INTRODUCTION
Agmatine (AGM), 4-(aminobutyl) guanidine, is a natural biogenic endogenous dicationic amine metabolite mainly present in the deprotonated form at physiological pH and produced by decarboxylation of L-arginine via arginine decarboxylase in bacteria, plants, invertebrates, and mammals[1-5]. It is not supplied by nutritional components or bacterial colonization. AGM is metabolized by two distinct pathways depending on the tissue where it is contained: by “agmatinase” (AGM uryl hydrolase) to putrescine with cleavage of urea, mainly in the brain, and by “diamineoxidase” (DAO), in peripheral tissues, to 4-guanidinobutyraldehide, then dehydrogenated and hydrolyzed by specific enzymes and excreted out of the body. The heterogeneous location of DAO suggests that certain tissues or organs may have the capacity to regulate local AGM levels[6,7]. AGM is transported to organs by an energy-dependent mechanism which is inhibited by dose-dependent administration of putrescine, suggesting a correspondence between the transport mechanism of polyamines and AGM, probably using a carrier[8,9].
After its discovery in the brain, AGM was demonstrated in nearly all organs of rats, with organ-specific distribution. Its highest concentrations were found in the stomach (71 ng/g wet weight), followed by the aorta, small and large intestine, and spleen[10,11]. AGM was also shown in vascular smooth muscle and endothelial cells[12], and in plasma of rats at a concentration of 0.45 ng/mL, which is similar to that of catecholamines[10]. The source of circulating AGM remains undefined. In humans, higher plasma concentrations (47 ng/mL) were determined in comparison to rats[13]. The reasons underlying this large difference remain to be clarified.
It is becoming clear that AGM has multiple physiological functions in the body. It acts as a potential neurotransmitter in the brain[14,15], and a regulator of polyamine concentration[16] by acting on different enzymes involved in the polyamine pathway. It inhibits all isoforms of nitric oxide synthase (NOS), providing evidence of its important role in modulating NO production as an endogenous regulator[17]. In particular, AGM irreversibly inhibits the endothelial NOS and downregulates the inducible form (iNOS), and exhibiting a neuroprotective role since NO contributes to ischemic brain injury[18].
It has been reported that AGM is protective against ischemia reperfusion (I/R) injury in different organs including the brain, retina, kidney and heart[19-22]. However, no previous reports on its protective effect in gastric reperfusion injury have been investigated. Despite the fact that AGM is a strong base[23] and is found in mucous-secreting cells and in parietal cells where it localizes in the canaliculi, it was reported to be deleterious in ethanol-induced gastric lesions,[5] as well as in gastric stress-induced lesions[24,25]. Therefore, the aim of the present study was to investigate whether or not the administration of AGM is protective to rat stomach subjected to I/R injury, and the mechanisms involved.
MATERIALS AND METHODS
Animals
Male Wistar rats weighing 170-210 g were obtained from the College of Medicine Animal House at King Saud University. Rats were maintained on standard rat chow and tap water ad libitum. Rats were kept in an air-conditioned room with a 12 h day/light cycle. Animals were fasted 12 h prior to the experimental procedure. All studies were approved by the Ethics Committee of King Saud University.
Experimental design
Rats were divided into 3 experimental groups (6 rats/group): (1) control sham-operated group; (2) a gastric I/R group; and (3) I/R + AGM (100 mg/kg) group, administered AGM 15 min prior to I/R injury induction. Rats were anesthetized by i.p. injection of urethane at a dose of 125 mg/100 g body weight (BW). To investigate whether or not the protection induced by AGM is mediated by the Akt/IPK3 pathway, wortmannin (WM), an inhibitor of this pathway, was given at a dose of 15 μg/kg, i.p.[26], 15 min prior to AGM treatment in an additional I/R injury group. The stomach was observed macroscopically for hemorrhages and ulceration. The dose of AGM, selected for the current study was based on the previously published doses used in models of brain[19] and myocardial I/R injury[22]. AGM was dissolved in normal saline and controls received saline in an equivalent volume. WM was dissolved in dimethyl sulfoxide (10%).
Experimental model
Gastric IR lesions were produced as described by Yoshikawa et al[27]. Briefly, the stomach was exposed and the esophagus and the pylorus were occluded using bulldog clamps. The celiac artery was clamped and 100 mmol hydrochloric acid (HCL, 1 mL/100 g BW) was placed in the stomach to maintain acid levels during ischemia. The acid was then removed 25 min after ischemia and clamps were removed 30 min after ischemia. The tissues were allowed to re-perfuse for 30 min and then the stomach was removed and examined. AGM 100 mg/kg was found to be effective depending on histological examination and Evans blue (EB) dye extravasation.
At the end of the experiment gastric tissues were collected. Gastric samples were snap-frozen in liquid nitrogen and stored at -80 °C for subsequent assays of vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein-1 (MCP-1). A piece of each stomach was fixed in 4% phosphate-buffered formalin, embedded in paraffin, and cut. Paraffin sections were hydrated and stained with hematoxylin and eosin (HE) for assessment of mucosal damage or stained with sera specific for angiopoietin-1 (Ang-1) and Ang-2, (R and D Systems, United States).
Determination of vascular permeability
EB dye, an azo dye, is widely used as an indicator of increased capillary permeability[28-30]. Systemic administration of EB leads to the formation of a dissociable complex with serum albumin, and when there is microvascular tissue damage, EB extravasates. In another three experimental groups, 1 mL of EB (0.5% v/w) was injected i.v. after reperfusion or sham operation. The amount of EB that accumulated in the stomach within the reperfusion period was measured. Briefly, the animals were killed and the stomach was removed. After collecting the gastric content carefully by lavage with 5 mL cold distilled water, the stomach was opened along the greater curvature and the corpus mucosa was scraped off and put into a tube containing 5 mL distilled water. The EB was extracted by a modified method of Lange et al[29] and its concentration was spectrophotometrically quantified. The EB present in the gastric contents and mucosa was extracted by adding 5 mL formamide to each tube and kept in a shaking water bath at a temperature of 50 °C for 24 h. This was followed by centrifugation at 3000 g for 10 min and the absorbance of supernatant was measured at 612 nm (Lambada 5, Perkin-Elmer, Pomona, CA, United States). The amount of EB was calculated from a previously prepared standard curve and expressed as μg per stomach.
Histological study
Gastric tissues from the studied groups were fixed in 10% phosphate-buffered formalin, embedded in paraffin and 4 μm sections were made, followed by staining with HE and were examined histologically for mucosal damage.
Enzyme-linked immunosorbent assay
VEGF and MCP-1 were assayed in a supernatant of gastric tissue homogenate and calculated according to protein concentration in each sample. Protein was determined in each sample using Bradford Reagent (Biorad, United States). Concentrations of VEGF and MCP-1 were measured using an ELISA kit according to the manufacturer’s instructions (R and D Systems, United States).
Immunohistochemistry
Immunostaining was performed using formalin fixed, paraffin-embedded sections (4 μm) after dewaxing and rehydration. Endogenous peroxidase was quenched with 3% H2O2 for 30 min and sections were blocked with 10% normal goat serum (Sigma). Sections were incubated with Ang-1 and Ang-2 (Santa Cruz, Biotech., United States) at a concentration of 1:200 and were kept at room temperature for 2 h. Sections were then washed and incubated with secondary antibody, and immunoperoxidase staining was carried out using the Vectastain ABC Elite reagent kit (Vector Laboratories, CA, United States). Di-amino-benzidine was used as a chromogen. All slides were counterstained with HE.
Chemicals and reagents
All chemicals were purchased from Sigma (St, Louis, MO, United States) unless otherwise specified.
Statistical analysis
All values are expressed as the mean ± SD. Statistical significance of differences was determined using one-way analysis of variance. Further statistical analysis for post hoc comparisons was carried out using the Tukey test. A level of P < 0.05 was considered statistically significant.
RESULTS
Effect of AGM on gastric tissue vascular permeability
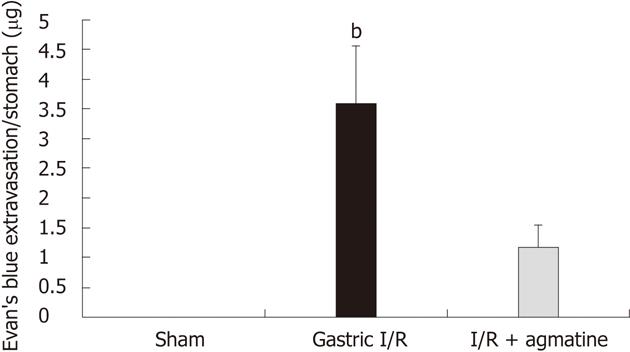
Gastric perfusion for 30 min following 30 min of ischemia induced a marked leakage of EB dye in the stomach lumen (3.58 ± 0.975 μg/stomach). The administration of AGM 100 mg/kg prior to induction of ischemia attenuated the leakage by about 60% when compared with no treatment (1.173 ± 0.374 μg/stomach, P < 0.05) (Figure 1).
Figure 1 Evan’s blue extravasation as a measure of vascular permeability in the studied groups.
Gastric ischemia reperfusion (I/R) injury induced extravasation of Evan’s blue dye due to increased vascular permeability. Agmatine (AGM) pre-treatment (100 mg/kg, i.p.) significantly attenuated the vascular leakage (gastric I/R vs I/R + agmatine, bP < 0.001). Results are expressed as mean ± SD.
Histological assessment
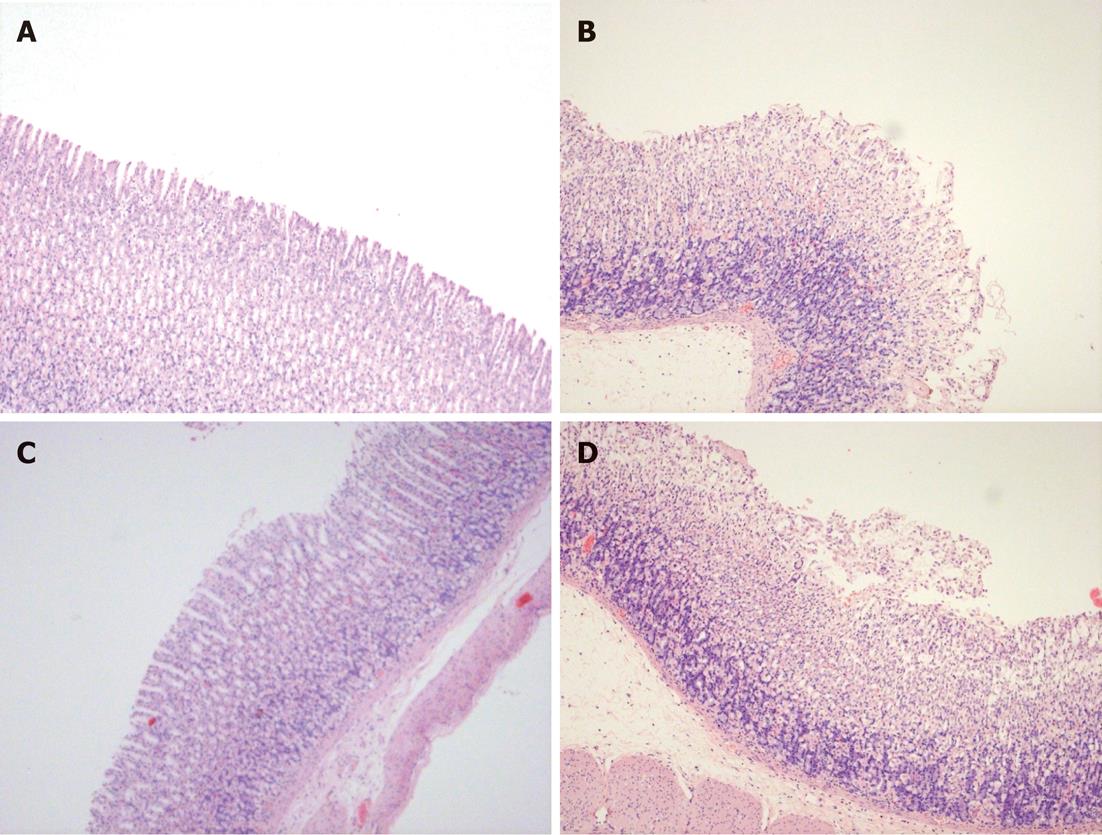
The histological features of gastric I/R injury included hemorrhage, and ulceration of the mucosa with inflammatory cell infiltration (Figure 2B). Gastric mucosa of normal rats showed intact mucosa and sub-mucosa (Figure 2A). The administration of AGM at a dose of 100 mg/kg attenuated the mucosal damage by some of 80% of the surface exposed (Figure 2C) with reduction in hemorrhage, ulceration and cellular infiltration. WM prevented the protective effect resulting in extensive ulceration, edema, and hemorrhage (Figure 2D).
Figure 2 Histological appearance of the gastric mucosa of HE stained sections of the studied groups.
A: Sham-operated showing intact mucosa; B: Gastric ischemia reperfusion (I/R) showing hemorrhages, edema and ulceration; C: Agmatine (AGM) treated group (100 mg/kg, i.p. prior to I/R) with marked preservation of gastric mucosa and disappearance of ulceration and hemorrhages; D: Effect of inhibition of Akt/phosphatidyl inositol-3-kinase (PI3K) (wortmannin 15 μg/kg, i.p.) prior to AGM treatment, showing extensive lesions and salvage of gastric mucosa into the lumen (× 20 magnification).
Effect of AGM on VEGF gastric tissue level
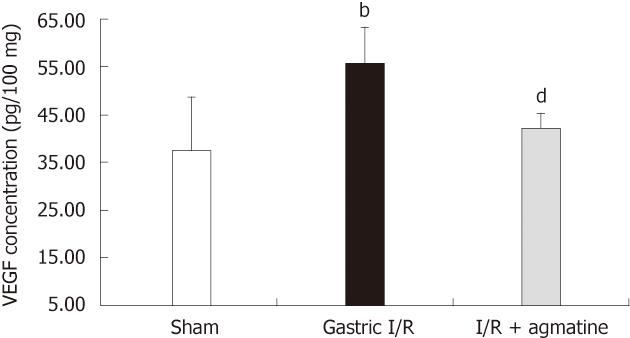
Following ischemic injury to the stomach, VEGF demonstrated an increase in gastric tissue homogenate (48.4 ± 6.53 pg/100 mg protein) compared with normal gastric tissue (32.725 ± 37.7 pg/100 mg protein). AGM treatment significantly reduced this increase (36.87 ± 2.71 pg/100 mg protein vs 48.4 ± 6.53 pg/100 mg protein, P < 0.05) (Figure 3).
Figure 3 Effect of administration of agmatine on gastric vascular endothelial growth factor tissue level.
Gastric vascular endothelial growth factor (VEGF) protein significantly increased after ischemia reperfusion (I/R) injury, bP < 0.01 vs sham group. Administration of agmatine (100 mg/kg, i.p.) 15 min prior to gastric I/R reduced the VEGF level in gastric tissue homogenate, dP < 0.01 vs I/R group. Results are expressed as mean ± SD.
Effect of AGM on MCP-1 gastric tissue level
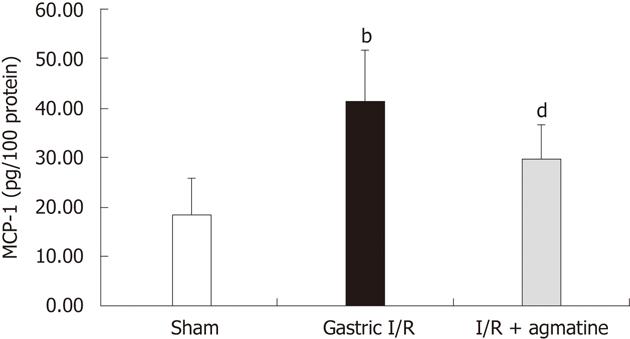
As shown in Figure 4, the I/R injury of the gastric mucosa induced an increase in the level of MCP-1 compared with the mucosa in control sham rats (41.17 ± 10.3 pg/100 mg protein vs 18.3 ± 7.4 pg/100 mg protein, P < 0.01). AGM pretreatment markedly reduced the MCP-1 levels relative to I/R injury (29.5 ± 7 pg/100 mg protein vs 41.17 ± 10.4 pg/100 mg protein; P < 0.01).
Figure 4 Effect of AGM administration on gastric monocyte chemoattractant protein-1 tissue level.
Gastric monocyte chemoattractant protein-1 (MCP-1) protein significantly increased after ischemia reperfusion (I/R) injury, bP < 0.01 vs sham group. Administration of agmatine (100 mg/kg, i.p.) 15 min prior to gastric I/R reduced the MCP-1 level in gastric tissue homogenate, dP < 0.01 vs I/R group. Results are expressed as mean ± SD.
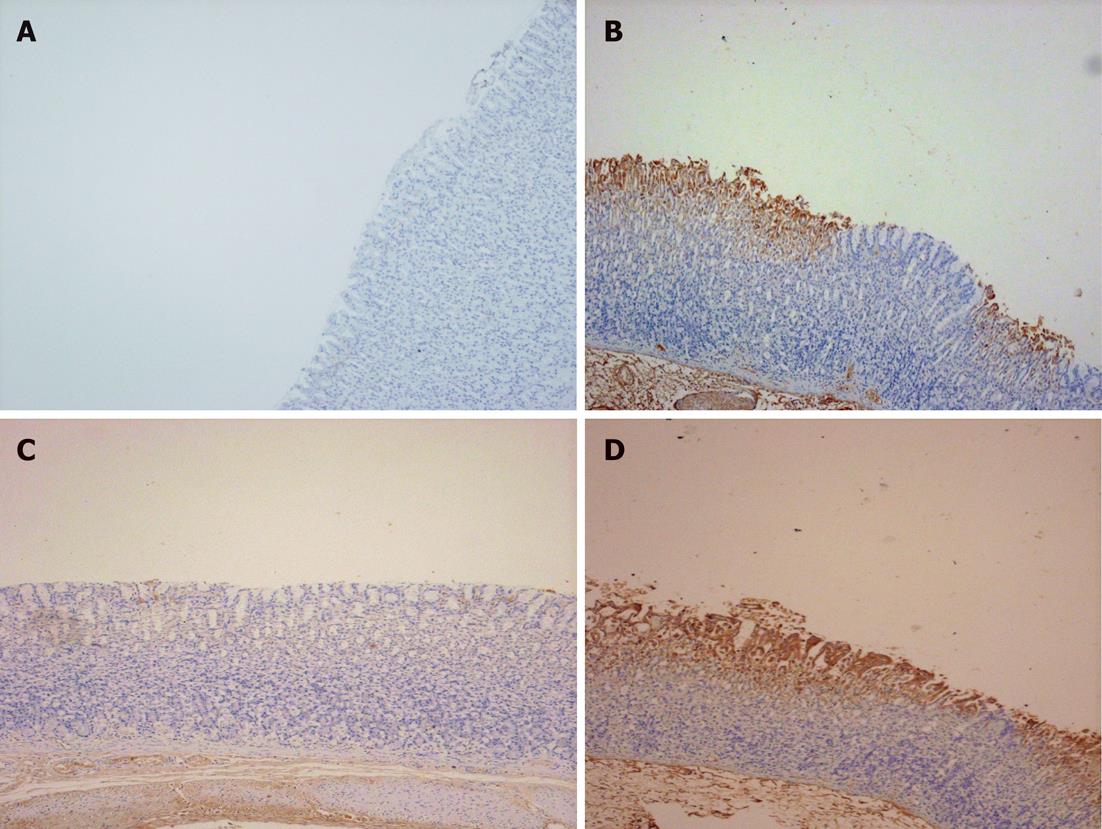
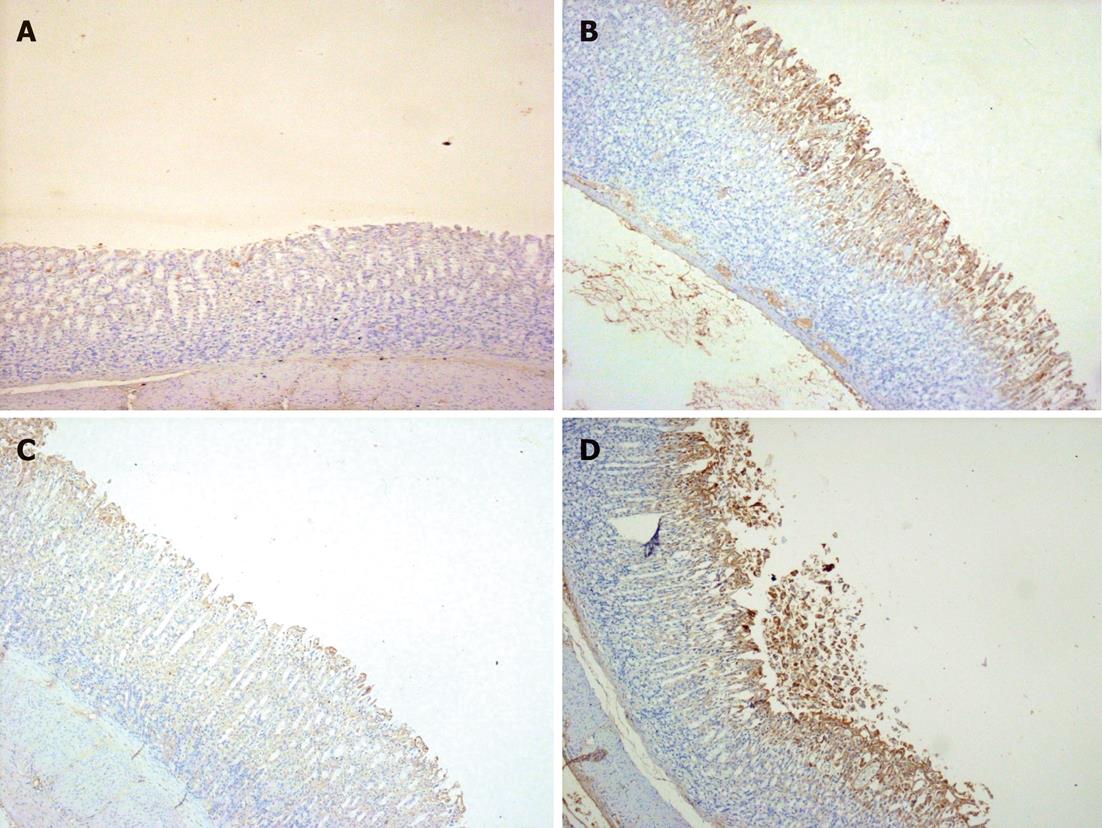
Ang-1 and Ang-2 immunostaining
A very faint staining for Ang-1 was seen in normal rat stomach (Figure 5A), while it was extensively expressed in areas where congestion and damage occurred in ischemia reperfused stomach (Figure 5B). However, AGM pretreatment (100 mg/kg) markedly attenuated the expression of Ang-1 (Figure 5C). Ang-2 expression of normal rat stomach (Figure 6A), was almost undetected. Extensive expression of Ang-2 was seen mostly in the mucosa of ischemia reperfused stomach (Figure 6B). AGM markedly attenuated Ang-2 expression and protected the mucosa from injury (Figure 6C).
Figure 5 Photomicrographs representative of angiopoietin-1 immunostaining of gastric sections of the studied groups.
A: Sham-operated showing lack of expression of angiopoietin-1 (Ang-1); B: Gastric ischemia reperfusion (I/R) group showing extensive cytoplasmic expression of Ang-1 particularly in areas of congestion and damage; C: Agmatine (AGM) treated group (100 mg/kg, i.p.), with marked attenuation of Ang-1 expression in intact mucosa; D: Wortmannin (15 μg/kg, i.p.) treated group prior to AGM administration abolished the effect of AGM and the expression of Ang-1 is marked (× 20 magnification).
Figure 6 Photomicrographs representative of angiopoietin-2 immunostaining of gastric sections of the studied groups.
A: Sham-operated showing faint expression of angiopoietin-2 (Ang-2); B: Gastric ischemia reperfusion (I/R) group showing extensive cytoplasmic expression of Ang-2 in areas of congestion and damage, and in the submucosa; C: Agmatine (AGM) treated group (100 mg/kg, i.p.), with marked attenuation of Ang-2 expression in intact mucosa; D: Wortmannin (15 μg/kg, i.p.) treated group prior to AGM administration abolished the effect of AGM and the expression of Ang-2 is marked in mucosa and sloughed tissues (× 20 magnification).
Effect of blocking the phosphatidyl inositol-3-kinase pathway on protection induced by AGM
AKt/phosphatidyl inositol-3-kinase (PI3K) is described as an important component of cell survival pathways in many cell types[26]. Pretreatment of rats with WM (15 μg/kg per i.p.), an inhibitor of AKt/PI3K, 15 min prior to AGM administration, markedly prevented its protective effect and extensive hemorrhages and ulceration were demonstrated microscopically (Figure 2D), suggesting that AGM probably acted on this signaling pathway to protect the stomach from I/R injury. The administration of WM prior to AGM also induced marked expression of both Ang-1 and Ang-2 in the mucosa and even in the submucosa (Figures 5D and 6D).
DISCUSSION
As mentioned earlier, the present study examined whether or not the administration of AGM prior to gastric I/R injury has a protective effect. Our results revealed a gastroprotective effect of AGM by reducing vascular permeability of the gastric mucosa as evidenced by reduction of EB dye extravasation, Ang-1 and Ang-2 protein expression in gastric sections, as well as VEGF and MCP-1 concentrations in gastric tissue homogenate. This effect is probably mediated by the Akt/PI3K pathway.
The gastric injury model we used in the present study is a gastric ischemia reperfusion model. In this model we injected 100 mmol HCL (1 mL/100 g BW) to maintain the gastric acid level during the procedure. The acid was then removed 25 min after ischemia and clamps were removed 30 min after ischemia. I/R injury was demonstrated macroscopically and microscopically in the form of congestion, hemorrhages, and mucosal ulceration. With prior administration of AGM, hemorrhages, blood clots and ulceration were attenuated. This protective effect, while supported by some studies, was opposed by others.
Circumstantial evidence was provided in a recent study that AGM is protective to the gastric mucosa[23]. That study, utilizing immunohistochemistry, showed that AGM is found in the mucus-secreting cells of the stomach and in the parietal cells where it is localized in the lumen of the canaliculi. Biochemically, basic amino acids are ideally suited to act as a source of carbon dioxide in the stomach, and the most basic amino acid is arginine (isoelectric point 11.15). A significant source of arginine is found in the stomach[23]. CO2 can be produced from the decarboxylation of arginine. The activation segment of pepsinogen in the parietal cell canaliculi can provide a source of arginine[31]. AGM is the decarboxylation product of arginine. Interestingly, the highest concentration of AGM is found in the stomach[10]. The fact that AGM is such a strong base and its cellular localization is in the gastric mucosa makes AGM a strong candidate for a protective role against HCL formed in the stomach[23]. In addition, Helicobacter pylori (H. pylori) infection is associated with a decrease in the amount of mucous-secreting cells in the stomach. This change is associated with a decrease in the amount of AGM in these mucous-secreting cells[32]. It was speculated that such a decrease in the amount of AGM in the epithelium of the H. pylori-infected stomach would make this epithelium more vulnerable to damage by gastric acid[23]. On the other hand, a group of investigators reported higher AGM concentrations in gastric juice from H. pylori-positive patients than from H. pylori-negative patients, and concluded that AGM is deleterious to the stomach and may be involved in the pathogenesis of gastroduodenal lesions[33]. However, these results suggest an association rather than a causal link. Furthermore, these results might implicate AGM as a counter-regulatory molecule to H. pylori. Supporting our speculation, a recent report demonstrated that bacteria such as Escherichia coli and H. pylori utilize AGM to survive the highly acidic medium of the stomach and even prevent AGM being taken up by stomach cells[34]. This explains the high levels of AGM previously reported in gastric juice of H. pylori patients, suggesting a compensatory mechanism.
The pattern of gastric response to AGM in our study is opposite to what has been reported by some other previous studies[24,25], which showed that AGM augments gastric acid and pepsin secretion, decreases gastric adherent mucus and worsens experimental gastric mucosal injury in rats in a pylorus-ligated ischemic stress model. Similar results demonstrating exacerbation of gastric mucosal injury by AGM pretreatment in ethanol-induced stress model in rats have been reported[5]. The inconsistency of these results with ours could be attributed to the models used and the duration of gastric stress. In our model, the rats were exposed to 30 min of ischemia and 30 min of reperfusion, while these investigators exposed the animals to 4 h of stress. In addition, these studies administered AGM at a dose of 10 mg/kg[24] or 20 mg/kg[5], which is much lower than that used in the present study or other studies. For example in a rat model of brain I/R injury AGM was given at a dose of 100 mg/kg[19].
The mechanisms by which AGM induced gastric protection were the focus of the present study. Increased vascular permeability occurs after insult to the gut[35] and hence, reduction of hyper-permeability can induce tissue protection. The current work provides evidence that AGM works by reducing vascular permeability of the stomach in response to I/R injury. This was investigated using EB dye extravasation as a measure of vascular permeability. Also, we measured VEGF concentration in gastric tissues and Ang-1 and Ang-2 distribution in gastric sections. The present study showed an elevation of VEGF content in the stomach following 30 min of ischemia and 30 min of reperfusion. This increase is most probably due to increased blood to the tissue upon reperfusion, but the possibility of increased expression of VEGF protein could be a factor. The prevention of this increase by AGM could be explained by the capability of AGM to reduce vascular permeability as was seen in the present study by the EB dye experiment. Interestingly, previous studies showed that transgenic mice overexpressing VEGF induced hyper-permeable vessels[36], providing evidence that VEGF increases vascular permeability. VEGF has been implicated in the pathophysiology of liver I/R injury, and its increase during reperfusion injury was seen to mediate leukocyte trafficking and ischemic injury response early after reperfusion, whereby VEGF acts via MCP-1[37]. Interestingly, the administration of VEGF antibodies was reported to block reperfusion injury[37]. The proinflammatory functions of VEGF have been demonstrated by previous studies[37-39]. Therefore, agents attenuating VEGF during the early period of reperfusion could possibly indirectly reduce the inflammatory response resulting from reperfusion injury.
Other vascular molecules studied in the current work were Ang-1 and Ang-2. We demonstrated the attenuation of both Ang-1 and Ang-2 expression in rats receiving AGM prior to induction of gastric I/R, compared with untreated rats. Ang-2, in contrast to Ang-1, was shown to increase vascular permeability, and to promote vascular leakage from blood vessels in vivo[40]. AGM seems to reduce vascular permeability at least partly by reducing VEGF and Ang-2 in gastric tissues.
MCP-1 is an inflammatory mediator molecule[41] whose upregulation is responsible for recruitment of inflammatory cells after I/R injury[42]. Importantly, the interaction of MCP-1 with its receptor CCR2 has been attributed a central role in experimental cardiac, renal and cerebral I/R models[43]. Therefore, decreased MCP-1 production will attenuate attraction and recruitment of monocytes and subsequently ameliorate the post-ischemic inflammatory responses. The present study demonstrated an increase in gastric tissue content of MCP-1 after I/R which was suppressed by AGM pre-treatment. Indeed, we provided evidence for the protective anti-inflammatory effect of AGM, whereby it reduced gastric MCP-1 in the ischemic group. In support of our observation, previous reports also showed an anti-inflammatory effect of AGM[44,45], a mechanism by which AGM can attenuate I/R injury. Furthermore, AGM was shown to inhibit the production of NO in macrophages, thus providing a molecular basis for the anti-inflammatory actions of AGM[42]. We, previously demonstrated that iNOS is upregulated in the gastric mucosa in response to I/R injury[46]. Interestingly, Mu et al[18] reported that iNOS was also upregulated in brain in response to I/R injury and was significantly downregulated by AGM. With these data in mind we may speculate that AGM could contribute to gastric protection against I/R injury by reducing NO production, possibly by inhibiting iNOS. Other suggested mechanisms that might be involved in the protection offered by AGM could be via preservation of endothelial dysfunction[47] and inhibition of matrix metalloproteinase-9, which is known to be upregulated in ischemic injury and degrades the basement membrane of blood vessels[48].
The signaling pathway by which AGM induced gastric protection is possibly mediated via Akt/PI3K. The present study provided indirect evidence, as inhibition of this pathway by WN, prior to AGM administration, prevented protection offered by AGM. A limitation to this study is that we did not measure Akt/PI3K activity in gastric tissues.
There are many reasons making AGM a good candidate for gastric protection. First, it is a strong base[22]; second, the highest concentration of AGM is found in the stomach[10]; third, AGM is localized in the mucus-secreting cells[22]. In the parietal cells, it is localized to the lumen of the canaliculi. Finally, a decrease in AGM concentration in mucus-secreting cells in H. pylori infection of the stomach is also associated with a decrease in the amount of mucus in the mucus-secreting cells of the stomach[32]. Recently, it has been reported that bacteria such as E-coli and H. pylori utilize AGM to survive the highly acidic medium of the stomach and even prevent AGM being taken up by stomach cells[34].
In conclusion, the present study revealed that AGM protects the rat stomach exposed to I/R injury at least for brief periods by reducing vascular permeability possibly mediated via Akt/PI3K pathway.