoexpression of COX-2 in primary gastric carcinomas and respective lymph node metastases.
Thirty-six gastric cancer specimens were obtained from patients surgically treated at the Cancer Institute of Ceará, Brazil. Formalin-fixed, paraffin-embedded tissue samples of primary gastric adenocarcinomas and their respective lymph node metastases were stained with hematoxylin and eosin and histologically analyzed. Gastric carcinomas were classified as intestinal (n = 10), diffuse (n = 12), mixed (n = 8), or unclassified (n = 6) (according to Lauren’s classification system)[21].
Tissue microarrays (TMAs) were constructed from the primary samples and metastatic lymph node samples. One donor block was identified in each case, and a tissue core 2 mm in diameter representative of the neoplasm without necrotic or hemorrhagic areas was punched (TMA-builder LabVision™ catalog #-TMA 001), then placed into receptor blocks of TMAs (24 samples/block). Sections 2 μm in thickness were obtained from the TMA blocks to perform immunohistochemistry.
Paraffin sections were dewaxed and rehydrated. Endogenous peroxide was blocked with a 3% H2O2 solution in methanol for 10 min, and for unmasking antigens, a retrieval solution (Vector Co™) 1% in hot water (98 °C) was added and incubated for 20 min. Ultra V block (TA-125-UB, LabVision Co™) was added to the sample and incubated for 10 min to inhibit nonspecific background staining. A rabbit monoclonal antibody against human COX-2 (Clone SP21, pm 70 kDa, LabVision Co™, 1:200) was applied for 60 min. After washing with phosphate buffered saline (PBS), the sections were incubated with a secondary anti-polyvalent biotinylated goat antibody (TP-125-BN, LabVision Co™) for 15 min, washed with PBS, incubated with a streptavidin-coupled peroxidase complex (TS-125-HR, LabVision Co™) for 15 min, then washed again with PBS. Reactions were processed at room temperature (approximately 20 °C) in an automated immunostainer (Autostainer, LabVision Co™, model 480-2D) at the Institute of Molecular Pathology and Immunology of the University of Porto. A 3% H2O2 solution in methanol and 60 mg chromogen diaminobenzidine in PBS were applied for 10 min at 37 °C in the dark. After washing with distilled water for 3 min, sections were counterstained with hematoxylin, dehydrated, diaphanized with xylene, mounted and analyzed. Samples containing fewer than 100 cells were excluded from analysis.
The positivity criterion was cytoplasmic staining of COX-2 in malignant cells. Immunoexpression of COX-2 was evaluated regarding intensity and extension using a modified scoring table based on Rajnakova et al[12] (using intensity × extension, rather than intensity + extension). We used the following definitions: Intensity = degree of immunostaining which predominates in the TMA sample (0- absent; 1- mild; 2- moderate; 3- strong); extension = percentage of predominant staining intensity in the sample (0- negative or rare cells; 1- < 25%; 2- 25%-50%; 3- 50%-75%; 4- > 75% of immunoreactive neoplastic cells). A combined score of 0-12 was calculated by intensity × extension. Final COX-2 expression was classified as low (< 6) or high (≥ 6) based on median cut-off. Scores were established by two independent observers without knowledge of previous clinical findings, resulting in a high level of concordance (89%; P < 0.05). Cases out of concordance were defined by consensus.
COX-2 positive controls were obtained from colonic adenocarcinoma sections[22]. Gastric non-tumoral mucosa and normal colonic mucosa distant from the tumor were used as negative controls. In known positive cases, there was no background staining when the primary antibody was not included. Stained inflammatory cells in the tumor stroma represented internal positive controls in cancer negative cases and fibroblasts were considered as an internal negative control in samples with positive neoplastic cells.
The non-parametric Mann-Whitney unpaired test (two-tailed) was used to compare median scores among different gastric carcinoma histotypes. The non-parametric Wilcoxon paired test (two-tailed) was utilized to test whether the median staining scores were significantly different between each primary gastric histotype sample of carcinoma and respective lymph node metastases. A P value of less than 0.05 was regarded as statistically significant.
This study was approved by the Research Ethics Committee of the Cancer Institute of Ceará, Brazil (protocol number 32/2004) and conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Table 1 shows the primary gastric cancer distribution by histological type and detailed scores. There is a marked concentration of scores at 4 and 8 and dispersion of other scores.
| Histotype | COX-2 (combined scores) | ||||||||
| 0 | 1 | 2 | 3 | 4 | 6 | 8 | 9 | 12 | |
| Intestinal | 1 | 2 | - | 1 | 1 | 3 | 1 | - | 1 |
| Diffuse | - | 1 | - | 1 | 4 | 2 | 4 | - | - |
| Mixed: Intestinal component | - | - | - | - | 4 | - | 2 | 1 | 1 |
| Diffuse component | - | - | - | - | 4 | - | 3 | - | 1 |
| Unclassified | 1 | - | - | 1 | 1 | 1 | 1 | - | 1 |
In lymph nodes, the highest score of 12 occurred at a much higher frequency (13 cases) than in primary tumors (only 4 cases), notably in diffuse and mixed carcinomas, while intestinal histotype was given a score of 8 more frequently than in primary carcinomas (Tables 1 and 2).
| Histotype | COX-2 (combined scores) | ||||||||
| 0 | 1 | 2 | 3 | 4 | 6 | 8 | 9 | 12 | |
| Intestinal | 1 | 1 | - | - | 2 | 1 | 4 | - | 1 |
| Diffuse | - | 1 | - | - | 1 | - | 4 | - | 6 |
| Mixed | 1 | - | - | 1 | - | - | 1 | 1 | 4 |
| Unclassified | 2 | - | - | - | 1 | - | 1 | - | 2 |
Analysis of low (< 6) and high (≥ 6) COX-2 expression levels showed no differences among primary and metastatic respective histotypes (Table 3).
| COX-2 scores | Histotype | ||||
| Intestinal | Diffuse | Mixed | Unclassified | Total | |
| < 6 | 5 | 6 | 4 | 3 | 18 |
| ≥ 6 | 5 | 6 | 4 | 3 | 18 |
| Total | 10 | 12 | 8 | 6 | 36 |
In lymph nodes (Table 4), high COX-2 expression (score ≥ 6) was found in most cases (25/36 = 69%), mainly in diffuse (10/12 = 83%) and mixed histotypes (6/8 = 75%), but also in intestinal tumors (6/10 = 60%; Table 4). The highest lymph node expression difference was not statistically significant in the whole sample (P = 0.1488).
| COX-2 scores | Histotype | ||||
| Intestinal | Diffuse | Mixed | Unclassified | Total | |
| < 6 | 4 | 2 | 2 | 3 | 11 |
| ≥ 6 | 6 | 10 | 6 | 3 | 25 |
| Total | 10 | 12 | 8 | 6 | 36 |
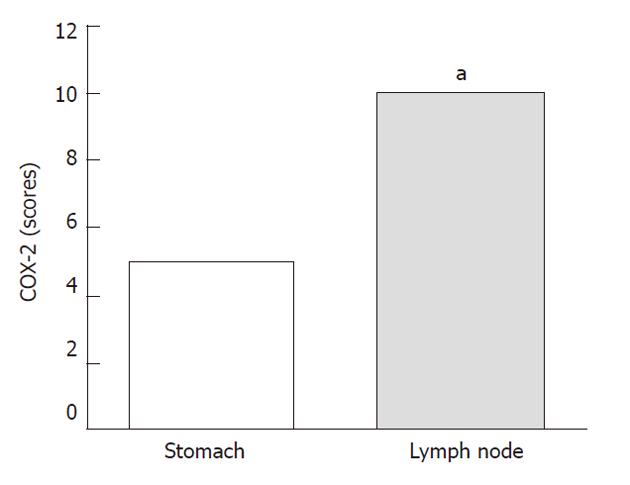
Nevertheless, in the diffuse histotype, COX-2 immunoexpression in lymph nodes was significantly higher than that in the stomach (P = 0.0108), as shown in Figure 1.
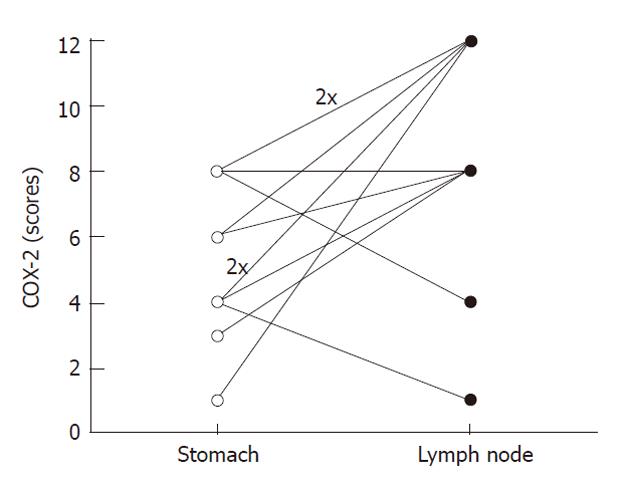
The highest COX-2 expression in metastatic diffuse carcinomas occurred not only in the whole sample of this histotype. Analysis of each case at primary and metastatic sites revealed increased expression in 75% of cases (9/12), similar expression levels in 1/12 cases (8%) and reduced expression levels in only two cases (17%). In five of nine cases with increased immunoexpression, there was a change from low to high COX-2 expression (Figure 2).
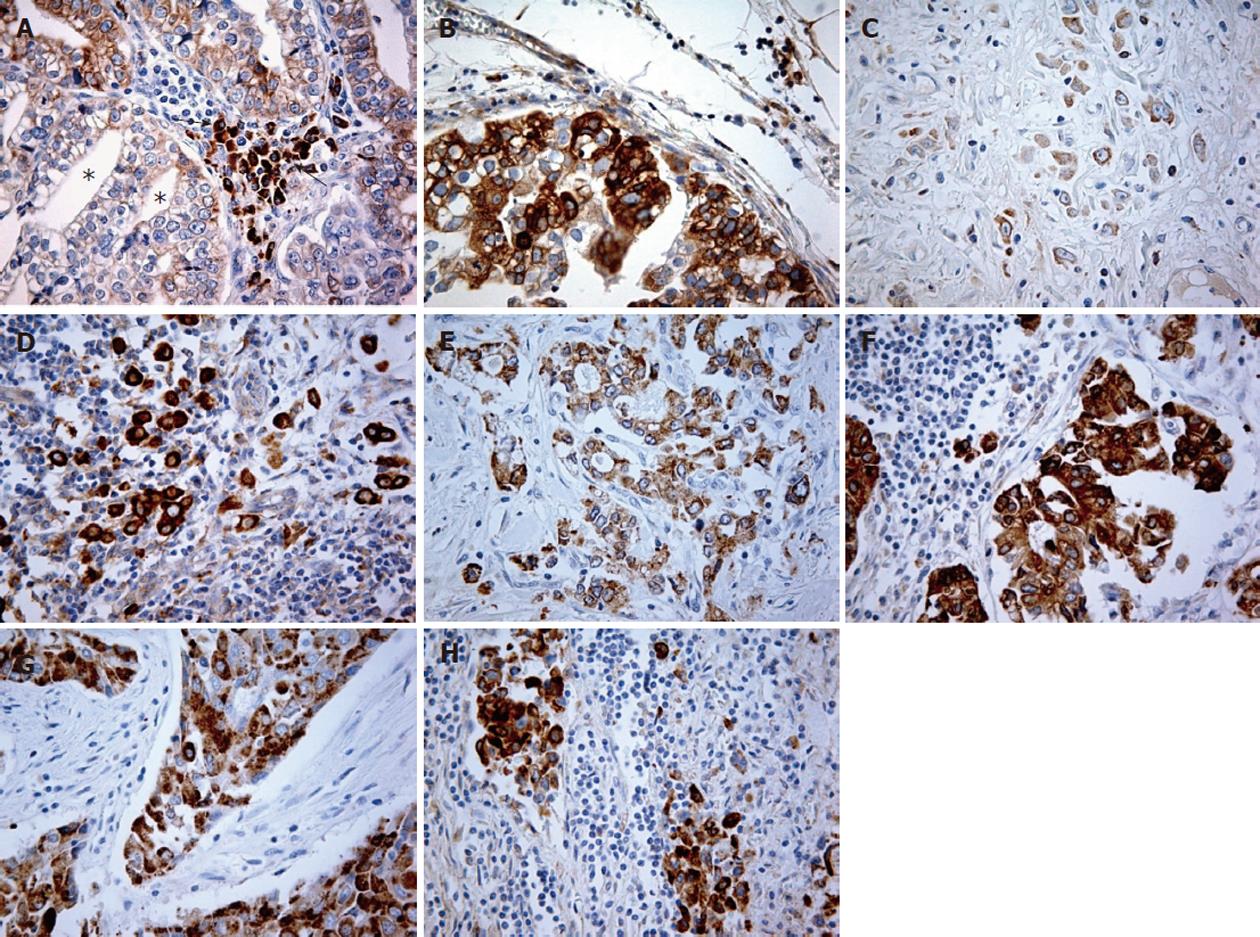
Figure 3 shows high COX-2 expression in metastatic tumors compared to respective primary carcinomas in every histological type (except unclassified).
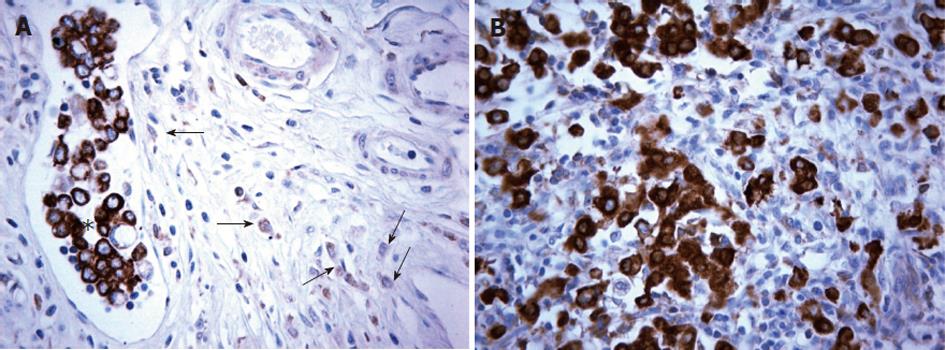
An interesting isolated finding was observed in case 12 (diffuse histotype), shown in Figure 4. The neoplastic cells invading the gastric wall were poorly stained but the cells in the vessel lumina had strong immunoexpression, similar to metastatic lymph node implants. We will comment on these findings in the discussion.
The study of simultaneous COX-2 immunoexpression in the primary tumor and its metastasis is rarely seen in the current literature. In a case report recently published, Scheer et al[23] revealed a strong multifocal COX-2 expression in about 80% of cells in the primary hypopharyngeal carcinoma and 10% in the frontotemporal bone metastasis. In addition to that, Kasper et al[24] found various degrees of cytoplasmic COX-2 expression in 57 colorectal carcinomas and in the corresponding liver metastases. As far as we know, there is no report regarding the simultaneous COX-2 expression in gastric cancer and in its respective lymph node metastasis.
In our study, we verified that COX-2 was expressed in 34/36 (94%) of the primary gastric carcinoma cases, similar to our results from a larger sampling (data not shown) and according to the frequency reported by other authors[14,20]. There is a broad variation in the frequency of COX-2 expression in gastric carcinomas, ranging from 43% to 100%[10,20]. In most reports, however, the frequency lies in the range of 60% to 80%[8,12,13,15,19,25].
Possible explanations for this variation are differences in sample characteristics, for instance, heterogeneity of sampling areas (inflammatory or ulcerated areas near cancer increase COX-2 expression) and variation in the usage of anti-inflammatory non-steroidal drugs, which reduce COX-2 expression. Other possibilities are methodological and technical procedures like time of fixation, sensitivity and specificity of monoclonal/polyclonal antibodies, and criteria for evaluation of immunoexpression. In many studies, including our own, separate scores were applied to intensity and extension and scores were then multiplied or added[8,10,12,19,20,25]. We used a scoring system ranging from 0 to 12 with a cut-off in the middle (low expression < 6; high expression ≥ 6), based on median.
When scores are sufficiently simplified, defined for example as low and high expression, reproducible results must be obtained. In our sample, elevated COX-2 expression was found in 50% of primary gastric carcinoma cases, similar to the findings of Sun et al[19] (55%), both with a cut-off based on median. Other reports with similar score criteria showed high COX-2 expression in 61% and 62%[8,25], percentages not so different from ours.
This study showed high COX-2 expression in primary gastric carcinomas in half of the cases and without significant differences between histotypes, in agreement with other reports[9,20]. Nevertheless, other authors have found a significantly higher frequency of positive cases[6,25,26] or cases with higher expression of COX-2[10,26] in the intestinal carcinomas than in diffuse tumors.
The importance of COX-2 expression on cancer bio-logy, in different malignant tumors including gastric carcinomas, must be emphasized. COX-2 influences neoplastic behavior in many ways, primarily through prostaglandin E2 (PGE2) synthesis promoting cellular proliferation[27-29], invasiveness[30-32], angiogenesis and inhibition of apoptosis[33,34]. These effects undoubtedly contribute to the process of tumor survival and progression.
In a larger series (intestinal and diffuse histotypes, results not shown), we found strong COX-2 immunostaining in 58% of tumors at stage T1 and in 48% of more advanced lesions (T2 to T4), in both histotypes (intestinal = 42%; diffuse = 55%). These percentages were higher in mixed carcinomas (data not shown). These findings reinforce the importance of COX-2 presence in local tumor progression of gastric carcinomas in all histological types.
In the lymph nodes, our results clearly showed higher COX-2 expression in metastatic tumors than in respective primary gastric carcinomas of the diffuse histotype. Diffuse carcinomas showed a strong increase in COX-2 lymph node immunostaining compared to primary gastric tumors, not only in the whole group but in most individual cases (Figure 2). To the best of our knowledge, this is the first report to evaluate COX-2 expression simultaneously in both sites and to demonstrate the strongest enzyme marking in a more advanced stage of gastric cancer compared to primary invasive tumors. Recently, Kim et al[35] compared COX-2 (and many other proteins) expression levels in primary gastric carcinomas and respective lymph node metastases and found no differences. Further investigation is needed to resolve this discrepancy.
We have no answer as to why, in the present study, the diffuse histotype showed the higher staining difference in metastatic lesions. Another question is “at what level of local invasion through the gastric wall does COX-2 expression increase?” There is an interesting finding in Figure 4 in which we show that neoplastic cells invading the gastric wall, near a vessel, were poorly stained while cells presented in the tumor embolus had a strong COX-2 immunoexpression, similar to the expression found in the respective lymph node metastases.
This is an isolated finding and we cannot make definitive conclusions. However, this observation does raise further questions: do the neoplastic cells in the neighboring vessel first increase COX-2 expression and, as a consequence, invade the vessel? Or, alternatively, do the cells invade the vessel wall and then the vessel microenvironment would induce COX-2 expression? Perhaps the latter would be a suitable answer, since there are no strongly stained cells in tissue around the vessel. These questions are outside the scope of this study and must be investigated using a larger sample size with many histopathological sections for vessel immunohistochemical markers and even better addressed with experimental models.
In summary, we have shown that COX-2 immunoexpression occurs frequently in primary gastric carcinomas and higher expression is seen in lymph node metastases of the diffuse histotype. These findings emphasize the importance of COX-2 as a potential marker of tumor progression and its possible use in diagnosis, prognosis or development of therapeutic tools against gastric cancer. The role of COX-2 in neoplastic invasion through the gastric wall, in vessel tumor emboli formation and in establishing distant metastases requires further study.
We thank Professor Manuel Sobrinho-Simões and Dr. Fátima Carneiro who made possible this international cooperation and Mrs. Dina Leitão for her excellent technical assistance. We are indebted to the Hospital of Cancer, Cancer Institute of Ceará (Brazil) for providing patient information and tissue blocks for the study.
Gastric carcinoma is one of the most common malignancies worldwide. It is widely accepted that cyclooxygenase-2 (COX-2) immunoexpression occurs in the lamina propria and epithelia in gastritis, in the intestinal metaplasia, in dysplasia, and more strongly in adenocarcinomas in a progressive manner according to the degree of the lesion. However, there is a lack of studies exploring the immunoexpression of COX-2 in primary gastric carcinomas and respective lymph node metastases.
In some reports, COX-2 positivity and COX-2 overexpression in primary carcinomas were associated with the presence of lymph node metastases, but some others found no correlation. In this study, the authors evaluated the immunoexpression of COX-2 in primary gastric carcinomas and respective lymph node metastases.
This study reports that COX-2 immunoexpression occurs frequently in primary gastric carcinomas and higher expression is seen in lymph node metastases of the diffuse histotype.
The importance of COX-2 expression on cancer biology, including gastric carcinomas, must be emphasized. COX-2 influences neoplastic behavior by promoting cellular proliferation, invasiveness, angiogenesis and inhibition of apoptosis, which contribute to the process of tumor survival and progression. The differential expression of molecular markers among cancer histotypes is of high importance regarding a possible improved therapeutic approach.
Gastric carcinomas are classified as intestinal, diffuse and mixed histotypes. The molecular characterization of these histotypes might provide a better knowledge concerning metastatic potential.
The authors performed immunohistochemistry on COX2 in primary gastric cancer and respective lymph node metastases, and found a higher COX2 expression in metastatic tumors compared to respective primary tumors, especially in diffuse type gastric cancer. Since the study on COX2 expression in the primary and its metastases is rarely seen, this work is significant.
Peer reviewer: Dr. Hiroki Sasaki, Department of Genetics Division, National Cancer Center Research Institute, Tsukiji 5-1-1, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Sun H L- Editor Webster JR E- Editor Li JY
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 3. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] |
| 5. | Wambura C, Aoyama N, Shirasaka D, Kuroda K, Maekawa S, Ebara S, Watanabe Y, Tamura T, Kasuga M. Influence of gastritis on cyclooxygenase-2 expression before and after eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2004;16:969-979. [PubMed] |
| 6. | Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359-363. [PubMed] |
| 7. | Tsuji S, Tsujii M, Murata H, Nishida T, Komori M, Yasumaru M, Ishii S, Sasayama Y, Kawano S, Hayashi N. Helicobacter pylori eradication to prevent gastric cancer: underlying molecular and cellular mechanisms. World J Gastroenterol. 2006;12:1671-1680. [PubMed] |
| 8. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [PubMed] |
| 9. | Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729-735. [PubMed] |
| 10. | Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923-1931. [PubMed] |
| 11. | Jang TJ. Expression of proteins related to prostaglandin E2 biosynthesis is increased in human gastric cancer and during gastric carcinogenesis. Virchows Arch. 2004;445:564-571. [PubMed] |
| 12. | Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177-185. [PubMed] |
| 13. | Lee TL, Leung WK, Lau JY, Tong JH, Ng EK, Chan FK, Chung SC, Sung JJ, To KF. Inverse association between cyclooxygenase-2 overexpression and microsatellite instability in gastric cancer. Cancer Lett. 2001;168:133-140. [PubMed] |
| 14. | Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64-73. [PubMed] |
| 15. | Kawabe A, Shimada Y, Uchida S, Maeda M, Yamasaki S, Kato M, Hashimoto Y, Ohshio G, Matsumoto M, Imamura M. Expression of cyclooxygenase-2 in primary and remnant gastric carcinoma: comparing it with p53 accumulation, Helicobacter pylori infection, and vascular endothelial growth factor expression. J Surg Oncol. 2002;80:79-88. [PubMed] |
| 16. | Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421-1426. [PubMed] |
| 17. | Yu JR, Wu YJ, Qin Q, Lu KZ, Yan S, Liu XS, Zheng SS. Expression of cyclooxygenase-2 in gastric cancer and its relation to liver metastasis and long-term prognosis. World J Gastroenterol. 2005;11:4908-4911. [PubMed] |
| 18. | Li HX, Chang XM, Song ZJ, He SX. Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:674-677. [PubMed] |
| 19. | Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707-713. [PubMed] |
| 20. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] |
| 21. | Joo YE, Chung IJ, Park YK, Koh YS, Lee JH, Park CH, Lee WS, Kim HS, Choi SK, Rew JS. Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med Sci. 2006;21:871-876. [PubMed] |
| 22. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [PubMed] |
| 23. | Scheer M, Drebber U, Breuhahn K, Möckel C, Reuther T, Kern M, Zöller JE. Expression of cyclooxygenase-2 (COX-2) in an advanced metastasized hypopharyngeal carcinoma and cultured tumor cells. Oral Maxillofac Surg. 2010;14:53-57. [PubMed] |
| 24. | Kasper HU, Konze E, Dienes HP, Stippel DL, Schirmacher P, Kern M. COX-2 expression and effects of COX-2 inhibition in colorectal carcinomas and their liver metastases. Anticancer Res. 2010;30:2017-2023. [PubMed] |
| 25. | Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222-229. [PubMed] |
| 26. | Liu G, Gong J, Cheng P, Dai F, Zhang J, Chang Y. Expression of COX-2 in different subtypes of gastric intestinal metaplasia and gastric carcinoma by tissue microarray. Chinese-German J Clin Oncol. 2005;4:151-154. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Shou M, Korzekwa KR, Krausz KW, Buters JT, Grogan J, Goldfarb I, Hardwick JP, Gonzalez FJ, Gelboin HV. Specificity of cDNA-expressed human and rodent cytochrome P450s in the oxidative metabolism of the potent carcinogen 7,12-dimethylbenz[a]anthracene. Mol Carcinog. 1996;17:241-249. [PubMed] |
| 28. | Harris RE, Robertson FM, Abou-Issa HM, Farrar WB, Brueggemeier R. Genetic induction and upregulation of cyclooxygenase (COX) and aromatase (CYP19): an extension of the dietary fat hypothesis of breast cancer. Med Hypotheses. 1999;52:291-292. [PubMed] |
| 29. | Yamagishi M, Noda M, Tatsumi Y, Mukaisho K, Mitsufuji S, Sugihara H, Okanoue T, Hattori T. Correlation between cyclooxygenase-2, proliferative activity, and mucin phenotype in human advanced gastric cancer. J Gastroenterol. 2004;39:1143-1149. [PubMed] |
| 30. | Tsujii M, Kawano S, Dubois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. |
| 31. | Jiang MC, Liao CF, Lee PH. Aspirin inhibits matrix metalloproteinase-2 activity, increases E-cadherin production, and inhibits in vitro invasion of tumor cells. Biochem Biophys Res Commun. 2001;282:671-677. [PubMed] |
| 32. | Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;10:181. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Tatsuguchi A, Matsui K, Shinji Y, Gudis K, Tsukui T, Kishida T, Fukuda Y, Sugisaki Y, Tokunaga A, Tajiri T. Cyclooxygenase-2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol. 2004;35:488-495. [PubMed] |
| 34. | Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-1322. [PubMed] |
| 35. | Kim JH, Kim MA, Lee HS, Kim WH. Comparative analysis of protein expressions in primary and metastatic gastric carcinomas. Hum Pathol. 2009;40:314-322. [PubMed] |