INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world and is estimated to cause approximately half a million deaths annually[1]. There are striking differences in the incidence of HCC related to age, gender, race, and geographic region, with hepatitis C virus (HCV) infection acquired 2-4 decades previously explaining at least half of the observed increase in HCC[2]. The survival rates remain generally dismal (median 8 mo)[2]. Undoubtedly, the best available treatment for all liver tumors is complete surgical resection. However, the synthetic somatostatin analogue octreotide has been found effective in inhibiting tumor growth in a variety of experimental models[3,4]. Octreotide did nοt influence hepatic or portal blood flow, although significantly increased reticuloendothelial system activity[5-7]. Apart from stimulation of the reticuloendothelial system, octreotide may have other mechanisms of action, to inhibit the growth of hepatic tumors. One of the mechanisms suggested may be a direct antiproliferative effect, through receptor-mediated growth inhibition[8]. In vitro studies have shown that octreotide demonstrates high affinity binding tοwards somatostatin receptors sstr2, sstr3 and sstr5, while nο binding affinity is found towards receptors sstrl and sstr4[9-11]. It has been reported that octreotide inhibits the proliferation and induces apoptosis of different HCC cell lines in vitro[12-18]. The mechanisms of apoptosis induction however are not well understood.
Tumor necrosis factor-α (TNF-α) is a well established as a means of apoptosis induction in a variety of cell types through specific responsive receptors, but other cells require transcriptional arrest[19]. Hepatoma cells treated with TNF-α and cycloheximide (CHX) undergo apoptosis, which is preceded by a strong activation of c-jun N-terminal kinase[20]. The human HCC cell line, SMMC-7721, was insensitive to TNF-α cytotoxicity, but quickly underwent apoptosis in the presence of TNF-α and CHX[21].
Apoptosis is a complex process characterized by caspase activation, cell shrinkage, chromatin condensation and internucleosomal DNA fragmentation[22-25]. Caspases are cysteine-containing aspartic acid-specific proteases and all have similar site-specific proteolytic activity. Caspases are divided into 3 distinct groups based on their substrate specificities. Group I (YVADase) includes caspase-1, -4 and -5 which are involved in cytokine production. Group II (DEVDase) caspases, e.g. caspase-3 and -7 are the main effector caspases during apoptosis. These are cleaved by group III (IETDase) caspases (e.g. caspase-6, -8, -9 or -10) early in the onset of apoptosis[26]. Upon activation, group II caspases act on various cell proteins[26]. Most, but not all, events in apoptosis appear to require a caspase-mediated proteolytic step[25].
Octreotide has been clinically used for treatment of HCC, with conflicting results. Both increased survival[27,28] and no effect[29,30] have been reported. Although negative studies have been criticized[31], the mechanisms by which octreotide may act have not been adequately clarified. Apoptosis may be a fundamental mechanism. In this study, we examined the effect of octreotide on cellular proliferation, apoptosis and caspase activation in HepG2 HCC cells. The model of TNF-α-induced apoptosis was chosen for comparison with octreotide in a study of the biological behavior of caspases, after treatment of HepG2 cells with octreotide.
MATERIALS AND METHODS
Octreotide was from Novartis (Basel, Switzerland) and was used at concentrations of 10-10 mol/L to 10-7 mol/L, to identify the optimal concentration for inhibition of cell proliferation. Incubations with TNF-α (R&D Systems, Minneapolis, USA) were made at concentrations from 0.1 to 100 ng/mL (0.1, 1, 10, 20, 100 ng/mL). According to the proliferation curve, the suitable concentrations for further experiments were 10-8 mol/L for octreotide and 20 ng/mL for TNF-α. These concentrations were used for all further combinations and measurements of apoptotic features.
Cell culture and incubation conditions
The HepG2 cell line is a human hepatocyte carcinoma cell line derived from a well-differentiated human hepatoblastoma and was purchased from the European Collection of Cell Cultures (ECACC, Porton Down, UK). HepG2 cells are maintained in continuous culture in our laboratory in RPMI supplemented with 10% fetal bovine serum (FBS, Gibco, Paisey, UK), at 37°C and in an atmosphere of 5% CO2. For experiments, HepG2 cells were seeded in 24-well plates at a density of 2 × 104/cm2.
Twenty four hours before the experiment, they were cultured in fresh medium without FBS, and then treated with different concentrations and combinations of all substances. Incubations were made at 37°C in 5% CO2. Supernatants were collected and stored in -80°C, while cell extracts where used for measurements of caspase activity. Control medium was complete media with 10% FBS. HepG2 cells cultured in control medium are referred to as control cells.
Proliferation assays
For measurement of growth inhibition, the sulforhodamine B colorimetric assay (SRB Assay; Biotium Inc., Hayward, CA, USA) was used, as previously described[32,33]. HepG2 cells were plated in 96-well plates, at an initial density of 5 × 103 cells, with 200 μL medium per well. All substances were added to cultures 1 d after seeding, in order to obtain best attachment of the cells at the beginning of the experiments. Cells were grown for a total of 6 d, with a change of medium and substances on the third day after treatment. Measurements were made as described in the original protocol. Briefly, 50 μL of 50% trichloroacetic acid were placed into the 200 μL medium and plates were stored at 4°C for 30 min. After washing 5 times with deionized water, plates were left to dry for 24 h at room temperature. Then, 70 μL of 0.4% sulforhodamine B in 1% acetic acid were placed in every well and left at room temperature for 20 min. Before air drying for a second time, plates were washed 5 times with 1% acetic acid. At the end of the procedure, 200 μL of unbuffered Tris-base solution (pH 10.5) were added to each well and measurements were made at 490 nm, subtracting the background at 620 nm. The mean of the optical densities of 8 different controls was considered to be 100% and all other values were expressed as a percentage of the controls.
Detection of apoptosis
DNA fragmentation: For detection of apoptosis, a sandwich, one step, colorimetric enzyme-linked immunosorbent assay, the Cell Death Detection ELISA Plus kit (Roche Diagnostics, Mannheim, Germany) was used. The assay allows for the specific determination of histone-complexed DNA fragments (mono and oligonucleosomes) from the cytoplasm of cells, after the induction of apoptosis. Briefly, after induction of apoptosis and 24-h incubation, the cells were pelleted by centrifugation (200 g, 10 min) and the supernatants (containing DNA from necrotic cells that leaked through the membrane during incubation) were discarded. Cells were resuspended and incubated for 30 min in lysis buffer. After lysis, intact nuclei were pelleted by centrifugation. Aliquots of the supernatants were transferred to a streptavidin-coated well of a microtiter plate with 2 monoclonal antibodies, antihistone (biotin-labeled) and anti-DNA (peroxidase-conjugated), so that nucleosomes in the supernatant created antibody-nucleosome complexes, which were continuously bound to the microtiter plate by the streptavidin. All samples were then incubated with peroxidase substrate and absorbance was measured at 405 nm. The mean of the optical densities of 8 different controls was considered to be 100% and all other values were expressed as a percentage of the controls.
Annexin-V/propidium iodide staining: For better evaluation of apoptotic features, the modified Annexin-V Apoptosis Detection Kit (BioVision, Mountain View, CA, US) was used, which is based on the observation that soon after initiation of apoptosis, cells translocate the membrane phosphatidylserine (PS) from the inner face of the plasma membrane to the cell surface, but they also shrink, increasing their side scatter (SS) and reducing their forward scatter (FS) characteristics. Once on the cell surface, PS can be easily detected by staining with a fluorescent conjugate of Annexin-V, that has a high affinity for PS. Cells that have bound Annexin-V-fluorescein isothiocyanate (FITC) (early apoptotic) show green staining in the plasma membrane, while cells that have lost membrane integrity will show red staining [propidium iodide (PI)] throughout the nucleus and a halo of green staining (FITC) on the cell surface (late apoptotic or necrotic cells).
After treatment and 24-h incubation in 24-well plates, adherent HepG2 cells were gently trypsinized and washed once with serum-containing medium. Then, 1-5 × 105 cells were collected by centrifugation (98 g, 5 min) and resuspended in 300 μL of 1 × Binding Buffer. After gentle pipetting to resuspend the cell pellets, 3 μL of Annexin-V-FITC and 3 μL of 50 μg/mL PI, were added, followed by a 5-min incubation at room temperature in the dark. Annexin-V-FITC binding was analyzed by flow cytometry (Epics Elite) (Ex = 488 nm; Em = 530 nm) using a FITC signal detector and PI staining by the phycoerythrin emission signal detector. Debris was excluded by scatter gating (forward vs side). At least 10 000 events were counted for each sample.
Caspase activity
The activities of caspase-3, caspase-9, caspase-8 and caspase-2 were measured. For the evaluation of caspase activity, colorimetric activity assay kits (Chemicon, Temecula, CA, USA) were used. The assays are based on spectophotometric detection of the chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate DEVD-pNA (caspase-3), LEHD-pNA (caspase-9), IETD-pNA (caspase-8) and VDVAD-pNA (caspase-2), respectively. Briefly, after treatment and 24-h incubation, supernatants were collected and cells were resuspended in 250 μL of chilled lysis buffer and incubated on ice for at least 10 min. After centrifugation (5 min, 10 000 g), supernatants (cytosolic extracts) were transferred to a fresh tube and placed on ice. The protein concentration for each sample set was assayed with BIORAD Protein assay (BIORAD, Munchen, Germany)[34]. Samples were incubated for 2-3 h at 37°C and measured at 405 nm, as indicated. The absorbance of pNA from every sample was compared with the uninduced controls and values were expressed as μmol/L of pNA per microgram of cytosolic protein (μmol/L per microgram).
Statistics
Statistical analysis was performed using Microsoft Excel 2007 and Instat software (GraphPad software inc., San Diego, California, USA). Results are expressed as mean ± standard error of the mean (SE). The Kolmogorov and Smirnov test was used to check the Gaussian distribution of data. Statistical comparisons were performed using one-way analysis of variance with Tukey’s post hoc comparisons. The non parametric Kruskal-Wallis test was used instead if Bartlett’s test indicated a significant difference between standard deviations. P < 0.05 was considered statistically significant.
RESULTS
Effect of octreotide on HepG2 proliferation
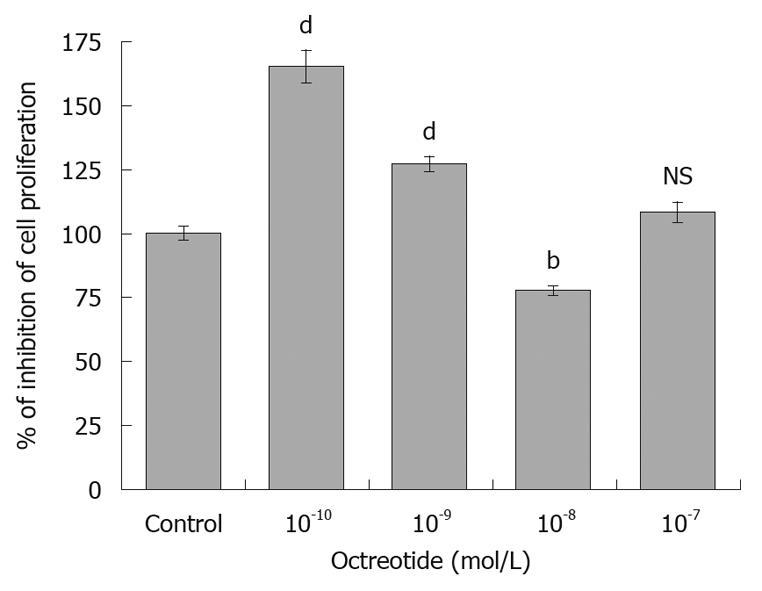
Octreotide caused an initial increase in HepG2 proliferation (165.2% ± 6.2% and 127% ± 3% with octreotide 10-10 mol/L and 10-9 mol/L, respectively) followed by a significant inhibition at a concentration of 10-8 mol/L (concentration expected in the blood of patients receiving treatment), reducing cellular proliferation to 77.5% ± 1.9% of control (Figure 1). No difference was observed at a concentration of 10-7 mol/L (108.3% ± 3.8%).
Figure 1 Octreotide at a concentration of 10-8 mol/L had a statistically significant inhibitory effect on cellular proliferation of HepG2 hepatocellular carcinoma cells, compared to untreated cells.
Lower concentrations caused an initial increase in proliferation. The results represent the mean of 8 different experiments ± SE (bP < 0.01, dP < 0.001). NS: Not significant.
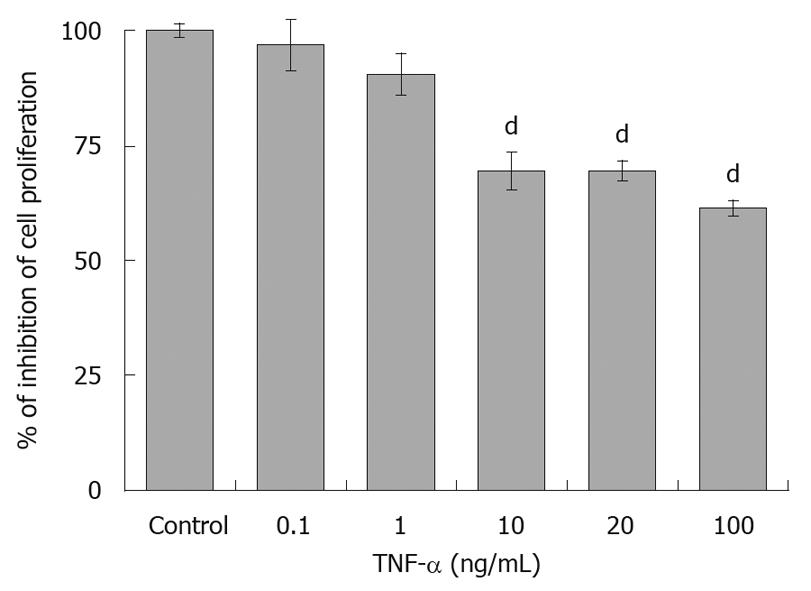
The effect of TNF-α was also examined, at concentrations from 0.1 ng/mL to 100 ng/mL. At the low concentrations of 0.1 and 1 ng/mL, TNF-α had no significant effect on the proliferation of HepG2 cells (96.9% ± 5% and 90.6% ± 4.5%, respectively), after 6 d of incubation. A marked inhibitory effect was detected with 10, 20 and 100 ng/mL TNF-α, which reduced cellular proliferation to 69.4% ± 4%, 69.6% ± 2.3% and 61.4% ± 1.7% of control, respectively (Figure 2). However, because TNF-α at a concentration of 20 ng/mL had the optimum inhibitory effect in another HCC cell line (SMMC-7721 cells)[19], this concentration was selected for further experiments.
Figure 2 Tumor necrosis factor-α at concentrations of 10, 20 and 100 ng/mL had a statistically significant inhibitory effect on cellular proliferation of HepG2 cells, compared to untreated cells.
The results represent the mean of 8 different experiments ± SE (dP < 0.001). TNF-α: Tumor necrosis factor-α.
Effect of octreotide on HepG2 apoptosis
Apoptosis was detected based on determination of histone-complexed DNA fragments (mono and oligonucleosomes) from the cytoplasm of apoptotic cells. Similar non significant detection of DNA fragmentation was noted after 24-h treatment of HepG2 cells with either octreotide or TNF-α (115.2% ± 6.95% and 115.2% ± 8.17%, respectively) (Figure 3).
Figure 3 Detection of DNA fragmentation revealed a non significant increase in DNA fragments, after 24-h treatment with octreotide or tumor necrosis factor-α (n = 8).
TNF-α: Tumor necrosis factor-α; NS: Not significant.
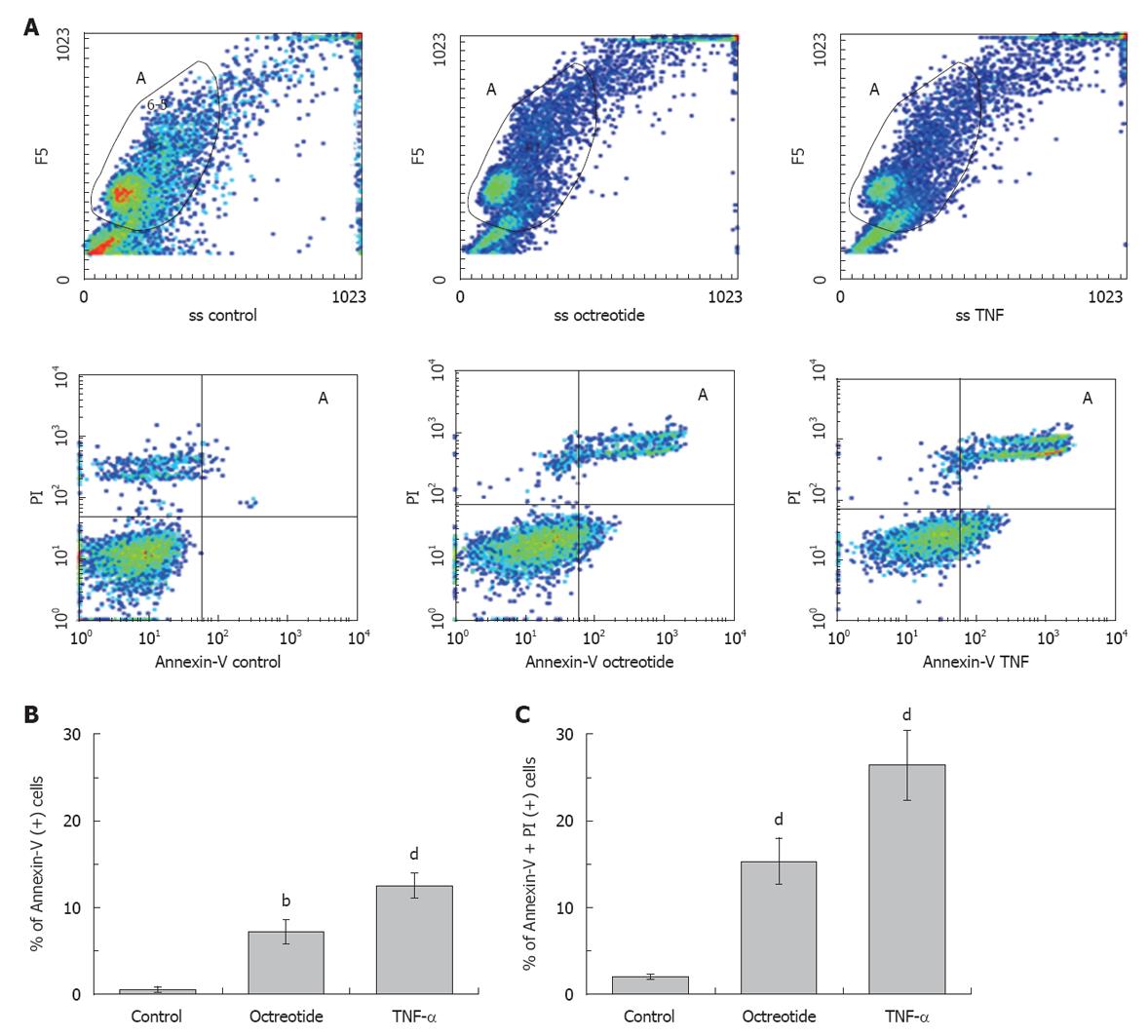
Necrotic cells should be visualized as double positive cells of large dimensions, as they rapidly lose membrane integrity and swelling occurs before destruction, or as fractured membranes of low FS and SS, PI only positive or double positive, but in the place where debris is usually detected and excluded. We also observed that all double positive cells had very small dimensions (data not shown), although they were still intact. We considered double positive cells as cells that followed the apoptotic rather than the necrotic procedure, having increased their SS and decreased their FS characteristics, but still retaining relatively small dimensions. That was the reason why we followed a specific gating strategy for analysis. Octreotide caused a significant increase in early apoptosis (7.2% ± 1.4%, P < 0.01, Annexin-V positive cells) and a highly significant increase in late apoptosis (15.3% ± 2.7%, P < 0.001, Annexin-V/PI double positive cells). TNF-α significantly increased early (12.5% ± 1.4%, P < 0.001) and more so late apoptosis (26.4% ± 4%, P < 0.001, Figure 4). All comparisons were made with untreated HepG2 cells used as control cells (0.5% ± 0.3% and 2 ± 0.3% for early and late apoptosis, respectively) (Figure 4).
Figure 4 The apoptotic effect of octreotide and tumor necrosis factor-α alone is shown.
A: Octreotide and tumor necrosis factor-α (TNF-α) significantly increased early (Annexin-V only positive, right lower quadrant) and more so late apoptotic cells [Annexin-V and propidium iodide (PI) positive, right upper quadrant]. Every sample was analyzed with the same gating strategy (Gate A) to exclude debris and non-specific binding of Annexin-V, while control refers to uninduced HepG2 cells; B: Annexin-V positive cells; C: Annexin-V/PI positive cells. B and C: Mean of 8 different experiments ± SE (bP < 0.01, dP < 0.001).
Effect of octreotide on caspase activity in HepG2 cells
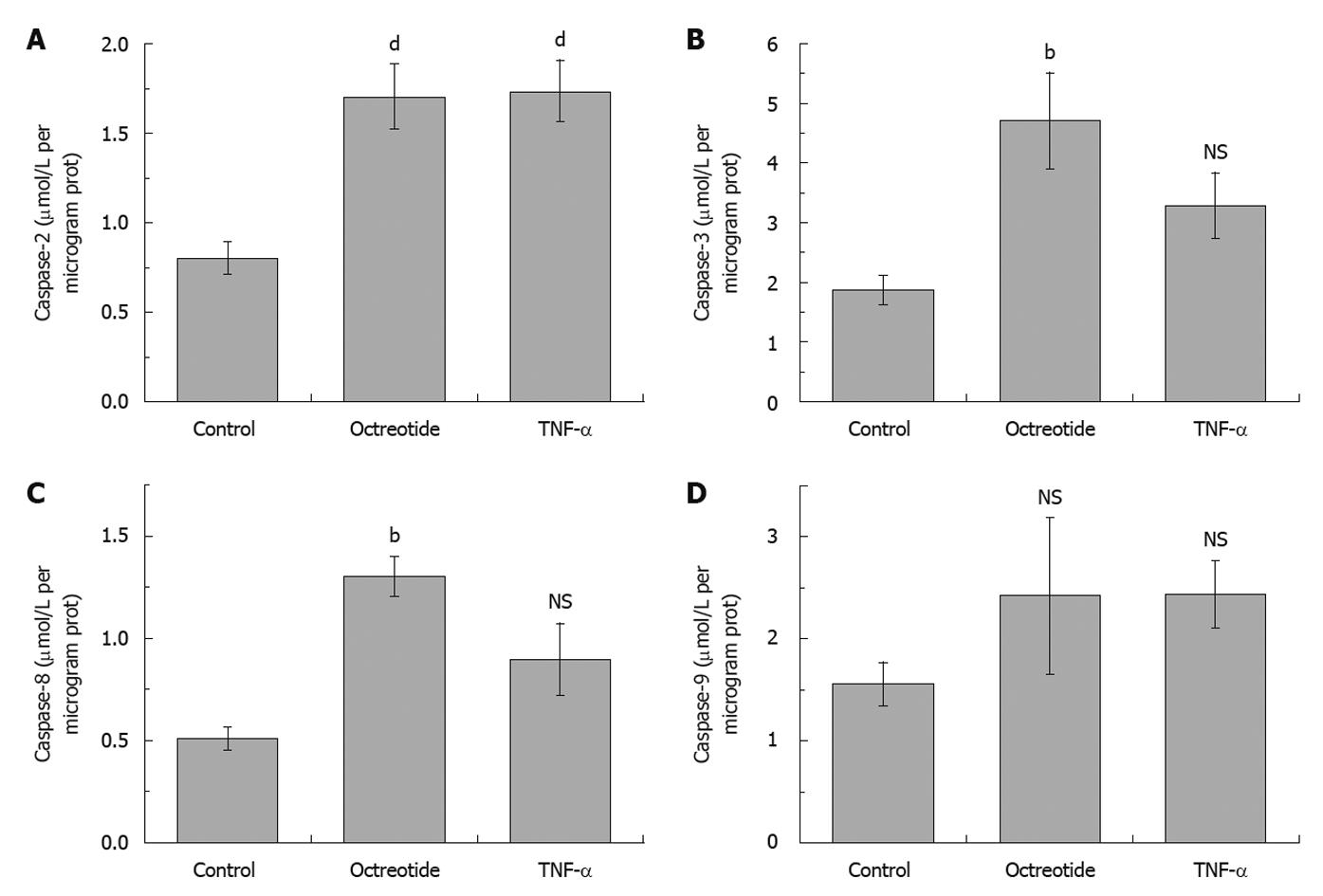
Caspase-3 activity was significantly increased after treatment of HepG2 cells with octreotide (4.71 ± 0.81 μmol/L per microgram protein, P < 0.01) alone, while after TNF-α only, a non significant increase was found (3.28 ± 0.55 μmol/L per microgram protein), compared with uninduced cells (1.87 ± 0.24 μmol/L per microgram protein) (Figure 5). A small but not significant increase in caspase-9 activity was detected after treatment of HepG2 cells with TNF-α (2.44 ± 0.33 μmol/L per microgram protein) or octreotide alone (2.42 ± 0.77 μmol/L per microgram protein) (Figure 5), compared to uninduced cells (1.56 ± 0.21 μmol/L per microgram protein). TNF-α caused a non significant increase in caspase-8 activity (0.9 ± 0.18 μmol/L per microgram protein) compared with control cells (0.51 ± 0.06 μmol/L per microgram protein) (Figure 5). However, octreotide caused a significant increase (1.3 ± 0.1 μmol/L per microgram protein, P < 0.01). TNF-α and octreotide caused a significant increase in caspase-2 activity (1.73 ± 0.17 μmol/L per microgram protein and 1.7 ± 0.18 μmol/L per microgram protein, respectively, P < 0.001), compared with control cells (0.8 ± 0.09 μmol/L per microgram protein) (Figure 5).
Figure 5 Caspase-2 (A), -3 (B), -8 (C) and -9 (D) activities, after treatment of HepG2 cells with 10-8 mol/L octreotide and 20 ng/mL tumor necrosis factor-α, compared to untreated cells.
The results represent the mean of 10 different experiments ± SE (bP < 0.01, dP < 0.001). TNF-α: Tumor necrosis factor-α; NS: Not significant.
DISCUSSION
Clinical studies of non-neuroendocrine tumors demonstrate that octreotide can inhibit the growth of a variety of tumors, either directly, through binding on the sstrs of tumor cells, or indirectly, through an immunomodulatory or an antiangiogenic effect[35-37]. Several reports indicate that octreotide inhibits the proliferation and induces apoptosis of HCC cells in vitro[12-18]. In this study we confirmed that octreotide inhibits HepG2 proliferation[12,15,18], but only at a concentration of 10-8 mol/L, although an initial increase at lower concentrations was observed.
In contrast to these findings, there are also reports that proliferation of HCC cells or hepatic stellate cells is not affected by octreotide[38,39]. In the study of Reynaert et al[39], shorter periods of culture compared to ours were used, while activation of sstrs was achieved with individual synthetic agonists, therefore a possible combined effect of concomitant receptor activation may have been missed. Similarly, clinical trials have demonstrated a survival benefit of patients with inoperable HCC treated with octreotide[27,28], but also negative studies have been published[29,30] and recently criticized[31]. Interestingly, in our study, lower concentrations of octreotide increase proliferation and this is possibly an additional reason for divergent results in both clinical trials and in vitro studies of octreotide in HCC. Our findings also indicate that measurements of serum octreotide levels may be important, at least in clinical trials, to verify optimal therapeutic drug concentrations.
Octreotide binds mainly to sstr2, sstr3 and sstr5[40], the presence of which has been recently documented in HepG2 cells[12,41]. The antiproliferative effect of octreotide is thought to be mediated by sstr2[42] and sstr5[43]. Even when a significant amount of sstr2 binding in cellular membranes is not evident, it is possible that octreotide is internalized either along with sstr2 or alone as reported by Dournaud et al[44] and Hornick et al[45]. Recently a desensitization of sstr2 has been reported after short term incubation of an HCC cell line with octreotide, which is probably reversed after long term incubation[46]. We have previously reported an IC50 of 1.25 nmol/L for the antiproliferative effect of octreotide for HepG2 cells[41] which is within the range of IC50 for sstr2 but it is lower from the IC50 reported for sstr5. In the case of sstr5, it is possible that a biological effect can be achieved without activation of the total number of receptors.
The antiproliferative effect of octreotide may be due to either cell necrosis or cell apoptosis[47]. Therefore, we investigated the apoptotic effect of octreotide, particularly in association with caspase activation, comparing this effect with the well-described pathways of TNF-α-mediated apoptosis. The machinery of apoptosis includes death receptors, adaptor proteins and proteolytic enzymes (caspases). Death receptors belong to the tumor necrosis factor receptor gene superfamily. Among these receptors, TNF receptor-1 (TNFR1) and Fas (CD95) are the most extensively characterized, and both are abundantly expressed in liver[20,48]. TNF-α at 20 ng/mL induced apoptosis in human hepatoma cell line SMMC-7721 in vitro, which was exacerbated by the hypoxanthine-xanthine oxidase system and CHX, but alleviated by superoxide dismutase, suggesting that TNF-α-induced apoptosis may be due to oxidative stress[49]. The SMMC-7721 cell line was insensitive to TNF-α cytotoxicity and underwent apoptosis quickly in the presence of TNF-α and CHX[21]. In accordance with this study, the optimal concentration of TNF-α was also found to be 20 ng/mL for HepG2 cells in our study.
Our findings with flow cytometry showed that both octreotide and TNF-α induced a significant early and late apoptosis of HepG2 cells. DNA fragmentation measurements also demonstrated a non significant induction of apoptosis. This possibly means that flow cytometry is a more sensitive method for quantification of apoptosis.
The mechanism by which octreotide induces apoptosis is not well understood. Changes in sstr expression because of downregulation or possible heterodimerization[27,50] of a receptor, together with changes in the expression of regulatory proteins required for correct trafficking of specific sstr subtypes, could affect the direct antitumor effect of octreotide, which has been previously demonstrated in models expressing sstr2[51,52]. Mediated by sstr2, octreotide upregulates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), death receptor 4 (DR4) and TNFR1, and downregulates Bcl-2, which results in apoptosis[53]. Mediated by sstr3, octreotide upregulates p53[47] or induces Bcl-2-associated protein Bax[47,54] and acidic endonuclease, resulting in apoptosis. In addition to these mechanisms, in this study we demonstrated a direct effect of octreotide on caspase activation. The effect of octreotide on caspase-mediated apoptosis, is limited to caspase-3 activation, as the main effector caspase supportive of apoptosis and it was detected in primary pheochromocytoma cells[55], in radiation-induced intestinal damage[56] and in activated lymphocytes[57]. Interestingly, decreased caspase-3 mRNA expression in Kupffer cells also indicates a possible additional beneficial effect of octreotide in HCC, through an antiapoptotic effect on Kupffer cells[58].
We suggest a caspase-mediated apoptotic pathway after treatment of HCC cells with octreotide, where, unlike TNF-α induced apoptosis, 3 out of 4 caspases tested were significantly increased. The activation of all caspases indicates a possible mitochondria-dependent apoptotic pathway. In contrast, findings from TNF-α-induced apoptosis possibly indicate a different pathway.
A TNF-α-mediated pathway is reported to activate caspase-8, which promotes cleavage of various downstream caspases, including caspases-3, -6 and -7. Caspase-8 can also cleave the Bcl-2 homologue Bid to reveal an active truncated Bid fragment inducing cell death through a mitochondrial pathway[59-62]. However in our study, caspase-8, caspase-3 and caspase-9 were all increased by TNF-α, but this was not statistically significant. Furthermore, caspase-2 was significantly increased by TNF-α, a finding not reported before. In a previous study, we presented a TNF-α-induced increase of caspase-2, but this increase did not reach the statistical significance[63]. In the present study, with an increased number of experiments, we found a significant increase in caspase-2 by TNF-α in HepG2 cells. This may be related to inefficient cleavage of Bid, so that all caspases can be significantly activated[59].
Caspase-2 seems to play critical and specific roles in programmed cell death[64]. It has been difficult to assign caspase-2 to the effector or initiator caspase groups. Cytokine-induced and stress-induced apoptosis act through conceptually similar pathways in which mitochondria are amplifiers of caspase activity rather than initiators of caspase activation[65]. In our study, caspase-2 appeared to be activated independent of significant (octreotide) or non-significant (TNF-α) activation of the mitochondria-mediated pathway. This may have been the result of intracellular events (such as pH or stress) or feedback activation by effector caspases (such as caspase-3).
Thus, our findings suggest that in HepG2 cells octreotide probably causes apoptosis by a mitochondrial apoptosis pathway, sequentially implicating caspase-8, -2, -9 and -3, although further experiments are required to define the exact initiator pathway. TNF-α on the other hand seems to induce caspase-2 activation, possibly mediated through oxidative stress, as suggested before[65]. The non-significant activation of the extrinsic pathway (caspase-8) or of the intrinsic pathway (caspase-9), perhaps due to inefficient Bid cleavage, is maybe the cause of the resistance observed in previous studies and of the eliminated TNF-α-mediated apoptotic effects observed in our study.
In summary, our results support the induction of a caspase-mediated apoptotic pathway by octreotide in HCC cells, implicating both the receptor-mediated and the mitochondrial-apoptotic pathway. The correlation of specific apoptotic, caspase-mediated pathways, with the expression of sstrs in HCC cells needs more investigation to better define and clarify the intracellular mechanisms of the antiproliferative effects of octreotide.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world and is estimated to cause approximately half a million deaths annually. Undoubtedly, the best available treatment for all liver tumors is complete surgical resection. However, the synthetic somatostatin analogue octreotide has been found effective in inhibiting tumor growth in a variety of experimental models.
Research frontiers
Apart from stimulation of reticuloendothelial system, octreotide may have other mechanisms of action, to inhibit the growth of hepatic tumors. It has been reported that octreotide inhibits the proliferation and induces apoptosis of different HCC cell lines in vitro. The mechanisms of apoptosis induction however are not well understood.
Innovations and breakthroughs
Several reports indicate that octreotide inhibits the proliferation and induces apoptosis of HCC cells in vitro. In this study, the authors confirmed that octreotide inhibited HepG2 proliferation at a concentration of 10-8 mol/L. Interestingly, lower concentrations of octreotide increased proliferation and this is possibly an additional reason for divergent results in both clinical trials and in vitro studies of octreotide in HCC. Also, their results support the induction of a caspase-mediated apoptotic pathway by octreotide in HepG2 cells, implicating both a receptor-mediated and mitochondrial-apoptotic pathway.
Applications
The findings of the present study indicate that measurements of serum octreotide levels may be important, at least in clinical trials, to verify optimal therapeutic drug concentrations. Also, based on the recently documented presence of sstr2, sstr3 and sstr5 in HepG2 cells, the need for further correlation of specific apoptotic, caspase-mediated pathways, with the expression of somatostatin receptors in HCC cells, is highlighted, to better define and clarify the intracellular mechanisms of the antiproliferative effects of octreotide.
Peer review
The authors evaluated the role of octreotide on cellular proliferation and apoptosis of HepG2 cells. Their results support the induction of a caspase-mediated apoptotic pathway by octreotide in HepG2 cells, implicating both a receptor-mediated and a mitochondrial-apoptotic pathway. They, also, indicated that measurements of serum octreotide levels may be important, at least in clinical trials, to verify optimal therapeutic drug concentrations.