Published online Nov 21, 2011. doi: 10.3748/wjg.v17.i43.4799
Revised: April 19, 2011
Accepted: April 26, 2011
Published online: November 21, 2011
AIM: To evaluate the effect of autoclaved diet on the jejunum neurons of the myenteric plexus of rats during their growth.
METHODS: The experimental groups were made up of rats going through weaning whose mothers received either an autoclaved or a non-autoclaved diet during gestation and lactation, and rats that were fed the same diet as their mothers during the post-weaning period. In order to measure the neurons’ body profile and to quantify the number of neurons per area, preparations were stained by the nicotinamide adenine dinucleotide-diaphorase method.
RESULTS: No significant changes were observed in rats’ body weight or in the number of neurons regardless of the diet used (P > 0.05). There was a decrease in the jejunum-ileum length in rats treated with an autoclaved diet (P < 0.05). An increase in the neuronal cross-sectional area was seen in rats that had received the autoclaved diet, an effect that was significant for animals undergoing weaning. In addition, all observed factors showed significant differences when related to the age of the animals.
CONCLUSION: The autoclaved diet did not alter the quantity of neurons, but increased their cell body area, suggesting changes similar to those observed in protein deficiency.
- Citation: Gonçalez PO, Clebis NK, Mari RB, Gagliardo KM, Stabille SR, Faria HG, Liberti EA, Jr JRK. Morphological effects of autoclaved diet on the myenteric neurons of rats. World J Gastroenterol 2011; 17(43): 4799-4803
- URL: https://www.wjgnet.com/1007-9327/full/v17/i43/4799.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i43.4799
The nutritional makeup of the diet provided to laboratory animals and the procedures used for its preparation, storage, and sterilization must be evaluated not only to ensure the proper development of the animal, but also to avoid contamination with pathogenic microorganisms[1-3].
Animal facilities have traditionally used the recommended autoclaving conditions of 120 °C for 15 min to sterilize commercial diet, as this is an easy, safe and low cost process[4]. However, this exposure to high temperature may compromise the components of the ration, destroying vitamins and proteins and affecting the nutritional value of the diet[2,4,5].
Proteins may become more chemically reactive after autoclaving, leading to their degeneration or even to reaction with other substances, and compromising their digestibility, functionality and nutritional value[4,6].
These characteristics have been evaluated through the KOH protein solubility test, which has been shown to be a good indicator of the reduction of the amount of protein in feed[6-9].
It is known that animal tissues are not homogeneously affected by protein deficiency[10]. Thus, tissues that show low rates of metabolism or cell renewal are later compromised[11]. Different conditions of protein malnutrition have been shown to cause changes in the amount and size of neurons in the myenteric plexus of the segment of the gastrointestinal tract of rats of different ages[12-18]. The results indicated that factors such as nutritional quality of the diet and animal age might interfere with functional morphology of the myenteric plexus, resulting in impairment of the function of the digestive system and, consequently, the performance of the animal.
For these reasons, in addition to the concern of maintaining the composition of the diet provided to laboratory animals, the integrity of nervous system elements that control the activities of the digestive system, such as the myenteric plexus, is important for animal nutrition and production, since a structural impairment of this plexus in animals with some type of protein deficiency should not be ignored.
Thus, considering the key role of the jejunum (and consequently the myenteric plexus) in the process of nutrient absorption[19], and the practice of autoclaving for diet sterilization in the care of laboratory animals, the myenteric neurons of the jejunum of rats fed autoclaved rations during pre- and post-weaning periods were qualitatively and quantitatively evaluated.
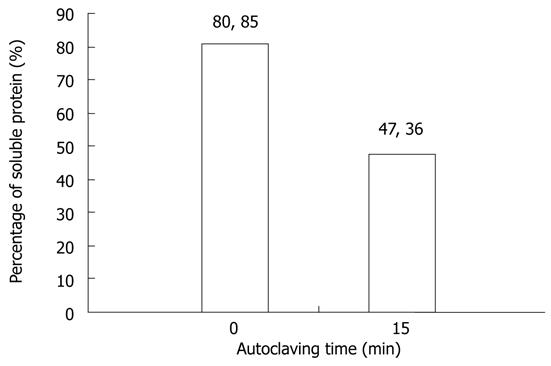
Four female Wistar rats from the central animal facility of the Maringa State University, were housed separately in polypropylene boxes equipped with an automatic feeder and drinker, kept in a temperature-controlled (22 °C) and photoperiod regulated room (12 h of light and 12 h of darkness). They received commercial water and food for rats (Nuvilab® CR1-Nuvital) ad libitum. After an adaptation period of one week, the animals were impregnated and divided into two groups, where two females made part of the control group (CG) and were maintained with a non-autoclaved diet (ND) with a soluble protein level of approximately 80.85% in KOH[20] (Figure 1). The remaining two females integrated into the experimental group (EG) and were fed with the same diet, except that the diet was autoclaved (120 °C for 15 min), leading to protein levels around 47.36% [autoclaved diet (AD)]. The composition of the diet is shown in Table 1.
| Nutrients | % |
| Dry matter | 89.88 |
| Crude protein | 22.23 |
| Crude Fiber | 5.73 |
| Calcium | 0.92 |
| Phosphorous | 0.87 |
| Crude energy (kcal/kg) | 3976 |
At birth, the offspring of each female were equalized to five young males, which were divided into the following groups according to diet and periods of life: (1) CG21, 21-day-old animals whose mothers were maintained with ND during pregnancy and nursing; (2) EG21, 21-day-old animals whose mothers were maintained with AD during pregnancy and nursing; (3) CG70, animals whose mothers were maintained with AD during pregnancy and nursing and were then maintained with the same diet until 70 d of age; and (4) EG70, animals whose mothers received AD during pregnancy and nursing and were then maintained with the same diet until 70 d of age.
After weighing, the animals were euthanized by cervical dislocation and laparotomized in order to remove the jejunum.
The jejunum was initially washed in Krebs solution, ligated with cotton threads at its extremities and its lumen was filled with a syringe needle until slightly distended. After incubation in Krebs solution at room temperature for 15-30 min, the specimens were transferred to a permeabilizing agent (0.3% Triton-X in Krebs solution) for 60 s and then submitted to three 10 min washes in Krebs solution.
The specimens were then incubated for 60 min at 20 min in 20 mL of a medium containing 0.5 mg/mL nitro blue tetrazolium (Sigma-Aldrich) in distilled water (5 mL), 0.1 mol/L sodium phosphate buffer (5 mL, pH 7.3), distilled water (10 mL) and 0.5 mg/mL β-nicotinamide adenine dinucleotide (reduced form)[21].
The reaction was stopped by immersion in 10% buffered formalin solution in which the viscera were fixed (24 h minimum). Fragments of each jejunum about 1 cm in length were opened longitudinally. The mucosal and submucosal layers of these fragments were removed and the specimens were thoroughly washed in distilled water. Finally, whole-mount preparations were laid in glycerol on a microscope slide and sealed with Entellan (Merck KGaA, Darmstadt, Germany).
The neuronal density and the profile areas of the nerve cell bodies were measured by examining the whole-mount preparations under a binocular microscope at 400x magnification. For each specimen, all neurons present in 40 microscopic fields (0.224 mm2 each) were counted (total area of 8.96 mm2). The profiles of 80 random nerve cell perikarya from each specimen were obtained on a semiautomatic device for morphometry analysis (Image pro Plus, 3.01). The data were expressed as means ± SD and compared by Kruskal-Wallis test. The level of significance was set at P < 0.05.
All experimental procedures were reviewed and approved by the Bioethics Committee of the School of Medicine and Veterinary of the University of São Paulo.
The animals fed with an autoclaved diet (EG21 and EG70) showed an increase in body weight of 0.83% and 6.3%, respectively, compared to animals in the CG21 and CG70 (Table 2). However, there was no statistically significant difference (P > 0.05) when comparing groups of same age (21 d and 70 d).
| Group | Body weight (g) | No. of neurons | Cell body area (μm2) |
| CG21 | 46.8 ± 1.61 | 1061.0 ± 50.721 | 230.0 ± 10.91 |
| EG21 | 47.2 ± 1.71 | 1168.0 ± 71.421 | 282.1 ± 7.02 |
| CG70 | 237.4 ± 10.32 | 881.4 ± 38.962 | 347.4 ± 13.43 |
| EG70 | 252.4 ± 11.12 | 969.4 ± 82.032 | 377.4 ± 22.03 |
Through the nicotinamide adenine dinucleotide (NADH)-diaphorase reaction, it was verified that the myenteric plexus was organized in elongated ganglia containing neurons of different sizes in all studied groups. These ganglia were scattered and arranged in parallel in the same direction as the muscle bundles of the circular layer of the muscular coat of jejunum.
The number of myenteric neurons present in 8.96 mm2 of jejunum differed between the 21- and 70-day-old animals, with lower amounts present in the 70-day-old animals (P < 0.05) (Table 2). However, when comparing the same age groups, (CG21 and EG21; CG70 and EG70), the number of neurons was shown to not change after use of autoclaved diet. Animals from the EG21 and EG70 groups showed an increase of 9.2% and 9% in the number of neurons when compared with the CG21 and CG70 groups, respectively, but this increase did not reach statistical significance (P > 0.05).
The area of the neuron cell bodies ranged from 105.1 μm2 to 553.9 μm2 in the CG21 group and from 101.1 μm2 to 640.7 μm2 in the EG21 group. In the CG70 group, the dimensions ranged from 95.2 μm2 to 713.2 μm2 and from 97.3 μm2 to 843 μm2 in the EG70 group.
The average size of myenteric neurons was smaller (P < 0.05) for younger animals (CG21 and EG21) compared to the 70-day-old animals (CG70 and EG70). The neurons from the CG70 and EG70 groups showed an increase in their average area of around 51% and 33.8%, respectively, when compared to their control groups (CG21 and EG21) (Table 2).
Statistically, it was found that the average area of the neuronal cell body differed between animals from the CG21 and EG21 groups (P < 0.05), with higher values for animals from EG21, whose mothers received an autoclaved diet during pregnancy and nursing (Table 2). Although it was verified that neurons in the CG70 group showed a cell body average area smaller than that observed in EG70, the differences in this parameter between the two groups were not significant (P > 0.05) (Table 2).
After autoclaving, the quality of the protein was altered in the diet sterilization procedure, reducing the usable protein content and indicating that animals in the EG21 and EG70 groups received feed with a lower protein quality than those in their respective control groups (CG21 and CG70).
Regardless of whether the diet was autoclaved, animals gained body weight during the experiments because of the natural growth and development from birth to adulthood. Although not statistically significant (P > 0.05), animals in the EG21 and EG70 groups had weight gain 0.85% and 6.3% higher than their respective controls (CG21 and CG70). In contrast, studies that examined rats of various ages and during different periods of protein malnutrition reported a decrease in body weight[13-17,22,23]. These differences are justifiable since the autoclaving temperature of the feed used in this study does not significantly alter the performance of rats in pre- or post-weaning periods. The compromise in animal performance is seen after autoclaving feed at temperatures higher than those used in our study[9]. Thus, the change in protein quality of the autoclaved ration given to animals in this study was not sufficient to influence a significant variation in animals’ body weight.
The reactive NADH-diaphorase myenteric neurons of the jejunum were organized predominantly in a dispersed ganglion distributed parallel to the direction of muscles fibers of circular layers of the muscular coat, as described for the myenteric plexus of rats[24].
In the quantitative analysis, although animals from the experimental groups (EG21 and EG70) had respective increases of 9.2% and 9% in the quantity of neurons compared to their controls (CG21 and CG70), the number of myenteric neurons present in 8.96 mm2 of jejunum in animals that were fed with an autoclaved diet did not change, since the average amount of neurons observed did not differ (P > 0.05) among animals from the CG21 (1061 ± 50.72) and EG21 (881.4 ± 38.96) groups, and even among animals from CG70 (881.4 ± 38.96) and EG70 (969.4 ± 82.03).
On the other hand, the number of myenteric neurons present in 8.96 mm2 of jejunum differed (P < 0.05) among 21- and 70-day-old animals, with lower values seen in 70-day-old animals. The decrease of 16.9% among the 21-day-old animals and 17% among 70-day-old animals indicates that the quantity of neurons was not influenced by the provided diet. It is believed that the numbers of neurons does not decrease, but increases during the animals’ growth period, and the observed decreases are only related to the greater dispersion of neurons in the organ[24,25].
In the morphometric analysis, we verified that the average area of cell body of reactive NADH-diaphorase myenteric neurons varied and differed (P < 0.05) during nursing and post-weaning periods. In general, the cell body area of neurons increased in animals from the CG70 (347.4 ± 13.4 μm2) and EG70 (377.4 ± 22 μm2) groups when compared to animals from the CG21 (230 ± 10.9 μm2) and EG21 (282.1 ± 7.0 μm2) groups, respectively.
However, animals that received an autoclaved diet (EG21) during pregnancy and lactation had a significant increase in neuron cell body area (22.65%) (P < 0.05) during the suckling period. On the other hand, there was an increase of 8.65% in the cell body area of neurons for animals from the EG70 group compared to animals from the CG70 group, but this effect was not statistically significant. These data suggest an effect of autoclaved diet on the area of myenteric neurons during the suckling period, which is different from the effect shown in the other study[15], which observed a small reduction in the size of neurons in the myenteric plexus under severe protein restriction.
Thus, an increase in the area of the cell body of myenteric neurons could be a response to nutritional deficiency associated with exposure time or the level of this deficiency. The increase in cell body area was mainly seen in animals from the EG21 group, whose mothers received an autoclaved diet during the pregnancy and lactation period, and this could be a neuronal response in order to remedy possible deficiencies. Neurons can increase metabolic activities to compensate for the decrease in protein quality of the ration, but our results could indicate neurons’ lower ability to achieve maximum expected development during the growth process.
The increase in neuronal size during animal growth is expected[24]. However, the fact that the neurons of animals that received an autoclaved diet during the post-weaning period had a growth level of 33.8% (lower than the 51.5% seen in CG70 animals) may suggest that an autoclaved diet causes a nutritional deficiency that inhibits the normal development of neurons during the post-weaning period. However, tests must be conducted to confirm this assumption.
Based on the solubility analysis of the protein in animal feed in KOH, it was found that the protein solubility level was 47.36% after autoclaving and 80.85% before autoclaving, indicating a decrease in the protein digestibility of 33.49%[8]. This means that the autoclaved diet had a usable protein level of 10.52% compared to the 17.97% of non-autoclaved diet, which falls below the 15% suggested by the National Research Council (NRC)[26] for rats undergoing pregnancy, lactation and growth.
Despite the low level of usable protein in the autoclaved diet (10.52%), it is still higher than the 8% used in some studies examining protein deficiency[14,16,17,22]. However, as the NRC[26] establishes a minimum protein level of 15% for rats during reproduction, pregnancy, lactation and growth periods and a level of 5% for maintenance phase, autoclaving the feed decreases the quality of the protein and may have interfered in neuronal development. This fact is corroborated by the size of neuronal cell bodies of animals that did not receive an autoclaved diet and those that did. Thus, although autoclaving the feed is more desirable, based on cost and safety, than other sterilization processes[4], its use must be considered when the objective of the research is to evaluate the nervous system.
In summary, the use of an autoclaved diet during pregnancy, lactation and post-weaning does not alter the quantity of reactive NADH-diaphorase myenteric neurons in the jejunum but does interfere with the increase in cell body area of neurons, preventing the neuron cell body from reaching a size similar to that observed using a non-autoclaved diet, suggesting a lower metabolic activity for those neurons.
The authors would like to thank Dr. Lilian J Oliveira and Dr. Janaina M Monteiro for their support with the laboratory procedures.
Neurons morphological characteristics influence their functional performance. Most of these characteristics develop early in neuron development, and therefore may be influenced by external factors such as nutritional quality, which must be well known and controlled.
The exposure to high temperature may compromise the components of the ration, destroying vitamins and proteins and affecting the nutritional value of the diet. It occurs during the autoclaving process, altering the expected performance of the provided diet.
Recent studies indicate that factors such as nutritional quality of the ration and animal age may interfere with the morphofunctional aspects of the myenteric plexus compromising digestive system function, and consequently the animal’s performance.
Changes in the nutritional values of autoclaved diets may be considered to correct possible tissue loss during animal growth and development.
The identification of myenteric neurons by the nicotinamide adenine dinucleotide (NADH)-diaphorase histochemical technique occurs through formation of formazan granules from an artificial electron acceptor (nitro blue tetrazolium), allowing the evaluation of respiratory activity of neurons, which provides evidence of their metabolic activity.
This manuscript is an interesting article in an attempt to evaluate the effect of autoclaved diet on the jejunum neurons of the myenteric plexus of rats during their growth phase. The results suggest that changes similar to those observed for protein deficiency occur in rats that have been fed with an autoclaved diet, but that this occurs to a lesser degree than for true protein deficiency.
Peer reviewer: Seng-Kee Chuah, MD, Division of Hepatogastroenterology, Chang Kaohsiung Gang Memorial Hospital, 123, Ta-Pei Road, Niaosung Hsiang, Kaohsiung 833, Taiwan, China
S- Editor Sun H L- Editor O’Neill M E- Editor Zhang DN
| 2. | Neves SP. Manual para técnicos em biotério. 2th ed. São Pulo: Rothschild 1996; . |
| 3. | Keenan KP, Ballan GC, Haught DG, Laraque P. Nutrition. The laboratory rat. London: Academic Press 2000; 453-462. |
| 4. | Tuffery AA. Laboratory animals. 2th ed. Chichester: John Wiley and Sons 1995; . |
| 5. | Coates ME. Diets for germ free animals: sterilization of diets. Laboratory animal handbook. London: Churchill Livingstone 1984; 85-90. |
| 6. | Sgarbieri VC. Proteínas em alimentos protéicos: propriedades, degradações e modificações. São Paulo: Livraria Varella 1996; . |
| 7. | Zhang Y, Parsons CM. Effects of overprocessing on the nutritional quality of peanut meal. Poult Sci. 1996;75:514-518. [PubMed] |
| 8. | Faria HG, Stabille SR. Efeito de diferentes tempos de autoclavagem sobre a qualidade nutricional da ração utilizada para ratos (Rattus norvegicus) em crescimento. Acta Scientiarum. 2001;23:645-648. |
| 9. | Faria HG, Stabille SR, Lee-ng PL, Brito RM, Mota VA. Effect of autoclave diets used for growing rats: digestibility and performance. Acta Scientiarum. 2004;26:113-119. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Deo MG. Cell biology of protein-calorie malnutrition. World Rev Nutr Diet. 1978;32:49-95. [PubMed] |
| 11. | Chaves N. Nutrição básica aplicada. Rio de Janeiro: Guanabara Koogan 1978; . |
| 12. | Santer RM, Conboy VB. Prenatal undernutrition permanently decreases enteric neuron number and sympathetic innervation of Auerbach's plexus in the rat. J Anat. 1990;168:57-62. [PubMed] |
| 13. | Natali MR, Miranda-Neto MH. Effects of maternal proteic undernutrition on the neurons of the myenteric plexus of the duodenum of rats. Arq Neuropsiquiatr. 1996;54:273-279. [PubMed] |
| 14. | Natali MR, Miranda-Neto MH. Effects of maternal proteic undernutrition on the neurons of the myenteric plexus of the duodenum of rats. Arq Neuropsiquiatr. 1996;54:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Castelucci P, de Souza RR, de Angelis RC, Furness JB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat large intestine: a quantitative morphological study. Cell Tissue Res. 2002;310:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Araújo EJ, Sant'Ana Dd Dde M, Molinari SL, de Miranda Neto MH. Effect of protein and vitamin B deficiency on the morpho-quantitative aspects of the myenteric plexus of the descending colon of adult rats. Arq Neuropsiquiatr. 2003;61:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Zanin ST, Molinari SL, Sant'Ana Dde M, de Miranda Neto MH. [NADH-diaphorase positive neurons of the jejunum of disnurtured adult rats (Rattus norvegicus): quantitative aspects]. Arq Neuropsiquiatr. 2003;61:650-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Liberti EA, Fontes RB, Fuggi VM, Maifrino LB, Souza RR. Effects of combined pre- and post-natal protein deprivation on the myenteric plexus of the esophagus of weanling rats: a histochemical, quantitative and ultrastructural study. World J Gastroenterol. 2007;13:3598-3604. [PubMed] |
| 19. | Costa M, Brookes SJ. The enteric nervous system. Am J Gastroenterol. 1994;89:S129-S137. [PubMed] |
| 20. | Costa M; Anfar. Proteína solúvel em KOH. In: Ministério da Agricultura e Reforma Agrária. Métodos analíticos de controle de alimentos para uso animal 1992; 1-3. |
| 21. | Gabella G. Detection of nerve cells by a histochemical technic. Experientia. 1969;25:218-219. [PubMed] [DOI] [Full Text] |
| 22. | Leite-Mello EV, Stabille SR, Miranda Neto MH. Effect of maternal protein deprivation on morphological and quantitative aspects of the myenteric plexus neurons of proximal colon in rats. Arq Neuropsiquiatr. 1997;55:106-113. [PubMed] |
| 23. | Sant'ana Dde M, Miranda Neto MH, de Souza RR, Molinari SL. Morphological and quantitative study of the myenteric plexus of the ascending colon of rats subjected to proteic desnutrition. Arq Neuropsiquiatr. 1997;55:687-695. [PubMed] |
| 24. | Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. J Anat. 1971;109:81-95. [PubMed] |
| 25. | Gaetani S, Mengheri E, Rossi A, Spadoni MA, Tochi G. Long term protein deficiency in adult rats: effects on different proteins of a sympathetic ganglion. Neurochemical Research. 1977;2:439-448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | National Research Council (NRC). Nutrients requirements of laboratory animals. 4th ed. Washington: National Academic Press 1995; . |