Published online Jul 28, 2011. doi: 10.3748/wjg.v17.i28.3335
Revised: November 29, 2010
Accepted: December 6, 2010
Published online: July 28, 2011
AIM: To evaluate the relationship between hepatic fat infiltration and abdominal fat volume by using computed tomography (CT).
METHODS: Three hundred and six patients who visited our obesity clinic between November 2007 and April 2008 underwent fat protocol CT scans. The age range of the patients was 19 to 79 years and the mean age was 49 years. The male to female ratio was 116:190. Liver and spleen attenuation measurements were taken with three regions of interests (ROIs) from the liver and two ROIs from the spleen. Hepatic attenuation indices (HAIs) were measured as follows: (1) hepatic parenchymal attenuation (CTLP); (2) liver to spleen attenuation ratio (LS ratio); and (3) difference between hepatic and splenic attenuation (LSdif). Abdominal fat volume was measured using a 3 mm slice CT scan starting at the level of the umbilicus and was automatically calculated by a workstation. Abdominal fat was classified into total fat (TF), visceral fat (VF), and subcutaneous fat (SF). We used a bivariate correlation method to assess the relationship between the three HAIs and TF, VF, and SF.
RESULTS: There were significant negative correlations between CTLP, LS ratio, and LSdif with TF, VF, and SF, respectively. The CTLP showed a strong negative correlation with TF and VF (r = -0.415 and -0.434, respectively, P < 0.001). The correlation between CTLP and SF was less significant (r = -0.313, P < 0.001).
CONCLUSION: Fatty infiltration of the liver was correlated with amount of abdominal fat and VF was more strongly associated with fatty liver than SF.
- Citation: Jang S, Lee CH, Choi KM, Lee J, Choi JW, Kim KA, Park CM. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol 2011; 17(28): 3335-3341
- URL: https://www.wjgnet.com/1007-9327/full/v17/i28/3335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i28.3335
Fatty liver is a disease in which excess fat, mainly triglycerides, accumulates to comprise more than 5% of the weight of the liver[1]. For patients without any history of excessive alcohol ingestion this condition is called non-alcoholic fatty liver disease (NAFLD). NAFLD is pervasive worldwide and its clinicopathologic spectrum ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which can advance to cirrhosis[1,2]. NAFLD is common in the obese and is correlated with type 2 diabetes mellitus (DM), dyslipidemia, and hypertension. Together, these abnormalities comprise insulin resistance syndrome (metabolic syndrome) and increase the risk of cardiovascular disease[3]. Among similar disease entities, abdominal obesity is more highly correlated with metabolic risk, independent of whole-body obesity. Within the category of abdominal obesity, visceral fat (VF) is more strongly correlated with metabolic risk than is subcutaneous fat (SF)[4].
In routine ultrasonography (US), computed tomography (CT), and magnetic resonance (MR) studies, we often detect fatty liver in patients who have normal body mass indices (BMIs). We also often find normal liver parenchyma in obese patients. As such, it is highly probable that fatty liver might be more strongly correlated with abdominal VF than with total fat (TF).
Histologic confirmation is the gold standard for diagnosing fatty liver[5]. However, biopsies are invasive, induce pain and require six or more hours of bed rest; they also modestly increase the risk of mortality.[6] Given the potential risks, biopsies are not performed in all patients. As a substitute for biopsy, imaging techniques, including US, CT, and MR, are now widely used. Of these, CT had been chosen as the method for this study[6]. CT attenuation values of the liver were strongly correlated with histological evidence of hepatic steatosis[7]. Hepatic attenuation was a reliable indicator of fatty liver if it was considerably lower than splenic attenuation[8]. Therefore, CT can be used as a non-invasive test to confirm the presence of hepatic steatosis.
To the best of our knowledge, no prior studies have explored the relationship between fatty liver and abdominal fat using CT. The purpose of this study was to identify any possible correlations between hepatic fat infiltration expressed as a CT liver attenuation value [in Hounsfield units (HU)] and abdominal fat volume, which was also measured directly from CT.
This prospective study included a total of 414 patients (160 men and 254 women; mean age, 50.19 years, ranging from 19 to 80 years) who visited our obesity clinic for self-perception of obesity from November 2007 to April 2008. Information including sex, age, height, body weight (WT), BMI, waist-hip ratio (WHR), history of alcohol intake, systolic and diastolic blood pressure, triglyceride level (TG), and low-density lipoprotein (LDL) was collected for each patient. Any patient with a history of significant alcohol consumption, bile duct dilatation, hepatic mass, hepatitis, liver cirrhosis, or history of hepatic surgery was excluded. A fat protocol CT was also conducted on each patient. Finally, the total number of actual participating patients was 306 (116 men and 190 women; mean age 49 years, ranging from 19 to 79 years). According to World Health Organization, a BMI over 25 kg/m2 is defined as overweight, and a BMI of over 30 kg/m2 as obese. 186 of the 306 total patients were normal; 95 of the 306 patients were overweight; 25 of the 306 total patients were obese.
All patients provided written informed consent and the Korea University Institutional Review Board approved this study protocol in accordance with the Declaration of Helsinki of the World Medical Association.
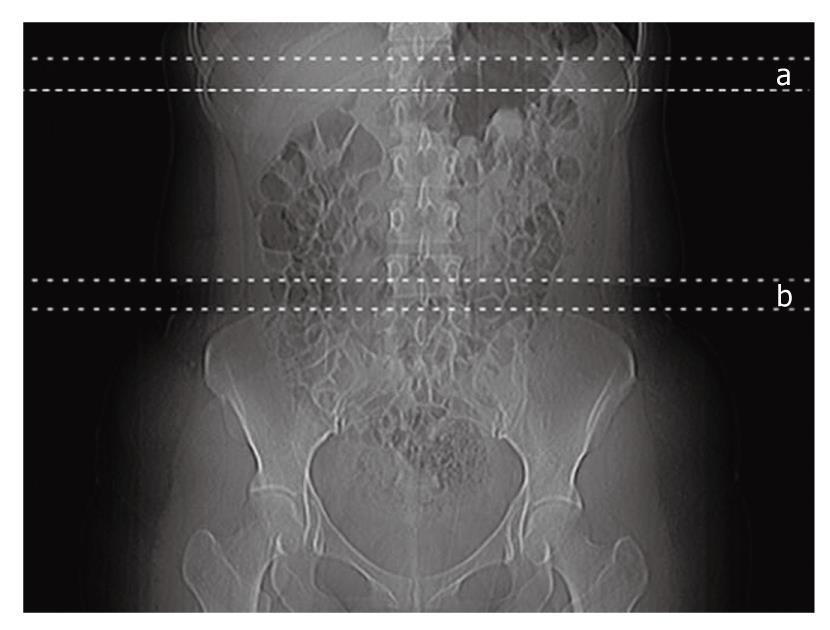
The attenuation of the liver and the spleen were measured using CT scans taken without intravenous contrast agent administration (Brilliance 64; Philips Medical Systems, Cleveland, OH, USA). The examination was done with a tube voltage of 120 kVp, a tube current of 50 mAs, and a tube rotation time of 750 ms. With the subject in the supine position, seven serial axial slices 3 mm in thickness were taken at approximately the mid-portion of the liver shadow on topogram (Figure 1).
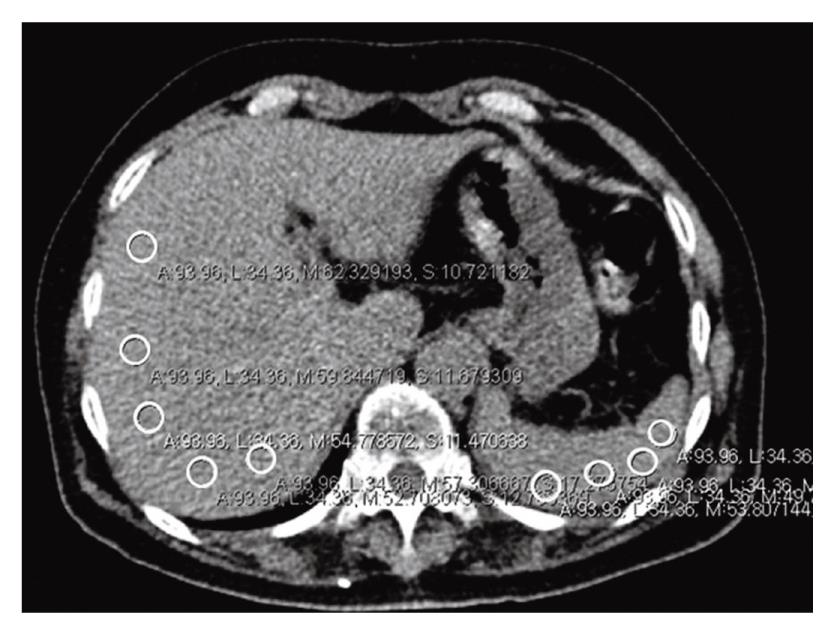
Among the seven serial slices, we chose one image for measurement of the hepatic and splenic attenuation in each patient. Five regions of interests (ROIs) were identified in the liver, avoiding vessels, bile ducts, calcifications, and artifacts; four ROIs were identified in the spleen in the same manner. The highest and lowest values were excluded when calculating the mean attenuation values of the liver and spleen. As such, three liver values and two spleen values were used to calculate the mean values (Figure 2).
We derived hepatic attenuation indices (HAIs) from the calculated mean attenuation values of the liver and spleen. The HAIs included: (1) hepatic parenchymal attenuation (CTLP; mean attenuation of the liver); (2) liver to spleen attenuation ratio (LS ratio; mean attenuation of the liver/mean attenuation of the spleen); and (3) difference between hepatic and splenic attenuation (LSdif; mean attenuation of liver - mean attenuation of spleen)[7].
The subjects were divided into two groups according to their LSdif. LSdif is among the popular reference values for grading fatty liver with CT[8]. Patients with an LSdif greater than 5 were classified as normal; 249 of 306 (81.4%) patients were included in this group. The remaining 57 patients with an LSdif less than 5 were classified as having fatty liver.
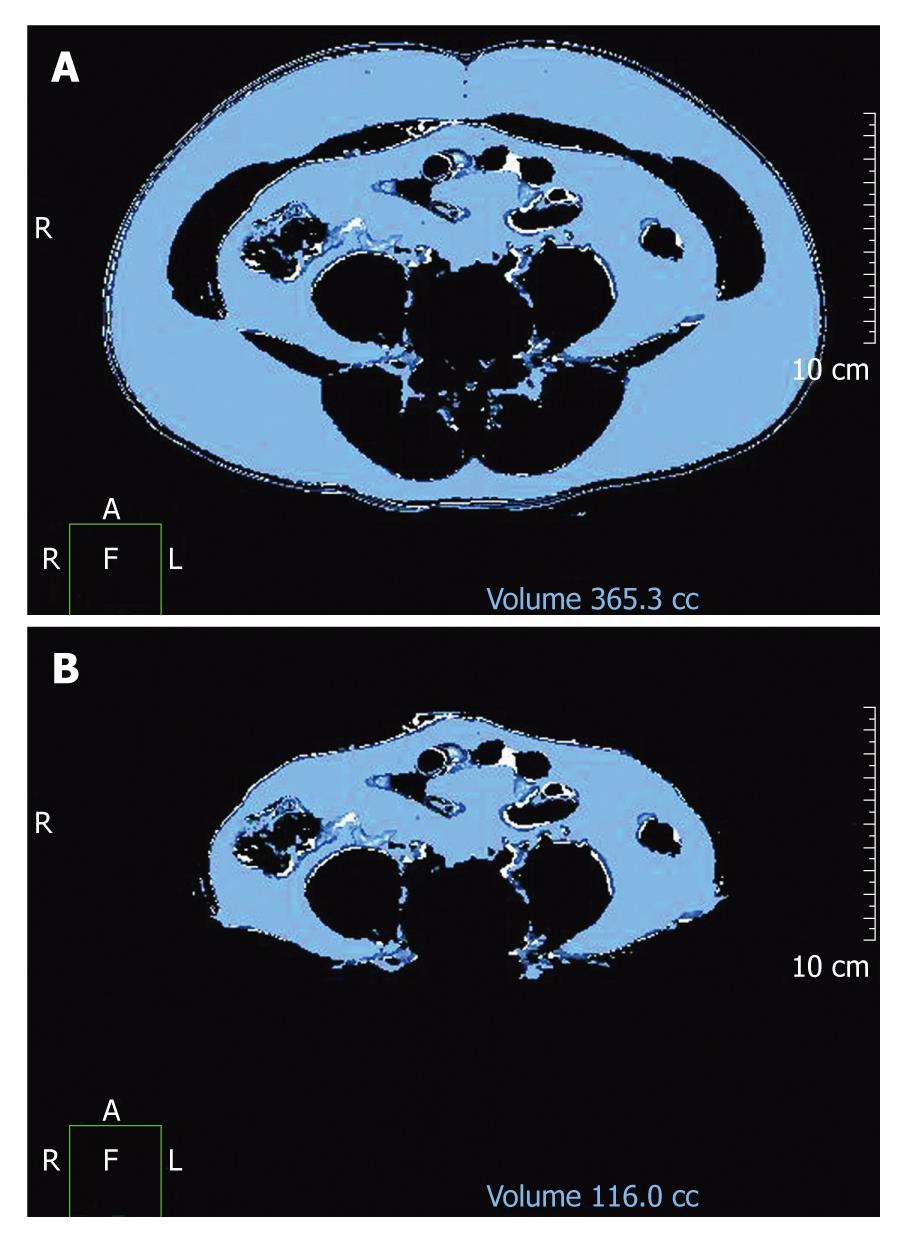
Abdominal fat distribution analysis was conducted on all patients. Starting at the level of the iliac crest, 7 slices 3 mm in thickness were scanned in a superior direction (Figure 1) to measure the VF and TF areas. The iliac crest corresponds to the L4/5 level and the level of the umbilicus. The cross-sectional surface areas (cm2) of different abdominal fat compartments were automatically analyzed in three dimensions using commercially available CT software (Philips EBW2 version 3.0). The adipose tissue area was determined electronically by setting the attenuation values for a region of interest within a range of -175 to -25 HU[9]. The window center was set at 100 and the width was set at 150. The VF area was quantified by determining the size of the intra-abdominal cavity at the internal aspect of the abdominal wall surrounding the cavity. Gas and intestinal contents were excluded (Figure 3).
Abdominal fat is composed of TF, VF, and SF. The SF area was derived by subtracting the VF area from the TF area. The ratio of VF to SF (VS ratio) was also calculated[7]. Furthermore, we assessed the difference of mean ± SD of TF, VF, and SF in men and women among three different groups in hepatic fat infiltration.
Statistical analysis was performed with SPSS statistical software (version 12.0 for Windows; SPSS Inc., Chicago, IL, USA). The alpha level was set at P = 0.05 for all tests. We used the bivariate correlation method to assess the correlation between HAIs and TF, VF, and SF. Bivariate correlation methods were also used to assess the relationships between the HAIs and BMI, WT, WHR, TG, and LDL and between BMI and TF, VF, and SF. We used Student’s t-test to compare the means of TF, VF, and SF between the two groups.
The mean ± SD of CTLP, LS ratio, LSdif, and the splenic attenuation value were 63.0 ± 10.7 (ranging from 15.4 to 78.6), 1.2 ± 0.2 (ranging from 0.3 to 1.8), 12.2 ± 10.4 (ranging from -30.4 to 33.4), and 50.8 ± 5.0 (ranging from 34.65 to 65.8), respectively. The mean values ± SD of TF, VF, and SF were 320.3 ± 144.7 (ranging from 68.8 to 869.6), 121.5 ± 68.1 (ranging from 15.7 to 377.2), and 198.8 ± 97.5 (ranging from 12.9 to 628.6), respectively. The mean values ± SD of BMI, WT, WHR, TG, and LDL were 24.5 ± 3.7 (ranging from 14.0 to 38.4), 64.7 ± 12.3 (ranging from 35 to 124), 0.87 ± 0.05 (ranging from 0.69 to 1.01), 137.2 ± 93.5 (ranging from 37 to 762), and 99.5 ± 32.0 (ranging from 12.6 to 331.6), respectively (Table 1).
| mean ± SD | Range | |
| CTLP | 63.0 ± 10.7 | 15.4-78.6 |
| LS ratio | 1.2 ± 0.2 | 0.3-1.8 |
| LSdif | 12.2 ± 10.4 | -30.4-33.4 |
| CTS | 63.0 ± 10.7 | 15.4-78.6 |
| TF (cm2) | 320.3 ± 144.7 | 68.8-869.6 |
| VF (cm2) | 121.5 ± 68.1 | 15.7-377.2 |
| SF (cm2) | 198.8 ± 97.5 | 12.9-628.6 |
| BMI (kg/m2) | 24.5 ± 3.7 | 14.0-38.4 |
| WT(kg) | 84.7 ± 12.3 | 65-124 |
| WHR | 0.87 ± 0.05 | 0.69-1.01 |
| TG (mg/dL) | 137.2 ± 93.5 | 37-762 |
| LDL (mg/dL) | 99.5 ± 32.0 | 12.6-331.6 |
There were significant negative correlations between CTLP, LS ratio, and LSdif with TF, VF, and SF, respectively. CTLP showed strong negative correlations with TF and VF (r = -0.415 and -0.434, respectively, P < 0.001). The correlation between CTLP and SF (r = -0.313, P < 0.001) was less significant. Among the three HAIs, CTLP demonstrated a greater tendency to correlate with the abdominal fat volume than the LS ratio or LSdif (Table 2).
| TF | VF | SF | |
| CTLP | r = -0.415 | r = -0.434 | r = -0.313 |
| P = 0.000 | P = 0.000 | P = 0.000 | |
| LS ratio | r = -0.258 | r = -0.298 | r = -0.172 |
| P = 0.000 | P = 0.000 | P = 0.003 | |
| LSdif | r = -0.297 | r = -0.330 | r = -0.210 |
| P = 0.000 | P = 0.000 | P = 0.000 |
BMI, WT, WHR, and TG were all negatively correlated with the respective HAIs. In contrast, LDL level was not correlated with HAIs (Table 3). BMI was strongly correlated with abdominal fat volume (r = 0.705, 0.601, and 0.624 for TF, VF, and SF, respectively, P < 0.001) (Table 4).
| BMI | WT | WHR | TG | LDL | |
| CTLP | r = -0.582 | r = -0.593 | r = -0.364 | r = -0.388 | r = -0.060 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.300 | |
| LS ratio | r = -0.331 | r = -0.405 | r = -0.219 | r = -0.314 | r = -0.036 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.531 | |
| LSdif | r = -0.392 | r = -0.454 | r = -0.257 | r = -0.341 | r = -0.036 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.531 |
| TF | VF | SF | |
| BMI | r = 0.705 | r = 0.601 | r = 0.624 |
| P = 0.000 | P = 0.000 | P = 0.000 |
The mean ± SD of TF, VF, and SF in normal patients were 299.8 ± 133.3, 110.1 ± 59.7, and 189.7 ± 92.8, respectively. The mean values ± SD of TF, VF, and SF in fatty liver patients were 409.8 ± 159.3, 171.4 ± 79.8, and 238.4 ± 108.0, respectively. The differences in TF, VF, and SF between the two groups of normal and fatty liver patients were all statistically significant (P < 0.001) (Table 5).
| n | mean ± SD | Minimum | Maximum | |
| TF (cm2) | ||||
| Normal | 249 | 299.8 ± 133.3 | 68.8 | 869.6 |
| Fatty liver | 57 | 409.8 ± 159.3 | 126.6 | 838.0 |
| VF (cm2) | ||||
| Normal | 249 | 110.1 ± 59.7 | 15.7 | 316.6 |
| Fatty liver | 57 | 171.4 ± 79.8 | 46.5 | 377.2 |
| SF (cm2) | ||||
| Normal | 249 | 189.7 ± 92.8 | 12.9 | 571.3 |
| Fatty liver | 57 | 238.4 ± 108.0 | 72.4 | 628.6 |
In the comparison between men and women, there were statistically significant differences in TF and SF among total or normal patients (Table 6).
| Men (n) | Women (n) | P value | |
| TF (cm2) | |||
| Total | 309.5 ± 131.3 (116) | 326.9 ± 152.3 (190) | 0.0051 |
| Normal | 292.6 ± 119.0 (89) | 303.9 ± 140.8 (160) | 0.0021 |
| Fatty liver | 410.6 ± 160.2 (27) | 409.1 ± 161.2 (30) | 0.545 |
| VF (cm2) | |||
| Total | 145.9 ± 66.6 (116) | 106.7 ± 64.8 (190) | 0.914 |
| Normal | 133.2 ± 54.3 (89) | 97.2 ± 58.8 (160) | 0.164 |
| Fatty liver | 190.2 ± 74.2 (27) | 154.5 ± 82.1 (30) | 0.444 |
| SF (cm2) | |||
| Total | 163.6 ± 80.6 (116) | 220.3 ± 100.8 (190) | 0.0001 |
| Normal | 159.3 ± 80.0 (89) | 206.6 ± 95.3 (160) | 0.0021 |
| Fatty liver | 220.4 ± 116.9 (27) | 254.5 ± 98.5 (30) | 0.916 |
The total radiation dose was 25-30 mGy/cm and the effective dose was less than (Deff) 0.37-0.45 mSv.
NAFLD is associated with metabolic syndrome and occurs in patients without a history of excessive alcohol ingestion. The presentation of NAFLD varies from asymptomatic elevated liver enzyme levels, through various levels of inflammation and fibrosis, to cirrhosis with complications of hepatic failure and hepatocellular carcinoma[10]. By using accurate non-invasive diagnostic methods that detect NASH (the most severe form of NAFLD and one that can advance to cirrhosis) at an early stage, it is possible to detect patients at risk of developing cirrhosis later in life[5]. Although liver biopsy is the gold standard for diagnosing NAFLD, it has limitations including sampling error and potential risks to the patients[2].
A hepatic attenuation value (CTLP) that is significantly lower than the splenic attenuation value is a reliable indicator for the presence of fatty liver[7]. CTLP and LS ratio both demonstrate strong inverse correlations with degree of histologic steatosis[6]. Therefore, CT can serve as a non-invasive test to confirm the presence of hepatic steatosis[7,8]. The effectiveness of confirming fatty liver using CT has been demonstrated in donor evaluation for liver transplantation[7,8]. A previous study used the difference between mean hepatic attenuation and mean splenic attenuation, which is presented in our study as LSdif, as a parameter for prediction of the degree of macrovesicular steatosis. In that study, an LSdif below -10 HU was correlated with greater than 30% macrovesicular steatosis, a level that is unacceptable for liver transplantation. An LSdif between -10 and 5 HU correctly predicted 6%-30% of steatosis, a relative contraindication for liver transplantation. An LSdif above 5 HU predicted 0%-5% of steatosis[8]. Another study indicated that the highest cut-off values that yielded 100% specificity for the diagnosis of macrovesicular steatosis of 30% or greater (the limit of acceptability for donation) were 0.8 for LS ratio, -9 for LSdif, and 42 for CTLP[11]. Accordingly, CT has functioned as a screening tool to avoid unnecessary liver biopsy in patients with fatty liver who are not suitable for transplantation[11]. Nonetheless, the diagnosis and grading of fatty liver using CT is limited because simple steatosis and NASH that would be demonstrated by biopsy cannot be differentiated using imaging techniques[7].
There are some other tools that can be used to evaluate fatty liver and abdominal obesity. US is relatively cheap and easy to use, but has no reproducibility. CT and MR allow accurate and objective quantification of each body fat components. MR has several disadvantages including long scan time, high cost, and contraindicant to patients with claustrophobia, so that there is a limitation to the use of MR as a screening tool in the obesity clinic. On the other hand, CT is a simple and reproducible tool, and is a popular technique to screen fatty liver and obesity. Though widely used, CT has limitations in the assessment of fatty liver because of its association with significant radiation exposure, especially in serial assessment[6].
Obesity is the cause of fatty liver in non-alcoholics. A previous study showed that BMI is directly related to the prevalence of NAFLD[1]. Although BMI is an independent predictor of fatty liver, many studies have indicated that body composition reflects an individual’s health status better than body WT or BMI. In a study involving 3 432 Japanese subjects by Omagari et al[1], 27.2% of non-alcoholic and non-overweight men and 59.2% of non-alcoholic and non-overweight women were found to have fatty liver. The body fat percentages of these patients as measured using a bipedal bioimpedance instrument were excessive compared to men and women without fatty liver. The present study assumed that a patient with a normal BMI had a central body fat distribution if the body fat percentage was excessive; we also assumed that central body fat distribution is associated with the development of fatty liver. Percentage body fat measurement is useful when determining the cause of fatty liver in non-alcoholic and non-overweight individuals, especially in women[1]. Another study categorized obesity into two types (Figure 4), “android” or “male-type” obesity (central or abdominal depot) showed a stronger correlation than “gyroid” or “female-type” obesity (lower body, gluteo-femoral, or peripheral depot) with increased mortality, DM, hyperlipidemia, hypertension, and atherosclerosis[9]. As such, obesity is not a homogeneous condition and the regional distribution of adipose tissue is important for understanding the link between obesity and disturbances in glucose and lipid metabolism[9]. In contrast, VF and central abdominal fat demonstrated a strong correlation with each other and a strong association with metabolic syndrome. Given that VF in the abdomen is more strongly associated with hyperglycemia, arteriosclerosis, and dyslipidemia than SF, it is useful to distinguish between VF and SF when examining a patient[12]. Anthropometric measurements that evaluate body fat distribution such as skin fold or WHR do not differentiate between VF and SF[9]. Imaging techniques such as abdominal CT and MR are suitable methods for examining abdominal VF because they allow direct measurement of abdominal fat and make an accurate distinction between VF and SF[4,9,13]. We used CT to measure each abdominal fat component and the degree of hepatic fat infiltration; we then analyzed the relationships between these values. To determine visceral intraabdominal and subcutaneous abdominal areas, a simple CT scan was performed at the level of the umbilicus (L4/5). Scanning at the level of the umbilicus was first proposed by Wajchenberg[9], who found that because body fat exists in the highest percentage at the level of the umbilicus, it is easiest to differentiate SF from intraabdominal fat at this level. Kvist et al[14] have revealed that VF areas from a single scan at L4/5 region are highly correlated to the total VF volume.
Our study showed that CTLP is more strongly correlated with the amount of abdominal fat than are LS ratio or LSdif. This is most likely because the subjects in this study were quite healthy. The fatty infiltration of the liver was found to be more strongly associated with TF and VF compared to SF. SF is different from VF in that venous drainage from SF is directed towards the systemic circulation while the drainage from VF is directed towards the portal vein. VF also acts as an endocrine organ and affects the risk of developing certain metabolic traits and vasoactive substances[4,15]. VF secretes free fatty acids and adipocytokines and allows fat accumulation in the liver[15]. There is a direct association between the amount of VF and the amount of free fatty acid delivered to the liver. Several circulating cytokines are increased with obesity and may combine with the influence of VF to generate insulin resistance, inflammation, and fibrosis in NAFLD[16].
There are a few limitations in our study. First, pathologic confirmation was not obtained. Diagnosing and grading fatty liver with imaging features alone has limited value without definitive pathologic confirmation[1]. The patients in our study population were primarily outpatients who desired an annual check-up or evaluation of obesity; we were thus ethically prohibited from obtaining liver biopsies. In addition, it is difficult to distinguish between simple steatosis and NASH using imaging alone[6]. Second, we did not consider patients with hemochromatosis or other conditions related to hepatic iron deposition. Increased hepatic iron causes increased attenuation of the liver resulting in misinterpretation of combined fatty liver. Third, CT induces a radiation effect. As part of an effort to minimize radiation exposure, only selected levels of the abdomen were obtained in evaluating the abdominal fat volume. Finally, daily alcohol consumption was not fully assessed[1].
We conclude that fat infiltration of the liver is well correlated with amount of abdominal fat. Fatty liver tends to be more strongly associated with VF compared to SF. In other words, if a non-obese patient exhibits fatty liver, the patient may in fact have visceral obesity. Likewise, not all patients who have a large amount of SF develop fatty liver.
It has been generally recognized that fatty liver can often be found among obese people. However, we often experienced that the obese patient might always not show the fatty liver on the hepatic ultrasound or computed tomography (CT) examination.
According to previous studies, fatty liver was found to be more strongly associated with visceral fat (VF) than subcutaneous fat, because of the portal venous drainage and endocrine effect of VF. In this study, the authors proved the result by CT.
The obese patient does not always show fatty liver in the imaging study. Fatty liver tends to be increased in incidence in the patients who have larger amount of VF.
By using body fat CT, we are able to quantify body fat distribution and degree of fatty liver and evaluate the correlation between the two variables. In the obesity clinic, we can standardize fatty liver and abdominal obesity and use these for the treatment response.
This is a good study to correlate the fatty liver with the abdominal fat using CT scan. This as a well designed study with nicely written manuscript. There are few limitations of the study, which are already mentioned by authors.
Peer reviewer: Ram Prakash Galwa, MBBS, MD, Dr., Department of Diagnostic Imaging, The Ottawa hospital, 751 Parkdale Avenue, Apartment 803, Ottawa, K1Y1J7, Canada
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098-1105. |
| 2. | Nugent C, Younossi ZM. Evaluation and management of obesity-related nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:432-441. |
| 3. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. |
| 4. | Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39-48. |
| 5. | Oliva MR, Mortele KJ, Segatto E, Glickman JN, Erturk SM, Ros PR, Silverman SG. Computed tomography features of nonalcoholic steatohepatitis with histopathologic correlation. J Comput Assist Tomogr. 2006;30:37-43. |
| 6. | Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol. 2003;15:539-543. |
| 7. | Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Hepatic steatosis in Cushing’s syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149:543-548. |
| 8. | Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, Saab S, Lu DS. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276-280. |
| 9. | Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738. |
| 11. | Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105-112. |
| 12. | Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27:2948-2953. |
| 13. | Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274-278. |
| 14. | Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351-1361. |
| 15. | Jakobsen MU, Berentzen T, Sørensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77-87. |
| 16. | Milner KL, van der Poorten D, Xu A, Bugianesi E, Kench JG, Lam KS, Chisholm DJ, George J. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology. 2009;49:1926-1934. |