Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7015
Revised: May 28, 2012
Accepted: June 28, 2012
Published online: December 21, 2012
AIM: To investigate the patterns and decisive prognostic factors for local recurrence of rectal cancer treated with a multidisciplinary team (MDT) modality.
METHODS: Ninety patients with local recurrence were studied, out of 1079 consecutive rectal cancer patients who underwent curative surgery from 1999 to 2007. For each patient, the recurrence pattern was assessed by specialist radiologists from the MDT using imaging, and the treatment strategy was decided after discussion by the MDT. The associations between clinicopathological factors and long-term outcomes were evaluated using both univariate and multivariate analysis.
RESULTS: The recurrence pattern was classified as follows: Twenty-seven (30%) recurrent tumors were evaluated as axial type, 21 (23.3%) were anterior type, 8 (8.9%) were posterior type, and 13 (25.6%) were lateral type. Forty-one patients had tumors that were evaluated as resectable by the MDT and ultimately received surgery, and R0 resection was achieved in 36 (87.8%) of these patients. The recurrence pattern was closely associated with resectability and R0 resection rate (P < 0.001). The recurrence pattern, interval to recurrence, and R0 resection were significantly associated with 5-year survival rate in univariate analysis. Multivariate analysis showed that the R0 resection was the unique independent factor affecting long-term survival.
CONCLUSION: The MDT modality improves patient selection for surgery by enabling accurate classification of the recurrence pattern; R0 resection is the most significant factor affecting long-term survival.
- Citation: Zhao J, Du CZ, Sun YS, Gu J. Patterns and prognosis of locally recurrent rectal cancer following multidisciplinary treatment. World J Gastroenterol 2012; 18(47): 7015-7020
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7015
Local recurrence of rectal cancer (LRRC) has dramatically fallen to 4%-10% after widespread application of total mesorectal excision (TME)[1-5]. Nevertheless, LRRC remains a significant clinical problem and is associated with severe morbidity, low quality of life, and poor survival in the majority of patients[6]. Although radical (R0) resection is the most effective way to improve prognosis, it is only achieved in 10%-50% of LRRC, and in general, the 5-year survival rate remains unfavorable, varying from 18% to 58% according to different reports[7-10]. One decisive factor for R0 resection is the pattern of recurrence[3,4,11,12], since an accurate assessment of the recurrence pattern before treatment is critical for the selection of indicated patients as candidates for surgery.
Currently, the treatment of rectal cancer has evolved toward a multidisciplinary team (MDT) modality[13], and this modality has been successfully delivered in our center for ten years[14]. The MDT approach can optimize treatment strategy by enabling accurate and integrative evaluation via discussion by the MDT before treatment[15]. However, whether the MDT approach can improve the R0 resection rate and long-term survival in patients with LRRC is still unknown. The aim of this study was to investigate the long-term outcome and its influential factors in LRRC under the MDT modality.
A total of 1079 consecutive patients who underwent curative surgery at the Beijing Cancer Hospital between January 1999 and December 2007 were reviewed. In this study, LRRC was defined as the occurrence of recurrent tumors after prior radical resection located within the pelvis, either alone or in conjunction with metastases[16]. Ninety eligible patients were ultimately included in the study according to the following criteria: (1) the initial surgery was R0 resection, and transabdominal surgery must be delivered strictly according to TME principles[17], without bowel resection margin or circumferential resection margin involvement; (2) no synchronic distant metastasis outside the pelvis; (3) no history of other malignant tumors; and (4) no severe surgical or medical complications that were likely to affect the long-term outcome after initial surgery.
Confirmation of LRRC by biopsy was obtained in 68 patients, and 10 patients were diagnosed with positron emission tomography. The remaining 12 patients were clinically diagnosed by assessing clinical symptoms, serum carcinoembryonic antigen (CEA) levels, and imaging, including computed tomography (CT), magnetic resonance imaging (MRI), and sonography.
Data for each patient was evaluated and discussed by a special MDT. Recurrence patterns were evaluated by senior radiologists of the MDT based on CT or MRI, using the Memorial Sloan-Kettering classification[6,12], namely, axial: recurrence at anastomotic, residual mesorectum, or perirectal soft tissue within the center of the pelvis or perineum following an abdominoperineal resection; anterior: involving the genitourinary tract; posterior: involving the sacrum and presacral fascia; and lateral: involving the muscles or soft tissue of the pelvic sidewall, major iliac vessels, sacral nerve plexus, and lateral bony pelvis.
Surgery for indicated patients was performed after discussion by the MDT, which included a group of experienced colorectal surgeons. Surgery for recurrent tumors was defined as curative (R0) if the area where resection was performed was grossly and microscopically free of residual cancer. Resections were considered palliative if either gross (R2) or microscopic (R1) cancer remained at the end of the procedure. For each patient, a thorough abdominal exploration was carried out to rule out extra-pelvic metastasis after division of adhesions. Recurrent tumors were resected, along with any adjacent structures involved. Frozen-section analysis was not routinely performed, except for the suspicious resection margin.
Decisions regarding whether and when patients needed to undergo chemoradiotherapy were arrived at via discussion by the MDT. In principle, preoperative external beam radiotherapy was applied in patients 70 years of age and under with the possibility of curative resection. A radiation dose of 50 Gy in 25 daily fractions of 2 Gy was planned if no neoadjuvant radiotherapy for the primary tumor had been delivered. Three fields (1 posterior and 2 lateral) were used. Chemotherapy with fluorouracil (350 mg/m2 per day) and leucovorin (20 mg/m2 per day) was administered concurrently in two 5 d courses. Surgery was performed 6-8 wk after the end of the preoperative treatment. None of the patients in this study underwent intraoperative or postoperative radiotherapy. Palliative chemotherapy for unresectable tumors was delivered by oncologists, and the regimens were based on fluorouracil, oxaliplatin, and irinotecan. If the patients could not receive radio- or chemotherapy due to poor status, supportive care, including nutrition support, analgesic therapy, and other measures were given to improve quality of life.
All included patients underwent regular follow-ups. Physical examination and laboratory testing (blood count, liver enzymes, and CEA) were performed at the time of each follow-up visit, every 3 mo for 2 years, and every 6 mo thereafter. Abdominal ultrasonography, a chest X-ray, and abdominal and pelvic CT were performed every 6 mo. Colonoscopy was performed every 12 mo. The follow-up was repeated for at least 5 years.
Statistical analyses were performed using the SPSS 16.0 Statistical Software (SPSS Inc., Chicago, IL). Categorical variables were compared using Pearson’s χ2 test. Survival rates were estimated using the Kaplan-Meier curve, and comparisons of survival between groups were made using the log-rank method. Multivariate analysis was performed using the Cox proportional-hazard method. All statistical tests were 2-tailed, and statistical significance was set at P < 0.05.
Of the 90 included patients, 54 were male and 36 were female. The mean age was 59.4 years (median 57.5; range 27-76). Median follow-up was 68.5 mo (range 6-96). Ten patients (11.1%) were lost to follow-up and their data were included in the survival analysis until the date of loss. The interval to recurrence was a median of 18.5 mo (3-102). Forty-one patients had recurrent tumors that were deemed resectable or potentially resectable; of these patients, 10 received preoperative chemoradiotherapy and 8 had R0 resection. Of the 41 patients who underwent surgery, 36 (87.8%) had R0 resection. In total, 9 patients underwent extended resection: 4 patients underwent total pelvic exenteration, 3 were R0 resection and 1 was R1 resection; 5 patients underwent combined organ excision, 3 were R0 resection, 1 was R1 resection, and 1 was R2 resection. Within the combined organ excision, 2 combined with uterus and vagina resection, 1 combined with partial prostate resection, and 2 combined with sacrum resection. The remaining 49 patients with unresectable tumors received conservative therapy: 18 received chemoradiotherapy and consequent chemotherapy, 25 received palliative chemotherapy alone, and 6 underwent supportive care. The demographic and clinicopathological characteristics are summarized in Table 1.
| Variables | Patient |
| Gender | |
| Male | 54 (60) |
| Female | 36 (40) |
| Age | |
| ≤ 60 yr | 50 (55.6) |
| > 60 yr | 40 (44.4) |
| Primary surgery | |
| Anterior resection | 54 (60) |
| Abdominoperineal resection | 32 (35.6) |
| Hartmann | 2 (2.2) |
| Local excision | 2 (2.2) |
| Neoadjuvant chemoradiotherapy for primary tumor | |
| Yes | 18 (20) |
| No | 72 (80) |
| Primary tumor stage | |
| I | 6 (6.6) |
| IIA | 8 (8.8) |
| IIB | 11 (12.2) |
| IIIA | 10 (11.1) |
| IIIB | 20 (22.2) |
| IIIC | 26 (28.8) |
| Unknown | 9 (10) |
| Serum CEA1 | |
| ≤ 5 ng/mL | 27 (30) |
| > 5 ng/mL | 48 (53.3) |
| Unknown | 15 (16.7) |
| Preoperative chemoradiotherapy for recurrent tumor2 | |
| Yes | 10 (24.4) |
| No | 31 (75.6) |
| Surgery for recurrent tumor | 41 (45.6) |
| Anterior resection | 2 (4.9) |
| Abdominoperineal resection | 30 (73.2) |
| Combined organ excision3 | 5 (12.2) |
| Total pelvic exenteration | 4 (9.8) |
| Conservative therapy | 49 (54.4) |
| Resection status | |
| R0 | 36 (40) |
| R1-2 | 5 (5.5) |
The pattern of local recurrence was classified by the MDT as axial type in 27 (30%) patients, anterior type in 21 (23.3%), posterior type in 8 (8.9%), lateral type in 23 (25.6%), and unclassifiable in 11.
The recurrence pattern had a strong association with resectability of the recurrent tumor, with the highest resection rate (88.9%) in the axial type and the lowest resection rate (21.7%) in the lateral type (P < 0.001) (Table 2). A significant difference in R0 resection percentage was also observed among the different patterns of recurrence: the R0 resection rates in axial, anterior, posterior, and lateral type were 85.2%, 33.3%, 25%, and 4.3%, respectively (P < 0.001; Table 2).
| Recurrence pattern | Resection rate (%) | P value | R0 rate (%) | P value |
| Axial | 88.9 (24/27) | < 0.001 | 85.2 (23/27) | < 0.001 |
| Anterior | 33.3 (7/21) | 33.3 (7/21) | ||
| Posterior | 25 (2/8) | 25 (2/8) | ||
| Lateral | 21.7 (5/23) | 4.3 (1/23) |
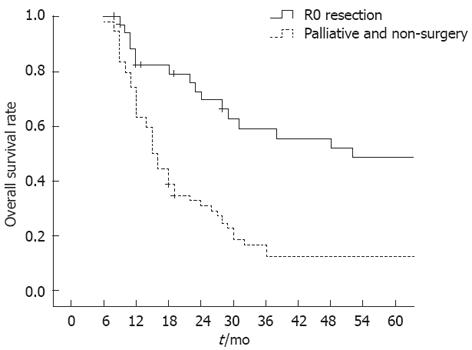
The 5-year overall survival rate of all patients was 31.1%. Univariate analysis of patient survival with locally recurrent tumors was performed according to clinicopathological and surgical factors. Twenty-seven patients with a long recurrence interval (> 24 mo) had a significantly higher rate of survival than the 63 patients with a short recurrence interval (≤ 24 mo) (48.1% vs 23.8%, P < 0.05; Table 3). A significant difference in survival was seen in patients with different recurrence patterns: the 5-year survival rate was 63% in patients with the axial type, 28.6% in the anterior type, 12.5% in the posterior type, and 4.3% in the lateral type (P < 0.001). Univariate analysis also demonstrated that patients who underwent R0 resection had a significantly improved survival rate than those with palliative resection or conservative therapy (55.6% vs 14.8%, P < 0.001; Figure 1). Gender, age, primary surgery, pathological stage of primary tumor, and serum CEA were not associated with long-term survival (Table 3).
| Variables | n | 5 yr OS rate (%) | P value |
| Gender | |||
| Male | 54 | 29.6 | 0.803 |
| Female | 36 | 33.3 | |
| Age | |||
| ≤ 60 yr | 50 | 26 | 0.187 |
| > 60 yr | 40 | 37.5 | |
| Surgery of primary tumor | |||
| Anterior resection | 54 | 31.5 | 0.451 |
| Abdominoperineal resection | 32 | 31.2 | |
| Pathologic stage of primary tumor | |||
| I-II | 25 | 33.3 | 0.473 |
| III | 56 | 30.8 | |
| Serum CEA1 | |||
| ≤ 5 ng/mL | 26 | 38.5 | 0.35 |
| > 5 ng/mL | 49 | 28.6 | |
| Interval to recurrence (mo) | |||
| ≤ 24 | 63 | 23.8 | 0.011 |
| > 24 | 27 | 48.1 | |
| Recurrence pattern | |||
| Axial | 27 | 63 | < 0.001 |
| Anterior | 21 | 28.6 | |
| Posterior | 8 | 12.5 | |
| Lateral | 23 | 4.3 | |
| Surgery for recurrent tumor | |||
| R0 resection | 36 | 55.6 | < 0.001 |
| R1-2 and non-surgery | 54 | 14.8 | |
Multivariate analysis revealed that only the R0 resection independently influenced long-term survival of locally recurrent rectal cancer (P < 0.05). Although other factors, including the recurrence pattern, may associate with overall survival, they were not as significant as R0 resection in prognosticating long-term survival (Table 4).
| Variables | Hazard ratio (95%CI) | P value |
| R0 resection | 2.734 (1.212-6.168) | 0.015 |
| Recurrence pattern | 1.078 (0.716-1.625) | 0.718 |
| Interval to recurrence | 0.978 (0.953-1.004) | 0.102 |
| Gender | 1.776 (0.870-3.625) | 0.114 |
| Age | 0.657 (0.326-1.322) | 0.239 |
| Serum CEA | 1.088 (0.447-2.647) | 0.853 |
| Primary tumor stage | 1.423 (0.575-3.517) | 0.445 |
The aggressive use of multimodality therapy, including chemotherapy and perioperative or intraoperative radiotherapy, is being increasingly delivered in order to improve the outcomes of patients with LRRC[8,18,19]. However, the prognosis of LRRC is far from satisfactory: the 5-year survival rate fluctuates between 18% and 58%, and only a minority of patients received curative surgery[7-10]. To date, surgical resection with curative intent is still the most relevant prognostic factor for LRRC, and this was demonstrated in almost all reports published thus far[7-10]. Unfortunately, the traditional pre-operative evaluation by surgeons does not allow for an accurate selection of patients as candidates for radical surgery, even though this selection is particularly important for prognosis. Nearly 30% to 65% of patients who underwent surgery did not achieve microscopically negative margins according to the majority of reports, which means that almost half of the patients would not benefit from surgery, whereas they still had to risk surgical morbidity[8,9,20,21]. Therefore, the evaluation and treatment for LRRC needs more accurate and individualized strategies, and to this end, we propose the MDT approach.
MDT is a promising approach for identifying candidates who are most likely to benefit from surgical resection, since this modality concentrates the superiority of a series of colorectal cancer-associated disciplines, including imaging, radiology, surgery, and oncology[13,22]. Our data revealed that an R0 resection rate of 87.8% could be achieved in patients who underwent surgery following MDT evaluation, which was significantly higher than that in previous reports[8,9,20,21], and suggested that patient selection for surgery might be dramatically improved via discussion by the MDT. Additionally, 8 out of 10 patients who were evaluated as marginally resectable gained R0 resection after preoperative chemoradiotherapy, based on the downsizing of the recurrent tumor; which suggested that the MDT approach may improve resectability by individual and multimodality treatment for LRRC. However, our findings need further studies to verify this, and our conclusion needs to be definitely proven by well-designed prospective cohort studies.
The basis of improved patient selection for surgery is the accurate evaluation of the recurrence pattern, which was also the main concern of the discussion by the MDT. Several studies have demonstrated that the recurrence pattern is a decisive factor for resectability[3,4,11,12]. Moore et al[12] reported the axial and anterior type of recurrences had a R0 resection rate greater than 70%, whereas the lateral type had a R0 resection rate lower than 20%. Yamada et al[11] also reported that the involvement of the pelvic sidewall in LRRC is a dominant adverse factor for surgery and prognosis, with a 5-year survival rate lower than 5%. Our data revealed that the recurrence pattern was an optimal index to accurately select indicated patients for surgery, since the difference was dramatically significant in R0 resection and survival between each pattern, with a favorable outcome in the axial type and a poor prognosis in the lateral type. Therefore, based on the results of this study, we recommend including the recurrence pattern as an essential criterion in clinical decision making.
In terms of the independent prognostic factors of long-term survival, the conclusions from this study are in accordance with those of most current reports, in that R0 resection is the most critical factor compared to other clinicopathological variables[3,4,10,11,20,21]. Although some studies have mentioned that elevated serum CEA, primary tumor stage, recurrence pattern, and other factors, may also have predictive value for long-term survival of LRRC[5,9,10,23]; these factors were demonstrated to be not as strong as radical surgery in their influence on the 5-year survival rate[10]. Therefore, the most pragmatic and effective way to improve long-term survival is to enhance radical resection for indicated patients via an MDT discussion.
In summary, this study reveals that the MDT approach optimizes the treatment strategy of LRRC; and among all the clinicopathological factors, R0 resection is the most significant factor affecting long-term survival.
Local recurrence of rectal cancer (LRRC) remains a significant clinical problem. Although radical resection is the most effective way to improve prognosis, it is only achieved in limited patients. Currently, the treatment of rectal cancer has evolved toward a multidisciplinary team (MDT) modality, and this modality is expected to improve the R0 resection rate and long-term survival of patients with LRRC. The aim of this study was to investigate the long-term outcome and its influential factors in LRRC under the MDT modality.
This study addresses the role and effectiveness of MDT in the treatment of LRRC, which was rarely reported in other studies. The results of this study suggest that among all the clinicopathological factors, R0 resection is the most significant factor affecting long-term survival, and MDT modality could improve R0 resection by optimize the treatment strategy.
This study demonstrated that the MDT approach could optimize the treatment strategy of LRRC; and R0 resection is still the most significant factor affecting long-term survival.
The MDT modality should be widely applied in the treatment of LRRC, since it could optimize treatment strategy and improve R0 resection.
Local recurrence refers to the occurrence of recurrent tumors after prior radical resection located within the pelvis, either alone or in conjunction with metastases. A multidisciplinary team is a unit or working group composed of specialists from multiple disciplines which are associated with a certain disease.
This is an excellent study in which the authors analyzed the associations between recurrence pattern and resectability, as well as the influential factors of long-term survival. All the pretreatment evaluation and therapy were made by a special MDT, which is a distinctive characteristic of this study. The results are interesting, and suggest that the recurrence pattern is a decisive factor of resectability, and that R0 resection is still the most significant factor affecting long-term survival. This study has important clinical significance for the identification of the indicated patients for surgery and those who had a better prognosis.
Peer reviewers: Alexander Graham Heriot, Associate Professor, Department of Surgical Oncology, Peter Maccallum Cancer Centre, 1 Andrews Place, Melbourne 3002, Australia; Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, Graz A-8036, Austria
S- Editor Wu X L- Editor Rutherford A E- Editor Zhang DN
| 1. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1913] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 2. | Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, Langmark F, Myrvold HE, Søreide O. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 434] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, Lacasta A, Bujanda L. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Zak Y, Moore HG, Ghosh BC. Patterns of recurrence of colorectal cancer. J Surg Oncol. 2008;97:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI, Wrobleski DE. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Sagar PM. Extended surgery for local recurrence and advanced rectal cancer. Colorectal Dis. 2006;8 Suppl 3:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis. 2006;8:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Hahnloser D, Nelson H, Gunderson LL, Hassan I, Haddock MG, O'Connell MJ, Cha S, Sargent DJ, Horgan A. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Salo JC, Paty PB, Guillem J, Minsky BD, Harrison LB, Cohen AM. Surgical salvage of recurrent rectal carcinoma after curative resection: a 10-year experience. Ann Surg Oncol. 1999;6:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Caricato M, Borzomati D, Ausania F, Valeri S, Rosignoli A, Coppola R. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani M, Paty PB, Wong WD, Guillem JG. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Meredith KL, Hoffe SE, Shibata D. The multidisciplinary management of rectal cancer. Surg Clin North Am. 2009;89:177-215, ix-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol. 2011;17:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Wille-Jørgensen P, Bülow S. The multidisciplinary team conference in rectal cancer--a step forward. Colorectal Dis. 2009;11:231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Marsh PJ, James RD, Schofield PF. Definition of local recurrence after surgery for rectal carcinoma. Br J Surg. 1995;82:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Heald RJ. Total mesorectal excision. The new European gold standard. G Chir. 1998;19:253-255. [PubMed] |
| 18. | Hashiguchi Y, Sekine T, Kato S, Sakamoto H, Nishimura Y, Kazumoto T, Sakura M, Tanaka Y. Indicators for surgical resection and intraoperative radiation therapy for pelvic recurrence of colorectal cancer. Dis Colon Rectum. 2003;46:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Saito N, Koda K, Takiguchi N, Oda K, Ono M, Sugito M, Kawashima K, Ito M. Curative surgery for local pelvic recurrence of rectal cancer. Dig Surg. 2003;20:192-19; discussion 200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Bedrosian I, Giacco G, Pederson L, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, Vauthey JN, Delclos M. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum. 2006;49:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Nicholls J. The multidisciplinary management of rectal cancer. Colorectal Dis. 2008;10:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Lopez-Kostner F, Fazio VW, Vignali A, Rybicki LA, Lavery IC. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |