Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4288
Revised: August 13, 2012
Accepted: August 16, 2012
Published online: August 28, 2012
AIM: To explore the anti-inflammatory potential of adeno-associated virus-mediated delivery of Tregitope 167 in an experimental colitis model.
METHODS: The trinitrobenzene sulfonate (TNBS) model of induced colitis was used in Balb/c mice. Subsequently after intravenous adeno-associated virus-mediated regulatory T-cell epitopes (Tregitope) delivery, acute colitis was initiated by intra-rectal administration of 1.5 mg TNBS in 40% ethanol followed by a second treatment with TNBS (0.75 mg in 20% ethanol) 8 d later. Control groups included mice not treated with TNBS (healthy control group) and mice treated by TNBS only (diseased group). At the time of sacrifice colon weight, the disease activity index and histology damage score were determined. Immunohistochemical staining of the colonic tissues was performed to asses the cellular infiltrate and the presence of transcription factor forkhead Box-P3 (Foxp3). Thymus, mesenteric lymph nodes, liver and spleen tissue were collected and the corresponding lymphocyte populations were further assessed by flow cytometry analysis for the expression of CD4+ T cell and regulatory T cell associated markers.
RESULTS: The Tregitope 167 treated mice gained an average of 4% over their initial body weight at the time of sacrifice. In contrast, the mice treated with TNBS alone (no Tregitope) developed colitis, and lost 4% of their initial body weight at the time of sacrifice (P < 0.01). The body weight increase that had been observed in the mice pre-treated with Tregitope 167 was substantiated by a lower disease activity index and a decreased colon weight as compared to the diseased control group (P < 0.01 and P < 0.001, respectively). Immunohistochemical staining of the colonic tissues for CD4+ showed that inflammatory cell infiltrates were present in TNBS treated mice with or without administration with tregitope 167 and that these cellular infiltrates consisted mainly of CD4+ cells. For both TNBS treated groups CD4+ T cell infiltrates were observed in the sub-epithelial layer and the lamina propria. CD4+ T cell infiltrates were also present in the muscularis mucosa layer of the diseased control mice, but were absent in the Tregitope 167 treated group. Numerous Foxp3 positive cells were detected in the lamina propria and sub-epithelium of the colon sections from mice treated with Tregitope 167. Furthermore, the Foxp3 and glycoprotein A repetitions predominant markers were significantly increased in the CD4+ T lymphocyte population in the thymus of the mice pre-treated with adeno-associated virus serotype 5 (cytomegalovirus promoter-Tregitope 167), as cytomegalovirus promoter compared to lymphocyte populations in the thymus of diseased and the healthy control mice (P < 0.05 and P < 0.001, respectively).
CONCLUSION: This study identifies adeno-associated virus-mediated delivery of regulatory T-cell epitope 167 as a novel anti-inflammatory approach with the capacity to decrease intestinal inflammation and induce long-term remission in inflammatory bowel disease.
- Citation: van der Marel S, Majowicz A, Kwikkers K, van Logtenstein R, te Velde AA, De Groot AS, Meijer SL, van Deventer SJ, Petry H, Hommes DW, Ferreira V. Adeno-associated virus mediated delivery of Tregitope 167 ameliorates experimental colitis. World J Gastroenterol 2012; 18(32): 4288-4299
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4288.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4288
Inflammatory bowel diseases (IBD) are inflammatory diseases that affect mostly young adults[1,2]. Although the precise pathogenesis has not been identified, it is generally accepted that IBD result from inappropriate mucosal immune system responses against intestinal flora and other luminal antigens[3-5]. IBD are associated with a reduction in quality of life[6-8] and no curative treatments are available.
Despite the fact that novel treatment strategies, including tumor necrosis factor (TNF)-neutralizing antibodies, have greatly expanded the therapeutic armamentarium, these therapeutics do not prevent complications in IBD and many patients still have to undergo surgery[9]. New treatment strategies that would prevent the initiation of inflammation and enable long-term remission would improve the lives of millions of individuals who are affected by IBD world-wide[10,11].
Recently, biological therapies that target immune pathways have emerged as a new therapeutic approach for the treatment of immune-mediated diseases. They include administration of monoclonal antibodies against inflammatory cytokines[12] and those that influence immune responses such as certain small molecules, Helminths and stem cells[10,13,14]. Since IBD are immune-mediated diseases, these biological therapies are highly promising treatment approaches and have the potential to achieve mucosal tolerance and long-term remission in IBD[10,12-14]. Here, we introduce regulatory T-cell epitopes (Tregitopes)[15,16] as novel biological agents that could create new possibilities for the regulation of inflammation and postulate that Tregitopes, delivered by adeno-associated virus (AAV), could be developed as a new therapeutic modality for the treatment of IBD.
Tregitopes are a set of putative regulatory T cell epitopes present in the immunoglobulin G molecule, which have been identified by using computational epitope mapping[15,16]. Tregitope sequence 167 (Tregitope 167) and an additional sequence (Tregitope 289) were synthesized and shown to bind to multiple Major Histocompatibility complex (MHC) class II molecules and to suppress immune response when co-administered with an antigen. Tregitopes 167 and 289 were also shown to expand natural occurring regulatory T (nTreg) cells and to induce a regulatory phenotype and function in peripheral T (iTreg) cells[15,16]. Harnessing the potential of Treg cells activated or induced by Tregitopes to regulate pathological immune responses in IBD may reduce the requirement for systemic immunosuppressive therapies. However, the use of immunomodulatory peptides in clinical applications for IBD so far have shown that the in vivo delivery of these peptides for therapeutic purposes is hindered by difficulties in obtaining sufficient and stable peptide concentrations[17-19]. Therefore, novel means for stable delivery of regulatory peptides have to be explored. AAV present a good safety profile and have been shown to be effective as gene delivery vectors in the clinic for the treatment of a broad range of diseases[20-22]. Therefore, AAV-mediated delivery represents an attractive approach to deliver the immuno-modulatory Tregitope peptides.
In the present study, the potential of AAV-mediated gene therapy for the therapeutic delivery of Tregitope 167 was explored. Systemic AAV-mediated administration of Tregitope 167 was shown to ameliorate the clinical and histo-pathological severity of trinitrobenzene sulfonate (TNBS) induced inflammatory colitis in mice. Hence, AAV-mediated delivery of regulatory T-cell epitopes appears to be a promising novel therapeutic approach for the treatment of IBD and could represent an alternative or adjunct to the use of immunosuppressive drugs.
Mouse Tregitope cDNA was synthesized (Integrated DNA Technologies, IDT, Inc) according to the published corresponding sequence[15,16] and cloned into the plasmid pPSC10[23] under the control of the cytomegalovirus (CMV) promoter. The Woodchuck hepatitis virus post-transcriptional enhancer was incorporated behind the Tregitope 167 cDNA to further optimize gene expression[24]. The AAV vector, AAV5 (CMV-Tregitope 167) was produced according to a technology adapted from Negrete et al[23]. The AAV vector was purified with an anion column using the ÄKTA explorer system (GE-Healthcare). After purification, the concentration of AAV vector genomes copies (genome copies/mL) was determined at 9 × 1013 genome copies/mL by Taqman qPCR amplification. The biological infectivity of AAV5 (CMV-Tregitope 167) was demonstrated in vitro by PCR amplification of the “CMV-Tregitope 167” DNA fragment (product size 402 bp) on DNA isolated from HEK293T transduced with AAV5 (CMV-Tregitope 167). Primers designed and synthesized for Tregitope 167 and the CMV promoter were used.
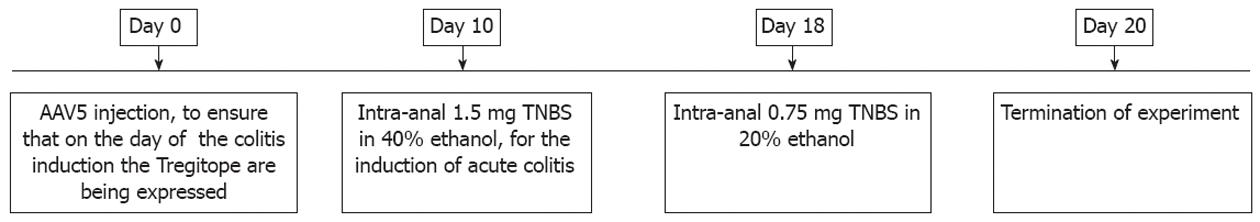
Balb/c mice (males, age 6-8 wk) were obtained from Harlan Laboratories, the Netherlands. The experimental protocol was approved by the ethical committee for animal welfare of the AMC (Academic Medical Center, Amsterdam, the Netherlands). Colitis was induced in mice by administration of TNBS (TNBS, Sigma-Aldrich), as described previously[25]. The general procedure is summarized in Figure 1.
Mice were injected intravenously with either phosphate-buffered saline or AAV5 (CMV-Tregitope) 10 d before acute colitis was initiated by intra-rectal administration of 1.5 mg TNBS in 40% ethanol. Consecutively, a second TNBS treatment (0.75 mg in 20% ethanol) was done 8 d after the first TNBS treatment as described precedently[25]. Mice not treated with TNBS (healthy control group) and mice treated with TNBS only (diseased control group) were used as references to monitor colitis development. A concomitant sham AAV control vector was not used in this study as this control has been shown to be equivalent to saline control[26,27]. Even though AAV-mediated gene transfer leads to the development of neutralizing antibodies against the vector capsid[28], preventing vector re-administration, no inflammatory responses against the AAV capsid were documented in in vivo gene transfer mice models using AAV vectors[27,29].
The body weights of the mice were recorded daily, and wasting disease progression was expressed by the percentage of weight loss as compared to body weight at the day of initiation of TNBS treatment (day 10, Figure 1). Animals were withdrawn from the study when their weight loss was > 25% of their original body weight. At the time of sacrifice, colons were collected and presence of loose stool and visible fecal blood was assessed.
At the time of sacrifice, a composite score [disease activity index (DAI)] was established as described previously[25]. Body weight loss was scored on a scale of 0-4 (0, < 1%; 1, 1%-5%; 2, 5%-10%; 3, 10%-15%; 4, > 15%). Loose stool was scored on a scale of 0-4 (0, normal; 1, loose droppings; 2, loose stools, colon filled with feces; 3, loose stool, feces only near cecum; 4, empty bowel). Visible fecal blood was scored on a scale of 0-4 (0, negative; 2, positive; 4, gross bleeding). The DAI consists of a combination of body weight loss, loose stool and visible fecal blood scores divided by 3 as described previously[25].
Colon tissue weights were recorded and used as an indicator of disease-related intestinal wall thickening. Increased colon weight has been shown to correlate with increased colon inflammation[25]. Colons were first divided longitudinally into two parts: one part was immediately frozen in liquid nitrogen for protein extraction and cytokine determination, while the second part was stored in formalin and embedded in paraffin for (immuno-) histological evaluation. Blood was collected by orbital puncture immediately following sacrifice and plasma was separated by centrifugation (5000 r/min for 5 min). Plasma samples were stored at -80 °C until analysis.
Colonic segments were fixed in 10% formalin overnight and thereafter stored in 70% ethanol before embedding in paraffin. Tissues sections (7 μm thick) were stained with haematoxylin and eosin (HE) for histology scoring. The histology damage score was calculated using the following criteria: percentage of area involved, number of follicle aggregates, edema, fibrosis, erosion/ulceration, crypt loss, and infiltration of mononuclear and polymorphonuclear cells, as described previously[30]. The percentage of area involved and crypt loss were scored on a scale of 0-4 (0, normal; 1, < 10%; 2, 10%; 3, 10%-50%; and 4, > 50%). Erosions were defined as 0 if the epithelium was intact, 1 if the lamina propria was involved, 2 if ulcerations involved the submucosa, and 3 when ulcerations were transmural. The severity of the other parameters was scored on a scale of 0-3 (0, absent; 1, weak; 2, moderate; and 3, severe)[30]. A certified pathologist scored all the tissue sections (blinded analysis).
Colon tissues sections of 7 μm were aceton-fixed and stained with rat-anti-mouse (ram) CD4 (1:100, BD550278), ram CD8a (1:50, BD550281), ram CD19 (1:50, BD550284), ram Foxp3 (1:100, eBiosciences14-5773-82) and ram F4/80 (1:500). Prior to anti-rat biotin conjugated secondary antibody (1:50, BD51-7605kc) and Streptavidin-HRP (BD) incubations, endogenous peroxidases were blocked by incubation with 0.3% H2O2 for 20 min. After 5 min of diaminobenzidine staining (BD), the sections were counter-stained with Haematoxylin, dehydrated and mounted in Entallan.
Thymus, mesenteric lymphoid nodes, liver and spleen tissue were collected upon sacrifice. Cell suspensions obtained from each of the tissue samples were prepared using 40 μm cell strainers (BD Biosciences) and stained for T cell surface markers CD4 (clone RM4-5, eBioscience), CD8 (Clone 53-6.7, Miltenyi) and Treg cell surface markers glycoprotein A repetitions predominant (GARP) and CD25 (clone YGIC86 and clone PC61.5 respectively, both eBioscience) as well as for the intracellular Treg cell marker Foxp3 (clone FJK16, eBioscience). The analysis was performed by flow cytometry (FACSCalibur, BD Biosciences).
The results are presented as mean ± SD or SE, where appropriate. Statistical analyses were performed using Prism 5.0 (GraphPad). Data were analyzed using a 1 way analysis of variance, followed by Dunn’s post hoc test for multiple comparisons.
We investigated the potential for AAV5-mediated delivery of regulatory T-cell epitopes to prevent the development of TNBS induced colitis. Mice treated intra-rectally with TNBS in ethanol developed a severe illness as reflected in the progressive body weight loss over time and an increase in disease activity index, histology damage score and mucosal inflammatory parameters at the time of sacrifice.
Development of colitis in the TNBS mice model is strongly associated with wasting disease[31]. Daily weight determination is therefore important to determine colitis severity and is indicative of differences in colitis development between experimental groups[31]. Animals were withdrawn from the study when their weight loss was > 25% of their original body weight.
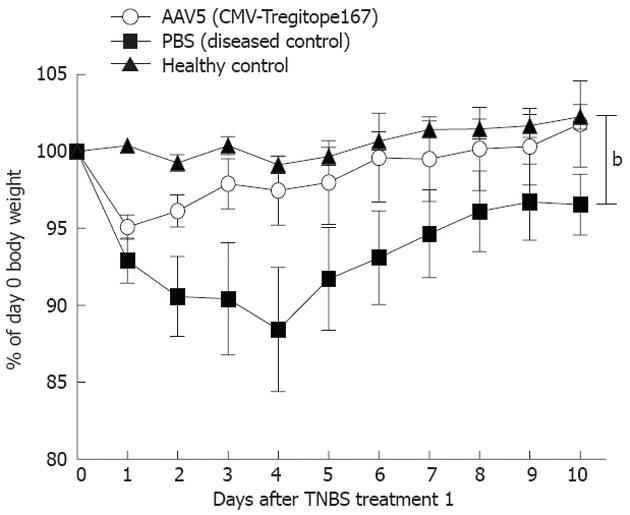
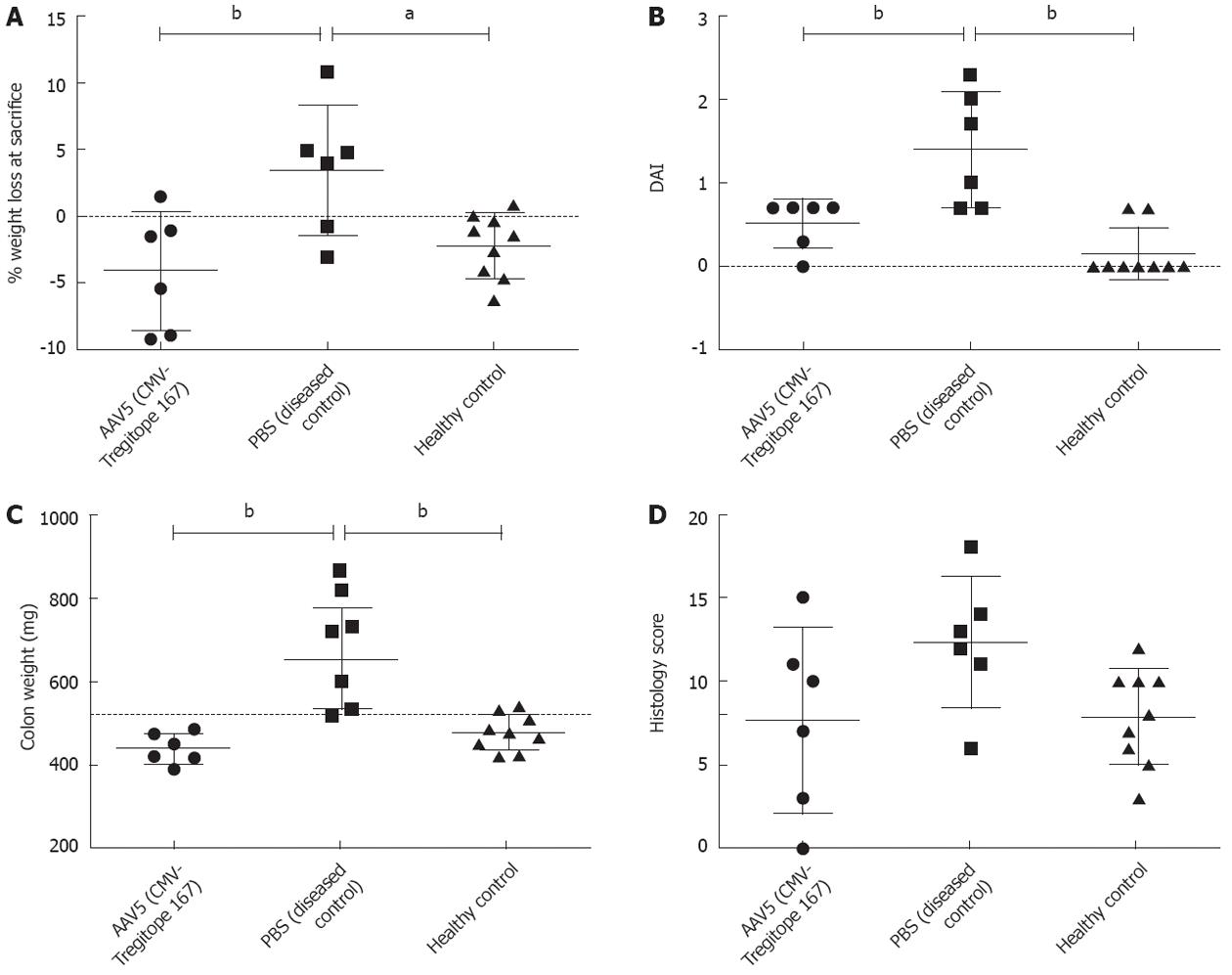
The body weight of the mice was monitored daily after the first TNBS treatment as an indication of the severity in the colitis development between experimental groups (Figure 2). TNBS treated mice that were pre-administered with Tregitope 167, showed a body weight that increased over time and was comparable to the weigh gain of untreated healthy control mice (Figure 2). The Tregitope 167 treated mice gained an average of 4% over their initial body weight at the time of sacrifice (Figure 3A). In contrast, the mice treated with TNBS alone (no Tregitope) developed colitis, and lost 4% of their initial body weight at the time of sacrifice (Figure 3A).
Increases in colon weight, as well as in the disease activity index are both indicative of colonic inflammation and were determined at the time of sacrifice (Figure 3). The body weight increase that had been observed in the mice pre-treated with Tregitope 167 was substantiated by a lower disease activity index (Figure 3B) and a decreased colon weight (Figure 3C) as compared to the diseased control group (P < 0.01 and P < 0.001 respectively).
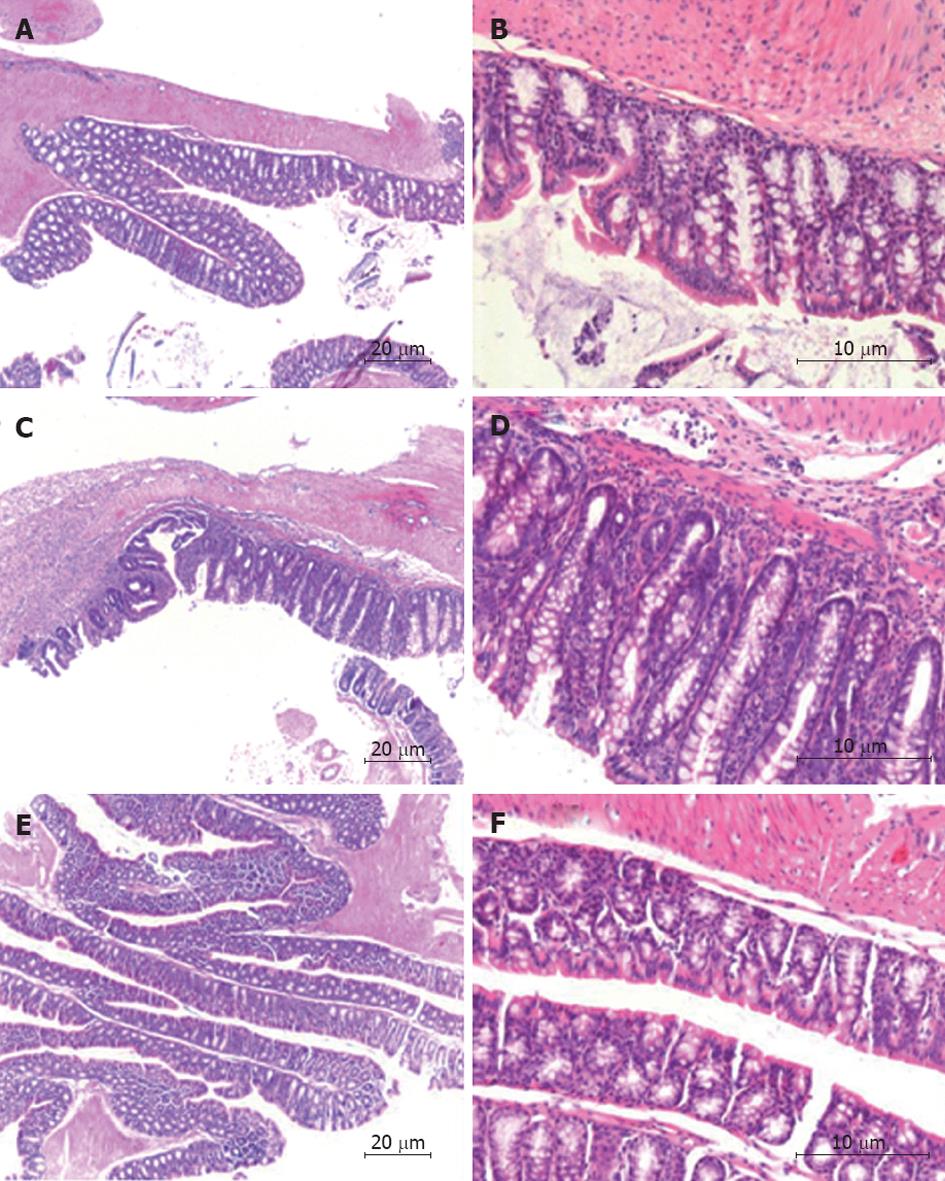
The histology damage score was performed on HE stained tissue sections. The score was calculated using the following criteria: percentage of area involved, number of follicle aggregates, edema, fibrosis, erosion/ulceration, crypt loss, and infiltration of mononuclear and polymorphonuclear cells. The histological scoring showed that the AAV5 (CMV-Tregitope 167) pre-treated mice presented a decreased severity of colitis as compared to the diseased control group (Figure 3D) as a result of lower levels of inflammation, namely decreased cellular infiltrations, little crypt loss and the absence of erosions and ulceration (Figure 4).
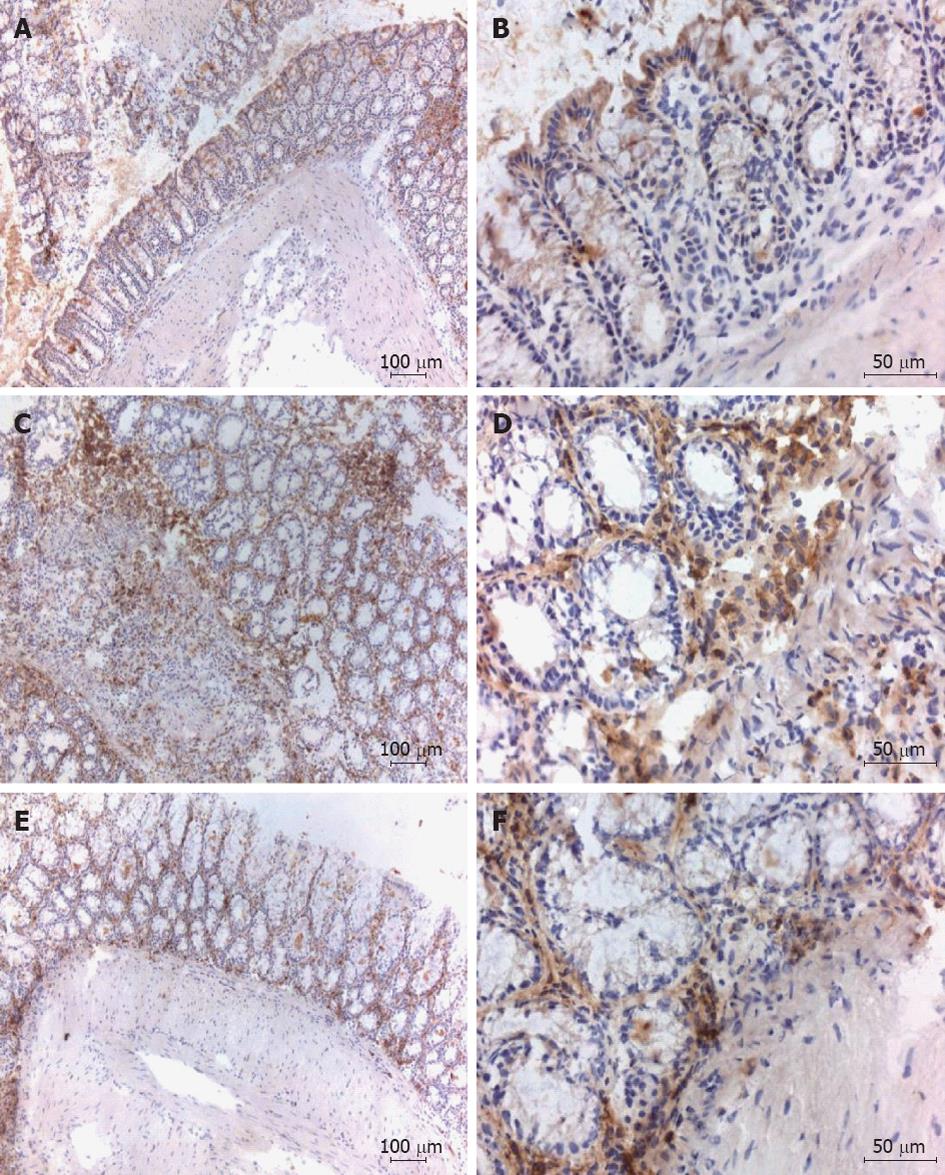
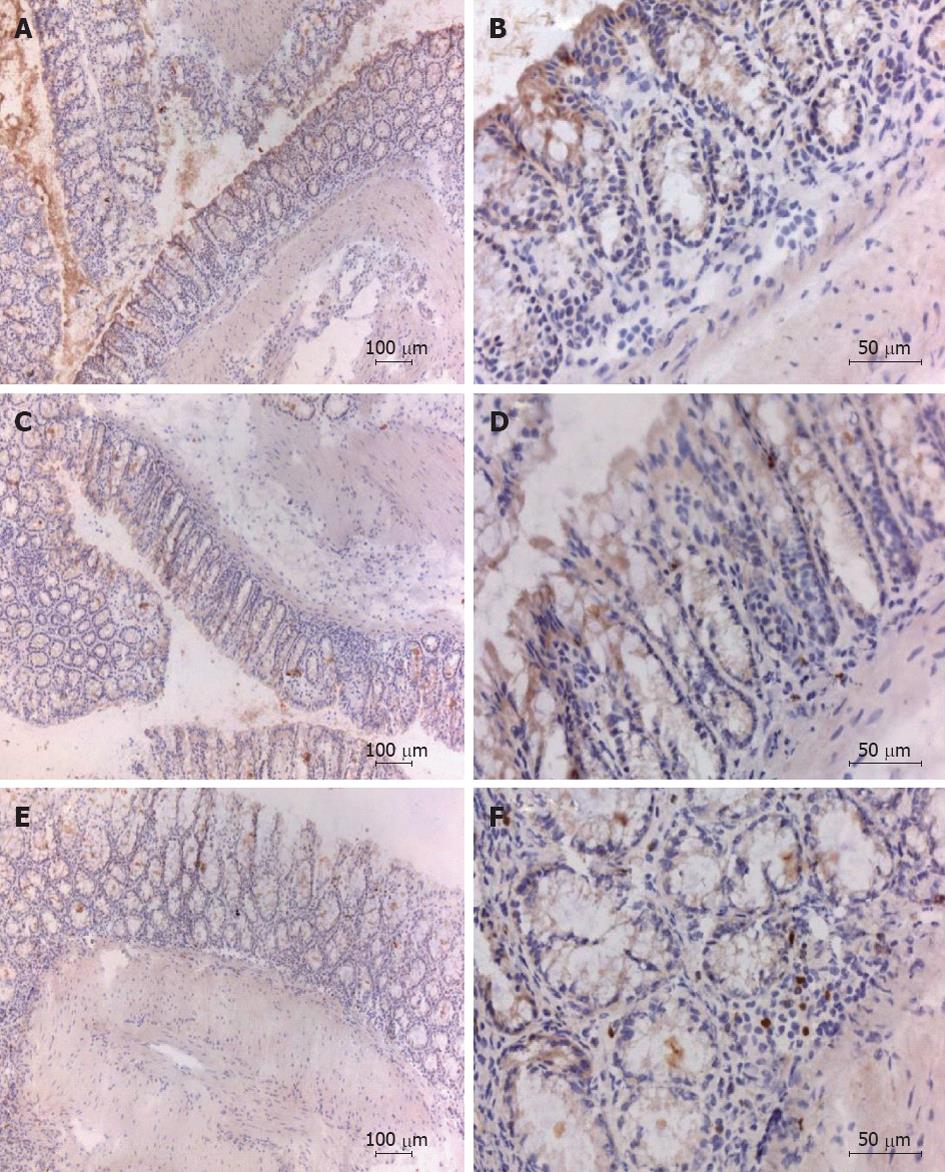
The TNBS induced colitis model is characterized by the local infiltration of CD4+ T cells in the intestinal mucosa[32]. Immunohistochemical staining of the colonic tissues for CD4+ showed that, at the day of sacrifice, inflammatory cell infiltrates were present in TNBS treated mice with or without administration with Tregitope 167 and that these cellular infiltrates consisted mainly of CD4+ cells. For both TNBS treated groups CD4+ T cell infiltrates were observed in the sub-epithelial layer and the lamina propria. CD4+ T cell infiltrates were also present in the muscularis mucosa layer of the diseased control mice, but were absent in the AAV5 (CMV-Tregitope 167) pre-treated group (Figure 5).
The reported ability of Tregitopes to both activate and induce Treg cells led us to further assess the presence of Treg-cell associated markers in the colonic tissues.
Colon tissues were prepared and the presence of transcription factor Foxp3 was assessed by immunohistochemistry, so as to determine whether regulatory T cells were present in the peri-colonic infiltrates[33]. Numerous Foxp3 positive cells were detected in the lamina propria and sub-epithelium of the colon sections from mice treated with Tregitope 167 (Figure 6). Foxp3 positive cells were absent or sporadic in the colon of healthy and diseased control mice (Figure 6).
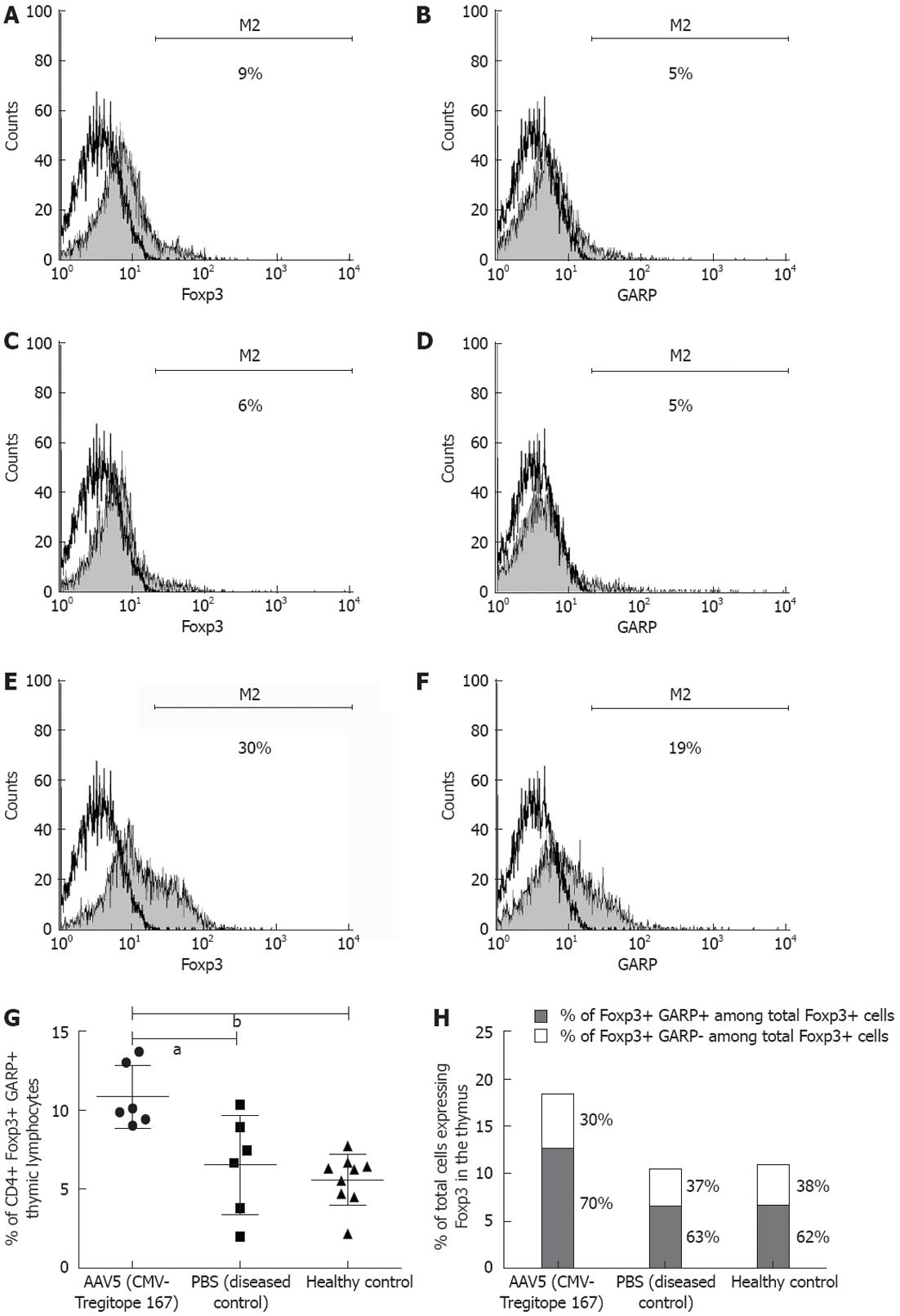
Thymus, mesenteric lymph nodes, liver and spleen tissue were collected at the time of sacrifice and the corresponding lymphocyte populations were further assessed by flow cytometry analysis for the expression of the CD4 T cell surface marker and the Treg cell associated markers Foxp3[34], CD25[35] and GARP[36,37] (Figure 7A-F). The Foxp3 and GARP markers were significantly increased in the CD4+ T lymphocyte population in the thymus of the mice pre-treated with AAV5 (CMV-Tregitope 167), as compared to lymphocyte populations in the thymus of diseased and the healthy control mice (P < 0.05 and P < 0.001 respectively, Figure 7G). CD4+ thymic lymphocyte population (mean ± SD, 11% ± 2%, n = 6) co-expressed Foxp3 and GARP in the thymus of AAV5 (CMV-Tregitope 167) pre-treated mice as compared to the thymic lymphocyte population of diseased (mean ± SD, 7% ± 3%, n = 6) and the healthy control groups (mean ± SD, 6% ± 2%, n = 9), respectively (Figure 7G). Both the relative and absolute number of Foxp3 expressing T cells were expanded in the thymus after AAV5 (CMV-Tregitope 167) pre-treatment (Figure 7H). No significant differences in the expression of Foxp3 and GARP in the lymphocyte populations of the mesenteric lymph nodes, liver and spleen were identified.
Curative treatment approaches for Crohn’s disease and ulcerative colitis represent a significant unmet medical need. Regulatory T (Treg) cells are key players in maintaining peripheral tolerance, preventing autoimmune diseases and limiting chronic inflammation[33,38]. Therefore, novel strategies that aim for therapeutic tolerance induction and leverage Treg cells are currently being explored[39]. In the present study, the potential for AAV-mediated delivery of an immunomodulatory peptide (Tregitope 167) was investigated.
In this study, we demonstrate that the systemic AAV-based delivery of Tregitope 167 has the potential to prevent the development of fulminant colitis in a TNBS-induced model of IBD. Tregitope 167 was used in our study as its binding affinity for the MHC molecule in Balb/c mice is superior to Tregitope 289 (De Groot, manuscript submitted for publication). The significant decrease of colonic inflammation in the Tregitope 167 pre-treated mice was reflected in an overall weight gain and substantiated by a decreased disease activity index, colon weight and histology damage score at the time of sacrifice. Additionally, there was less mucosal inflammation in the AAV (CMV-Tregitope 167) pre-treated mice. This therapeutic benefit corresponded with increases in the relative number of T cells expressing regulatory T cell markers in the colon tissues and among thymic lymphocytes of the Tregitope 167-treated mice.
IBD patients do not present defects in regulatory T cell function or phenotype[40-42] and by consequence are more likely to benefit from therapies aiming at inducing and expanding Treg cells than patients affected by other autoimmune diseases. Tregitope 167 has the potential to both activate nTreg cells and induce iTreg cells[15,16] and may be suitable as a novel therapeutic agent for IBD. However, the use of immunomodulatory peptides in clinical applications for IBD has been hindered by difficulties associated with the systemic delivery of the therapeutic peptides in sufficient quantity and concentration to the target tissues[17-19]. AAV has proven to be both effective and safe as a gene therapy delivery vector in the clinic[20-22]. Therefore, AAV-mediated delivery of Tregitopes was explored in this study. The AAV serotype 5 (AAV5) was used since pre-existing immunity to AAV5 in humans has been shown to be low[28,43]. Our data demonstrate that AAV5-mediated delivery may be an efficient approach for stable administration of Tregitopes in vivo. Further studies will need to be performed to determine the duration of the immunological tolerance that is evoked by induction and activation of Treg cells.
Regulatory T (Treg) cells are considered to be essential in the counter-regulation of inflammatory reactions and Foxp3 is considered as a marker of the regulatory phenotype[34,44,45]. Staining for Foxp3 in mice pre-treated with Tregitope 167 revealed the presence of Foxp3 positive cells in the lamina propria and sub-epithelium of the colon sections. Additionally, expression of both Foxp3 and GARP were increased in the thymic CD4+ T lymphocyte population in mice pre-treated with AAV5 (CMV-Tregitope 167), indicating an increase in activated regulatory T-cells[34,36,37,44,45]. The presence of higher numbers of activated regulatory T cells corresponded with the prevention of fulminant intestinal inflammation in vivo in this TNBS model of IBD.
No increase in the Treg cell populations was observed in the mesenteric lymph nodes, liver and spleen in the current study. We hypothesize that this could be due to the duration of the experiment and the timing of the Treg cell evaluation. In other mouse models such as the model of spontaneous encephalomyelitis, the de novo generation of Treg cells was initiated intrathymically, and was followed by Foxp3 induction in peripheral tissue at later time points[46].
Tregitopes are T cell epitopes naturally located in immunoglobulins that bind to multiple MHC class II alleles and induce Treg cell responses. We have demonstrated that antigen presenting cells (APCs) present Tregitopes to nTreg cells, engage feedback mechanisms promoting a tolerogenic APC phenotype, induce Treg cell expansion, and modulate antigen-specific effector T cell responses (Cousens and De Groot, manuscript submitted for publication). Proportions of APC expressing MHC II, CD80, and CD86 are suppressed, consistent with reported effects of intravenous immunoglobulin[47] and of the immunoglobulin (Ig) G-derived peptide hCDR1[48]. The basic mechanism of Tregitope tolerance induction is currently proposed to be as follows: (1) APC present Tregitopes to nTreg cells; (2) nTreg cells are activated to proliferate; (3) nTreg cells provide tolerogenic feedback signals to APC, modulating the APC phenotype; and (4) nTreg cells and tolerogenic APC together suppress antigen-specific T cell responses (Cousens and De Groot, manuscript submitted for publication).
A limitation of the colitis model used in this study was the acute necrotizing enterocolitis, occurring in the first 3 d after the first TNBS treatment, a presentation of disease which is unrelated to IBD[25]. Therefore the surviving number of mice, included in the analysis was lower than anticipated, which, for some analysis, conflicted with statistical analysis of the data. As a consequence, a large variability in the colon mucosa cytokines levels was observed after TNBS induced experimental colitis and prevented an accurate analysis of those parameters. Therefore, further development of AAV mediated delivery of Tregitope 167 in different experimental models of inflammatory disease will be necessary to confirm the obtained results.
In summary, our data provide preliminary evidence supporting the potential use of AAV-based Tregitope delivery as a therapy for the treatment of IBD. Further investigations will permit to define the mechanism by which Tregitope exert their immune regulatory properties, the duration of the effect, the ability of Tregitopes to reduce disease that has already been established and their eventual impact on systemic immunity.
Overall, this study identifies AAV-mediated delivery of regulatory T-cell epitope 167 as a novel anti-inflammatory approach with the capacity to decrease intestinal inflammation and induce long-term remission in IBD.
We would like to thank Daalhuisen JB (AMC, Amsterdam, The Netherlands) for his excellent technical expertise and the staff of the Central Animal Facility of the AMC for animal care. We would like to thank Dr. Cornelis FM Sier (LUMC, Leiden, The Netherlands) for carefully reviewing the manuscript and for statistical advice.
The focal point in the search for novel treatment options in inflammatory bowel diseases (IBD) is tolerance induction. In other words, exploring different mechanisms by which the immune system could maintain unresponsiveness to the antigens in the mucosal environment which cause destructive immune responses and thereby disease activity in IBD patients.
The recently discovered regulatory T cell epitopes (Tregitopes) are highly promising peptides that were demonstrated to both activate and induce regulatory T cells in vitro and in vivo. Modulation of T cell responses with Tregitopes may contribute to the regulation of pathological inflammatory responses in IBD. However, the use of immunomodulatory peptides in clinical applications for IBD so far have shown that the in vivo delivery of these peptides for therapeutic purposes is hindered by difficulties in obtaining sufficient and stable peptide concentrations.
This study identifies adeno-associated virus (AAV)-mediated delivery of regulatory T-cell epitope as a novel anti-inflammatory approach with the capacity to decrease intestinal inflammation and induce long-term remission in IBD.
The significance of the achievements to IBD relate to possible novel therapeutic approaches, which could aim at long term tolerance induction. The described study identifies a novel anti-inflammatory strategy with the capacity to ameliorate the intestinal inflammatory response and restore mucosal immune tolerance.
Tregitopes are T cell epitopes naturally located in immunoglobulins that bind to multiple major histocompatibility complex class II alleles and induce regulatory T cell responses. The non-pathogenic, replication-deficient AAV holds promise for gene therapy. The AAV vector has a good safety profile as it remains predominantly episomal. The therapeutic potential of AAV as a vector in gene therapy has also been demonstrated in a clinical setting in recent studies.
This interesting paper is investigating the possibility of AAV mediated delivery of regulatory trinitrobenzene sulfonate (TNBS) colitis. The major finding of the study was that systemic administration of AAV was associated with a marked decrease in the clinical and histological severity of the TNBS induced inflammatory colitis parallel with an increase of the T lymphocytes expressing regulatory markers in the colon and thymus. Overall, this manuscript presents an interesting approach opening new possibilities.
Peer reviewers: Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46, 1088 Budapest, Hungary; Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, H1083 Budapest, Hungary
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [PubMed] |
| 3. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [PubMed] |
| 4. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [PubMed] |
| 5. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1537] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 6. | Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Graff LA, Vincent N, Walker JR, Clara I, Carr R, Ediger J, Miller N, Rogala L, Rawsthorne P, Lix L. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882-1889. [PubMed] |
| 8. | Lix LM, Graff LA, Walker JR, Clara I, Rawsthorne P, Rogala L, Miller N, Ediger J, Pretorius T, Bernstein CN. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Cannom RR, Kaiser AM, Ault GT, Beart RW, Etzioni DA. Inflammatory bowel disease in the United States from 1998 to 2005: has infliximab affected surgical rates? Am Surg. 2009;75:976-980. [PubMed] |
| 10. | van der Marel S, Majowicz A, van Deventer S, Petry H, Hommes DW, Ferreira V. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 11. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [PubMed] |
| 12. | van der Woude CJ, Hommes DW. Biologics in Crohn's disease: searching indicators for outcome. Expert Opin Biol Ther. 2007;7:1233-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52-63. [PubMed] |
| 14. | Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. Activation of natural regulatory T cells by IgG Fc-derived peptide "Tregitopes". Blood. 2008;112:3303-3311. [PubMed] |
| 16. | Elyaman W, Khoury SJ, Scott DW, De Groot AS. Potential application of tregitopes as immunomodulating agents in multiple sclerosis. Neurol Res Int. 2011;2011:256460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Buruiana FE, Solà I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010;CD005109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [PubMed] |
| 19. | Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Crohn's Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461-1472. [PubMed] |
| 20. | Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231-2239. [PubMed] |
| 21. | Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240-2248. [PubMed] |
| 22. | Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1422] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 23. | Negrete A, Kotin RM. Strategies for manufacturing recombinant adeno-associated virus vectors for gene therapy applications exploiting baculovirus technology. Brief Funct Genomic Proteomic. 2008;7:303-311. [PubMed] |
| 24. | Lee YB, Glover CP, Cosgrave AS, Bienemann A, Uney JB. Optimizing regulatable gene expression using adenoviral vectors. Exp Physiol. 2005;90:33-37. [PubMed] |
| 25. | te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Conrad CK, Allen SS, Afione SA, Reynolds TC, Beck SE, Fee-Maki M, Barrazza-Ortiz X, Adams R, Askin FB, Carter BJ. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 1996;3:658-668. [PubMed] |
| 27. | Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, Virella-Lowell IL. IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 2010;17:567-576. [PubMed] |
| 28. | van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H, Hommes DW, Ferreira V. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis. 2011;17:2436-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Polyak S, Mach A, Porvasnik S, Dixon L, Conlon T, Erger KE, Acosta A, Wright AJ, Campbell-Thompson M, Zolotukhin I. Identification of adeno-associated viral vectors suitable for intestinal gene delivery and modulation of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G296-G308. [PubMed] |
| 30. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [PubMed] |
| 32. | Mariman R, Kremer B, van Erk M, Lagerweij T, Koning F, Nagelkerken L. Gene expression profiling identifies mechanisms of protection to recurrent trinitrobenzene sulfonic acid colitis mediated by probiotics. Inflamm Bowel Dis. 2012;18:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490-500. [PubMed] |
| 34. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5859] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 35. | Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487-7491. [PubMed] |
| 36. | Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS One. 2008;3:e2705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439-13444. [PubMed] |
| 38. | Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 39. | St Clair EW, Turka LA, Saxon A, Matthews JB, Sayegh MH, Eisenbarth GS, Bluestone J. New reagents on the horizon for immune tolerance. Annu Rev Med. 2007;58:329-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [PubMed] |
| 42. | Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849-859. [PubMed] |
| 43. | Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 44. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6389] [Article Influence: 290.4] [Reference Citation Analysis (0)] |
| 45. | Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2198] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 46. | Zelenay S, Bergman ML, Paiva RS, Lino AC, Martins AC, Duarte JH, Moraes-Fontes MF, Bilate AM, Lafaille JJ, Demengeot J. Cutting edge: Intrathymic differentiation of adaptive Foxp3+ regulatory T cells upon peripheral proinflammatory immunization. J Immunol. 2010;185:3829-3833. [PubMed] |
| 47. | Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Sela U, Sharabi A, Dayan M, Hershkoviz R, Mozes E. The role of dendritic cells in the mechanism of action of a peptide that ameliorates lupus in murine models. Immunology. 2009;128:e395-e405. [PubMed] |