Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2848
Revised: February 24, 2011
Accepted: March 3, 2011
Published online: June 21, 2011
AIM: To observe the effects of sargentgloryvine stem extracts (SSE) on the hepatoma cell line HepG-2 in vitro and in vivo and determine its mechanisms of action.
METHODS: Cultured HepG-2 cells treated with SSE were analysed by 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-Diphenyltetrazolium bromide and clone formation assay. The cell cycle and apoptosis analysis were conducted by flow cytometric, TdT-Mediated dUTP Nick End Labeling and acridine orange/ethidium bromide staining methods, and protein expression was examined by both reverse transcriptase-polymerase chain reaction and Western blotting. The pathological changes of the tumor cells were observed by haematoxylin and eosin staining. Tumor growth inhibition and side effects were determined in a xenograft mouse model.
RESULTS: SSE treatment could not only inhibit HepG-2 cell proliferation in a dose- and time-dependent manner but also induce apoptosis and cell cycle arrest at the S phase. The number of colonies formed by SSE-treated tumor cells was fewer than that of the controls (P < 0.05). SSE induced caspase-dependent apoptosis accompanied by a significant decrease in Bcl-xl and Mcl-1 and elevation of Bak expression (P < 0.05). Tumor necrosis factor α in the xenograft tumor tissue and the liver functions of SSE-treated mice showed no significant changes at week 8 compared with the control group (P > 0.05). Systemic administration of SSE could inhibit the HepG-2 xenograft tumor growth with no obvious toxic side effects on normal tissues.
CONCLUSION: SSE can induce apoptosis of HepG-2 cells in vitro and in vivo through decreasing expression of Bcl-xl and Mcl-1 and increasing expression of Bax.
-
Citation: Wang MH, Long M, Zhu BY, Yang SH, Ren JH, Zhang HZ. Effects of sargentgloryvine stem extracts on HepG-2 cells
in vitro andin vivo . World J Gastroenterol 2011; 17(23): 2848-2854 - URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2848
Although significant progress has been made over the past decades in cancer prevention and treatment, the development of effective treatment regimens remains one of the greatest challenges in the area of cancer chemotherapy. Recently, plant-derived natural products are becoming important as anti-cancer derivatives, including vincristine, vinblastine, paclitaxel and camptothecin, which are invaluable contributions of nature to modern medicine[1-4]. However, the quest to find novel therapeutic compounds for cancer treatment is a never-ending venture, and diverse plant species are being studied to identify prospective anti-cancer agents[5,6]. Sargentgloryvine stem of Sargentodoxa cunneata (Oliv.) has been widely used as an ingredient in Chinese medicine according to the Chinese herbal medicine principles for thousands of years in the treatment of several kinds of diseases, such as chronic pelvic cavity inflammation, rheumatism and appendicitis. Sargentgloryvine stem extract (SSE) as a chemotherapeutic adjuvant can enhance the efficacy and ameliorate the side effects of cancer chemo- or radio-therapy. Because SSE has been used in Chinese herbal medicine as a bioactive constituent in a complex herbal mixture, an important question is whether its biological activity can be largely or exclusively ascribed to one or more individual compounds present in this herb. To address this question, we prepared SSE and studied its effects. Our previous research showed that SSE possesses potent anticancer activities[7]. However, the molecular mechanisms underlying the anticancer effects of SSE are poorly understood and need to be elucidated. To identify potential anticancer mechanisms of SSE in human hepatocellular carcinoma (HCC), the molecular effects of SSE on HepG-2 cells were examined. Down-regulation of the two anti-apoptotic Bcl-2 family proteins Bcl-xl and Mcl-1 may be responsible for antiproliferative and cell apoptotic effects of SSE on HepG-2 cells. Meanwhile, HepG-2 xenograft nude mice treated systemically with SSE were also monitored in tumor growth inhibition and toxicity in vivo. The purpose of this study was to observe the effects of SSE on the hepatoma cell line HepG-2 in vitro and in vivo, and preliminarily analyse its mechanisms of action.
SSE was extracted from 10 g dried powder of Sargentgloryvine stem in a rotary shaker with 200 mL of 50 mL/L ethanol at 60°C for 24 h. The extract was then filtered, concentrated using a rotary evaporator to remove the solvent, and finally lyophilised in a freeze-dryer to obtain crude freeze-dried powder. The same batch of SSE was used in all studies in order to keep the results reliable. The powder was dissolved in DMEM culture medium at a stock solution of 5 g/L for further use. The hepatoma cell line HepG-2 was preserved in our laboratory. Specific pathogen-free male athymic BALB/c nude mice of 6-7 wk of age with a body mass of 20-30 g were purchased from the Animal Experimental Centre of the Fourth Military Medical University. HepG-2 cells were cultured in DMEM supplemented with 100 mL/L foetal calf serum, 100 kU/L penicillin, 0.1 g/L streptomycin and 250 μg/L amphotericin B, incubated at 37°C in a humified atmosphere of 50 mL/L carbon dioxide. The primers for PT-PCR detection of transcript are described in Table 1.
| Gene | Sense sequence 5'-3' | Antisense sequence 5'-3' |
| β-actin | GACTTAGTTGCGTTACACCTTTC | TGCTGTCACCTTCACCGTTC |
| Bax | ATGGACGGGTCCGGGGAG | TCAGCCCATCTTCTTCCAGAT |
| Bak | ATGGCTTCGGGGCAAGGC | TCATGATTTGAAGAATCTTCGTACC |
| Bal-2 | ATGGCGCACGCTGGGAGAACG | GTACTCAGTCATCCACAGGGC |
| Bcl-xl | ATGTCTCAGAGCAACCGGGAGCT | TCATTTCCGACTGAAGAGTGAGC |
| Mcl-1 | TGCCGCTGCTGGAGTTGGT | TTACAGTAAGGCTATCTTATTAGAT |
| Bcl-w | CTCTGGTGGCAGACTTTGTAG | CCGTCCCCGTATAGAGCTGTGA |
| Bcl-b | ATGGCCGACTCGCAGGACCCA | TTATAAACGTTTCCATATAAAA |
| Blf-1 | ATGAGTGATCCAGAAACCAG | TTAATCCTCTTCTGAACTTTCA |
Cell viability and clone formation assay: HepG-2 and ECV304 cells (control cells) were cultured in 96-well plates at a density of 3 × 108 cells/L with 100 μL per well in DMEM. Cells were treated with 1.5, 15, 45, 60 and 90 mg/L SSE for 6, 12, 24, 36 and 48 h or medium only as a control (DMEM-treated) group. After incubation, cell proliferation was detected by 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and cytotoxicity was studied with a commercial assay kit (Beyotime Biotechnology, China) following the manufacturer’s instructions. Absorbance was measured at 570 nm using the Bio-Rad 550 microplate reader. The protracted cell growth curves and the inhibition of cell growth were calculated based on the absorbance (A) value as follows: inhibitory rate = (1-Atreated/Acontrol group) × 100%. For the colony formation assay, a total of 3 × 102 HepG-2 and control cells, and ECV304 tumor cells were plated in 75 mm culture dishes and treated with SSE at a concentration of 30 mg/L. After incubation for an additional 10-14 d, the cells were fixed with methanol and stained with 1 g/L crystal violet (Sigma, USA), and colonies of > 50 cells were manually counted. All experiments were performed in independent triplicates.
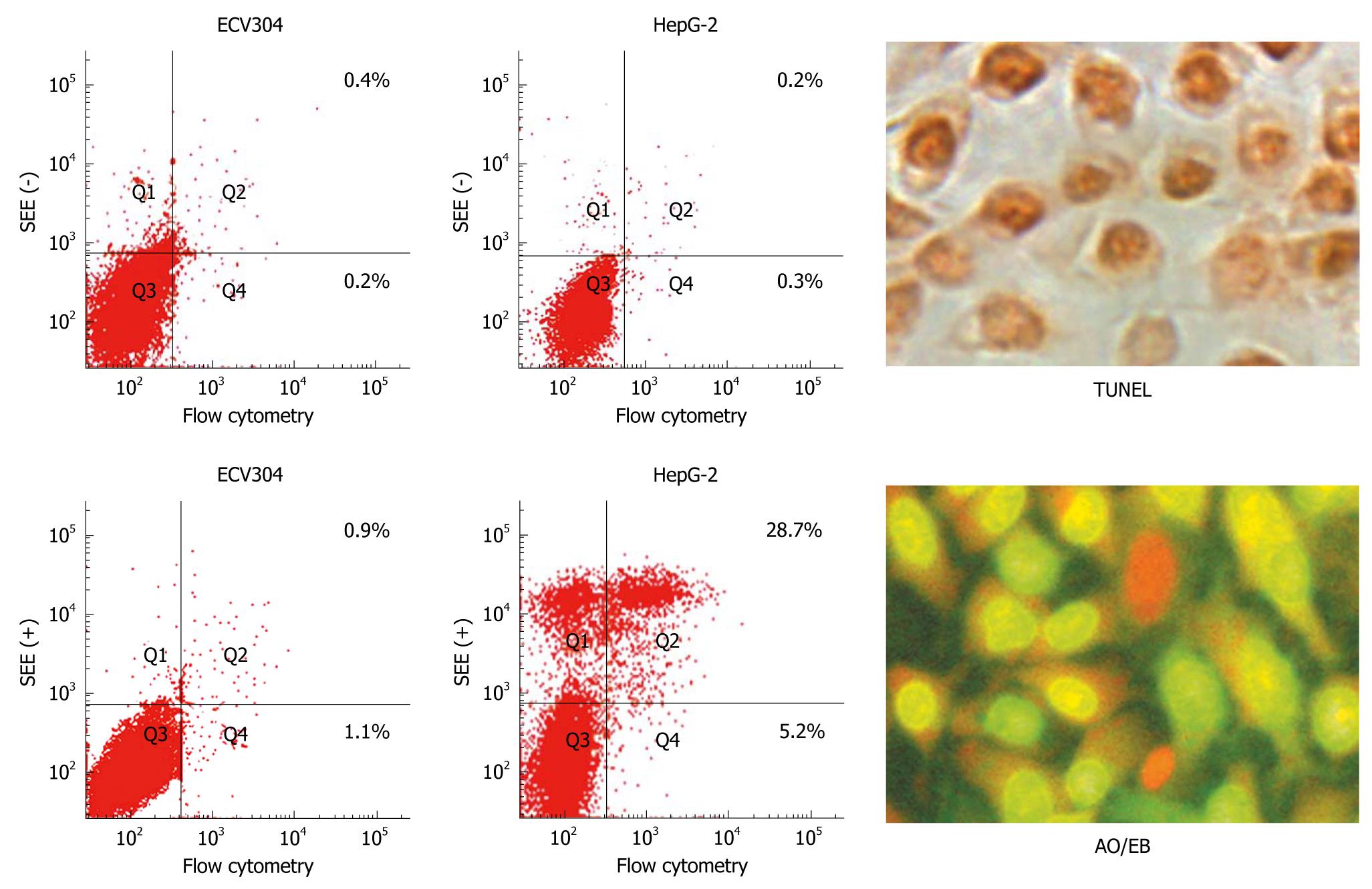
Cell apoptosis and cycle analysis: For apoptosis, HepG-2 cells and ECV304 cells were treated with SSE at concentrations of 15, 30 or 60 mg/L, or no drug as a control for 24 h. The cells were harvested and washed twice with cold PBS and resuspended in binding buffer. FITC-labelled annexin V and propidium iodide (PI) were added and incubated for 10 min at room temperature, and the cell suspensions were immediately analysed by flow cytometry. For cell cycle detection, the cells treated with 45 mg/L SSE for 24 h were fixed with 700 mL/L cold ethanol and resuspended in phosphate-buffered saline containing 20 mg/L PI and then analysed for PI fluorescence intensity by flow cytometry to measure the cellular DNA content. The HepG-2 cells were suspended in 75 mm plates and treated with SSE at a concentration of 45 mg/L for 24 h or an equal volume of culture medium as the control. The total volume of each well was one mL. The cells were collected, and a TdT-Mediated dUTP Nick End Labeling assay (Keygen Biotech Co., Ltd., Nanjing, China) was performed as suggested by the manufacturer to detect the incorporation of labelled nucleotides into DNA. At least 300 cells were counted under a light microscope, and apoptotic cells were identified. All experiments were performed in triplicate. The negative control cells were set up with no TdT enzyme added, and positive control cells were pretreated with DNase during the staining process.
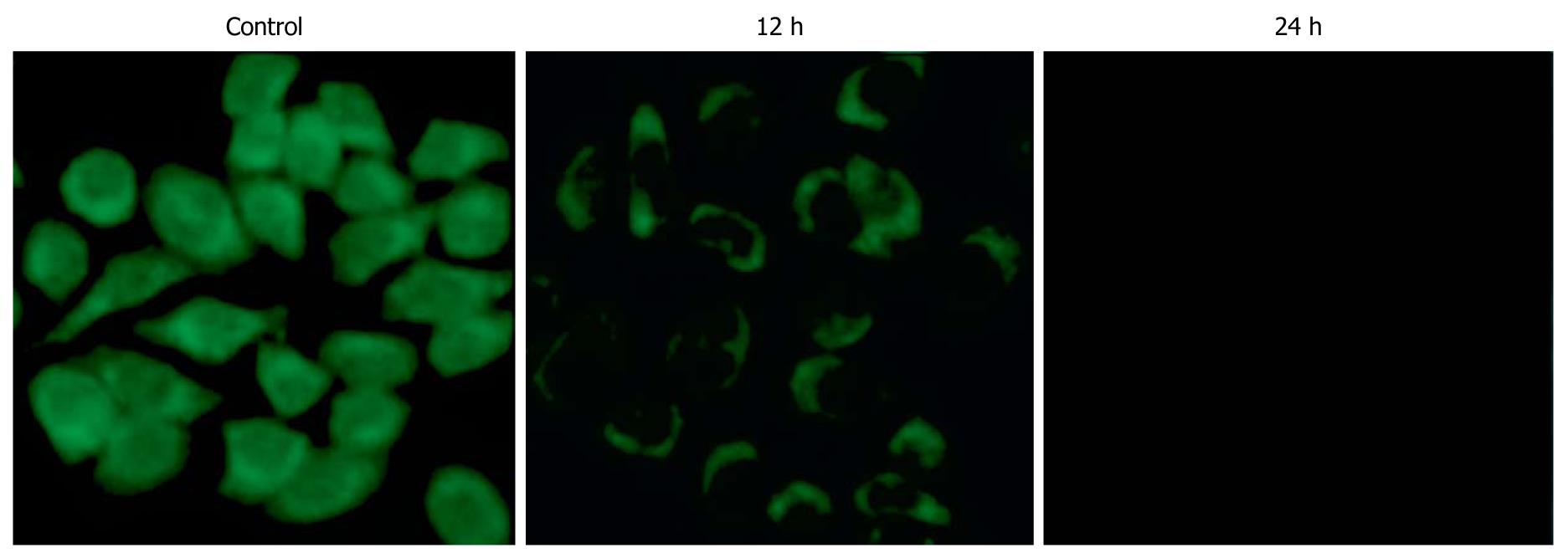
Apoptosis quantification and Δψm detection: HepG-2 cells were cultured in 6-well plates and treated with SSE at a concentration of 45 mg/L for 24 h. Acridine orange/ethidium bromide staining was performed following the manufacturer’s instructions (Keygen Biotech Co., Ltd., Nanjing, China). Acridine orange is a vital dye and can stain both live and dead cells. Ethidium bromide can only stain the cells that have lost membrane integrity. Live cells appear uniformly green, while early apoptotic cells stain green but contain bright green dots in the nuclei as a consequence of chromatin condensation and nuclear fragmentation. Late apoptotic cells incorporate more ethidium bromides and therefore stain orange and show condensed and often fragmented nuclei. At least 300 cells were counted under a fluorescence microscope to quantify apoptosis. All experiments were performed independently in triplicate. Additionally, HepG-2 cells were harvested following treatment with SSE at 45 mg/L for 12 and 24 h. After washing twice with PBS, 1 × 106 cells were incubated with 10 mg/L of Rh123 (Sigma, USA), a cationic lipophilic fluorochrome that is taken up by mitochondria in proportion to the Δψm, for 30 min at 37°C. Fluorescence intensities were determined by flow cytometry (Becton Dickinson Inc., USA).
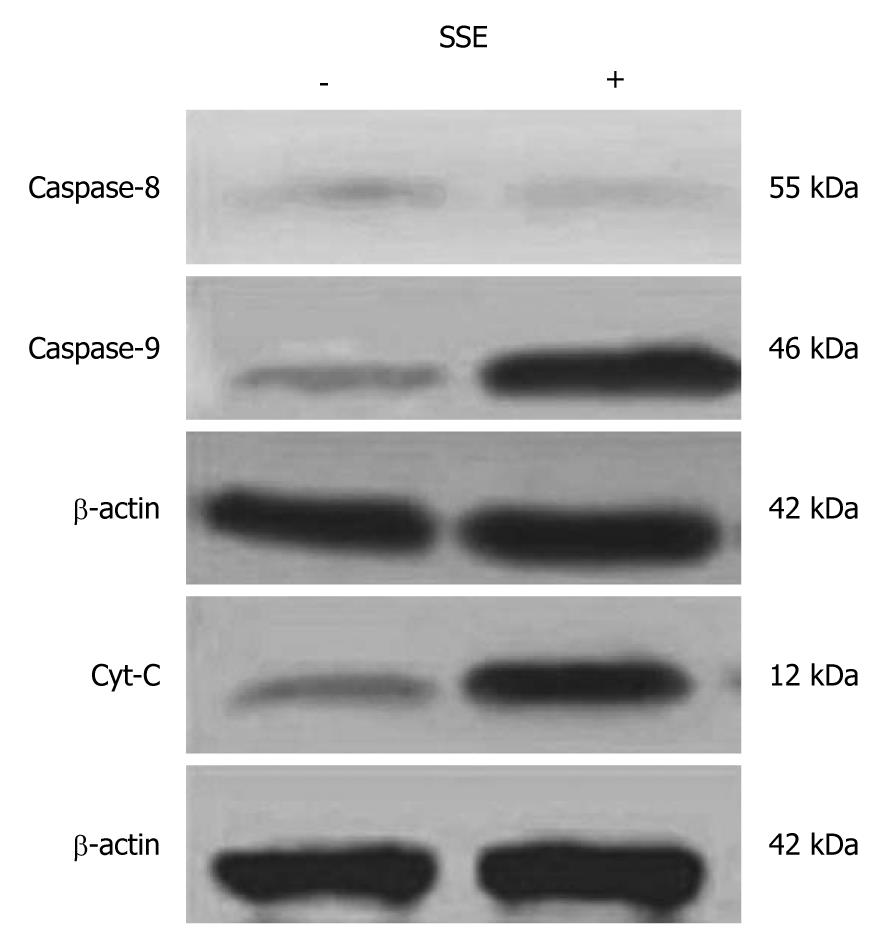
Gene expression study: Total RNA from 1 × 106 SSE-treated (45 mg/L for 24 h) HepG-2 cells and control cells were extracted by Trizol™ reagent (Invitrogen, USA), and 1 μg total RNA was used to synthesise cDNA with the superscript first-strand synthesis kit (Takara BioTechnology, Dalian, China) following the manufacturer’s instructions. One microliter cDNA was used to amplify the specific genes by reverse transcriptase-polymerase chain reaction (RT-PCR). To normalize cDNA loading, the β-actin gene was also amplified from each sample. RT-PCR was performed with the primers listed above. HepG-2 cells treated with SSE (45 mg/L for 24 h) and control cells were harvested by suspension in lysis buffer. The cell extracts were clarified by centrifugation, and the protein concentrations were determined using the Bio-Rad protein assay kit. Each protein extract (25 μg) was electrophoresed on a 100 g/L SDS-polyacrylamide gel, transferred to a membrane and blocked in 50 g/L skimmed milk in tris buffered saline-Tween 20 for 1 h at room temperature. Membranes were probed with anti-Bax, -Bak, -Bcl-xl, -Mcl-1, -caspase-8, -caspase-9, -cytochrome C and -β-actin antibodies overnight at 4°C. Primary antibodies were removed, and the blots were extensively washed with TBS-T three times. Blots were then incubated for 1 h at 37°C with the secondary antibodies in 10 g/L skimmed milk dissolved in TBS-T. After removal of the secondary antibody, blots were extensively washed and developed using the enhanced chemiluminescence kit in the dark (Santa Cruz Biotechnology). The primary antibodies used in this experiment were monoclonal mouse anti-human Bax, Bcl-xl, Mcl-1, cytochrome C, caspase-8 and-9 (latter two from Abcam, UK) and β-actin and polyclonal goat anti-human Bak (Santa Cruz). Goat anti-mouse and rabbit anti-goat IgG coupled to horseradish peroxidase (Santa Cruz Biotechnology) were used as secondary antibodies for detection of protein expression.
Solid tumor growth assay: Athymic BALB/c nude mice were housed in laminar flow cabinets under specific pathogen-free conditions. All animal studies were performed in compliance with the Institutional Guidelines of the Fourth Military Medical University. Aliquots of 1.0 × 106 HepG-2 cells were suspended 1:1 in PBS and subcutaneously inoculated into the right flank of each mouse. When 300 mm3 tumors were observed, the mice were randomly assigned to two groups (n = 5). The mice of the treatment group received 18 mg/kg SSE suspended in 50 μL DMEM, and the mice of the control group were treated with an equal volume of DMEM via vena caudalis injection every 2 d for 14 d. The tumor volume was measured each time before SSE administration with callipers using a standard formula as follows: width2× length × 0.5. An average tumor volume per mouse was used to calculate the group mean tumor volume ± SD (n = 5). Mice were sacrificed 24 h after the last administration of SSE to harvest the tissues of tumors and the heart, liver, spleen, kidneys and lungs. All tissues were then fixed in 40 g/L paraformaldehyde overnight and sectioned, and haematoxylin and eosin staining was performed to identify the toxicity of SSE in vivo. The supernatants of nude mice xenografts were preserved at -70°C. After protein concentration determination, tumor necrosis factor α (TNFα) levels were detected by ELISA in tumor tissues from nude mice according to the manufacturer’s instructions. Liver function (aspartate aminotransferase and alanine aminotransferase) and kidney function (creatinine) were also detected.
All statistical analyses were performed using SPSS13.0. Data were expressed as mean ± SD. Comparisons among all groups were performed with the one-way ANOVA analysis of variance test. Differences were considered significant at P < 0.05.
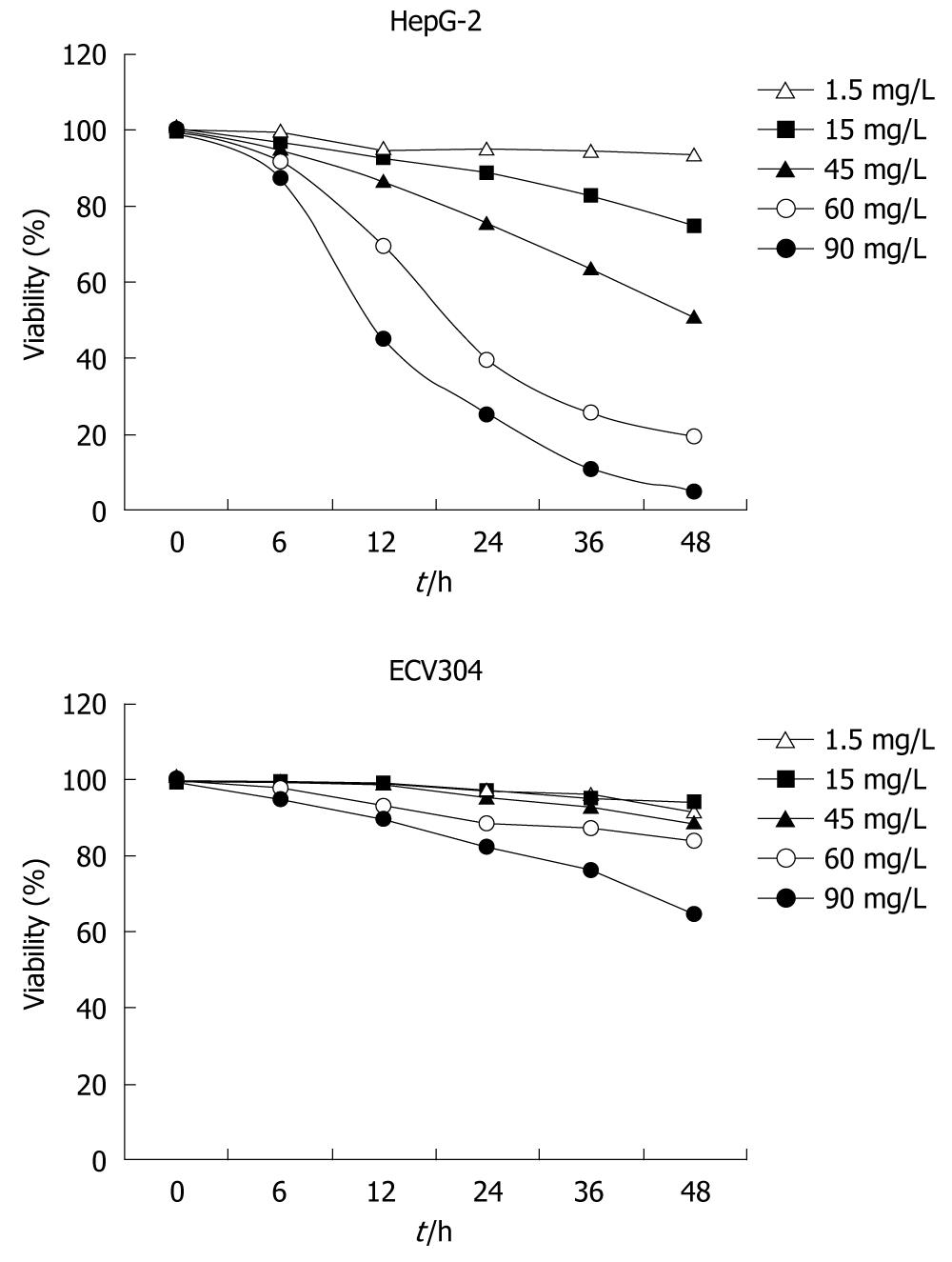
SSE-treatment at concentrations of 1.5 and 15 mg/L showed no significant growth inhibition of HepG-2 cells, but higher SSE concentrations of 30, 60 and 90 mg/L significantly inhibited the proliferation of the HepG-2 cells (Figure 1). At an SSE concentration of 30 mg/L, the inhibition rate (mean ± SD) increased with treatment time; the rates were 5.0 ± 1.4, 13.7 ± 2.7, 23.3 ± 6.0, 34.2 ± 5.3 h and 53.7 ± 3 at 6, 12, 24, 36 and 48 h, respectively (P < 0.05). All concentrations showed no obvious growth inhibition on ECV304 cells. Additionally, the number of colonies of SSE treated HepG-2 cells was lower than that of the control cells (261 ± 16 vs 492 ± 21, Student’s t test, P < 0.05), but no difference was observed on the plates of ECV304 cells. Finally, SSE treatment at 45 mg/L for 24 h significantly increased the proportion of S phase HepG-2 cells from 17.8 ± 1.9 to 63.3 ± 3.3 (P < 0.05), but decreased the proportion of G0/G1 phase and G2/M phase cells.
SSE treatment of HepG-2 cells increased the apoptosis rates in a dose-dependent manner (0.2%, 4.7%, 9.5%, 28.7%, as analysed by flow cytometry) (Figure 2). Western blotting analysis showed that SSE treatment (45 mg/L) for 24 h significantly increased caspase-9 but not caspase-8 cleavage (Figure 3), indicating that SSE induces apoptosis through the intrinsic pathway. Additionally, SSE-treated HepG-2 cells lost Δψm, as indicated by a decrease in Rh-123 fluorescence (Figure 4), which was significantly weaker than that of the control cells. Consistent with the results of fluorescence microscopy above, Western blotting analysis demonstrated that the expression of cytochrome C in plasmosin was also altered in SSE-treated HepG-2 cells (Figure 3).
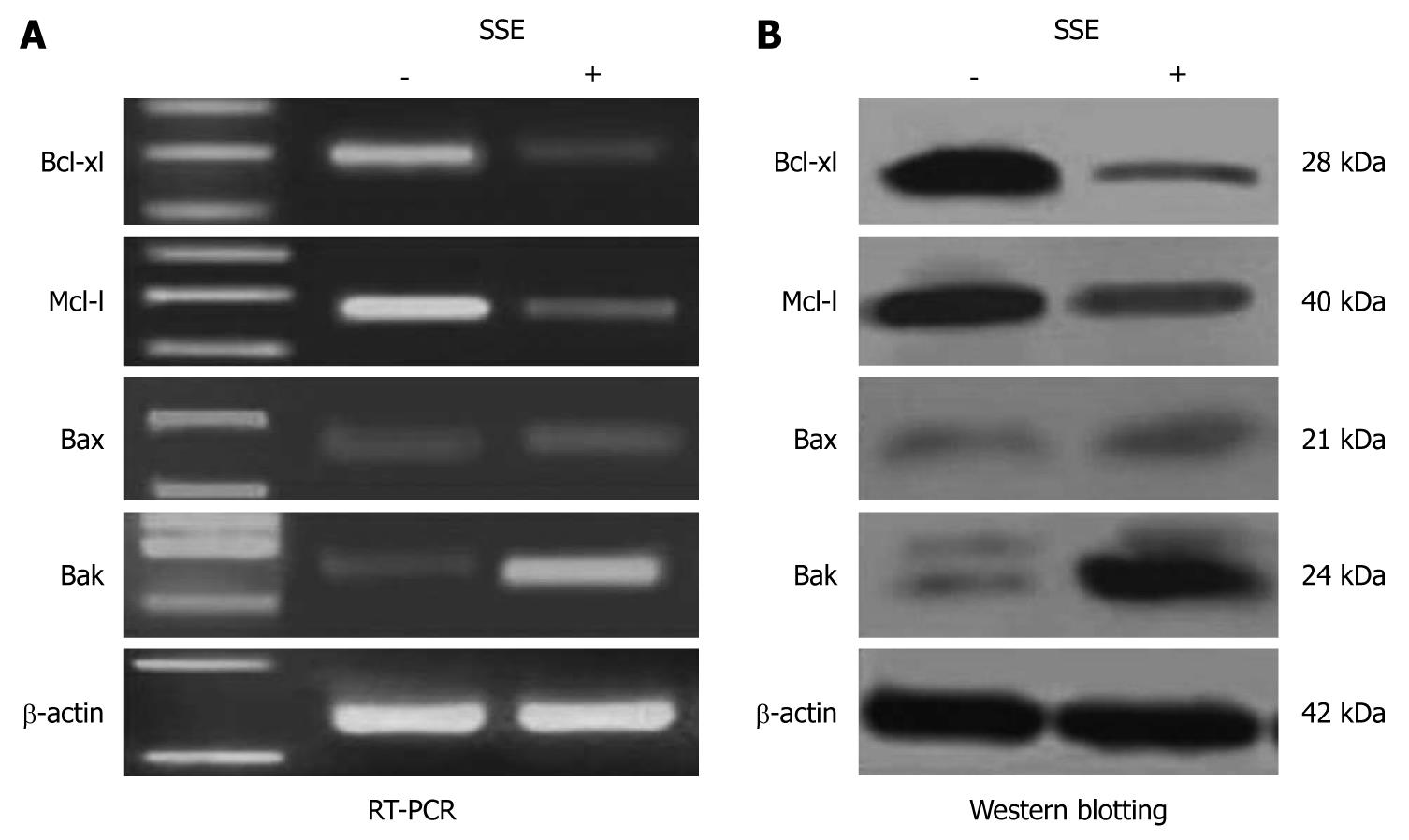
In SSE-treated (45 mg/L) HepG-2 cells, the expression of Bak but not Bax was significantly increased, while the expression levels of Bcl-xl and Mcl-1 were significantly down-regulated compared with the control cells (Figure 5A). Western blotting analysis further confirmed the up-regulation of Bax and down-regulation of Bcl-xl and Mcl-1 (Figure 5B).
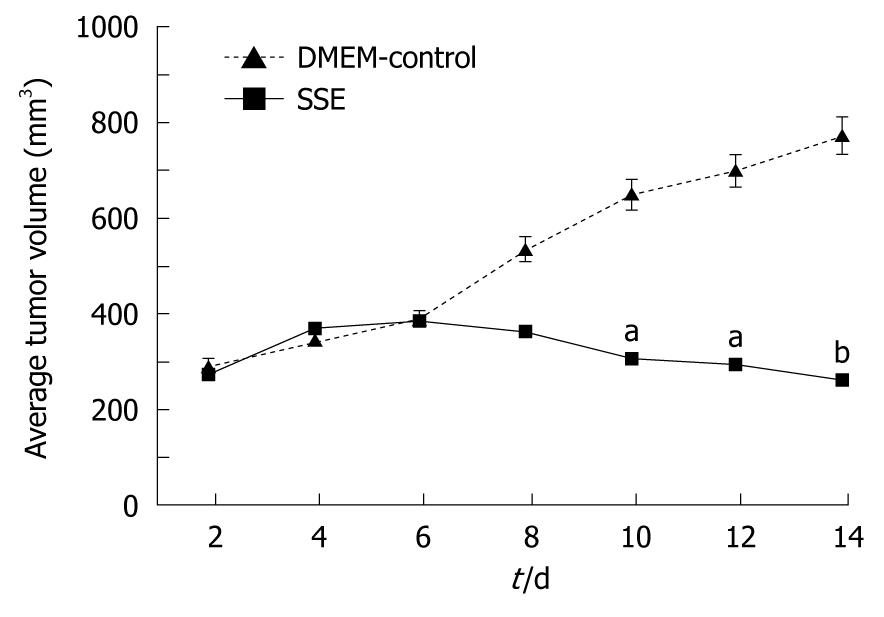
Compared with DMEM-treated mice, i.v. administration of 18 mg/kg SSE every 2 d for 14 d led to an inhibition of HepG-2 cell growth (Figure 6). No lesions were found in the heart, liver, spleen, lungs or kidneys in SSE-treated xenograft mice, and their functions were also normal. Compared with the control group, the TNFα levels in SSE-treated tumor tissue showed no significant difference at week 8 (9.9 ± 6.8 mg/L vs 9.1 ± 5.7 mg/L, P > 0.05). In addition, the food intake, mental status and activities were similar in the two groups during the treatment.
Recently, the discovery of active compounds from natural products has gained considerable attention as a new source of anticancer drugs[6-10]. The quest to find novel therapeutic compounds for cancer treatment is a never-ending venture. Sargentgloryvine stem, a traditional Chinese medicine, has been used for thousands of years in the treatment of several inflammatory diseases. Recently, we have successfully developed a novel extract from sargentgloryvine stem SSE that has a potent anticancer activity on HCC cells[1]. However, the molecular mechanisms underlying the anticancer effects of SSE have not been elucidated.
Our research shows that SSE can induce apoptosis of the hepatoma cell line HepG-2 in vitro and in vivo, and its mechanism of action may be through decreasing expression of Bcl-xl and Mcl-1 and increasing expression of Bax. Compared with the control group, TNFα in the tumor tissue and liver function in SSE-treated mice had no significant changes at 8 wk (P > 0.05). In addition, this study provides evidence that SSE may be a potent therapeutic agent in the treatment of HCC without obvious toxic side effects.
In this study, the inhibitory effect of SSE on HepG-2 cells was tested, and ECV304 cells as the control were also studied. It was demonstrated that SSE profoundly inhibited the growth of HepG-2 cells in a concentration- and time-dependent manner but not in ECV304 cells. MTT and colony formation assays indicate that SSE possesses specific anti-HCC cell activity rather than general cytotoxicity. Additionally, the study suggested that SSE may be safe for normal cells, thus, SSE may have advantages for clinical application.
To reveal the mechanisms of the inhibitory effect of SSE on HepG-2 cells, changes in the cell cycle were analysed. It was demonstrated that the cell cycle of HepG-2 cells was blocked at S phase after 45 mg/L SSE treatment for 24 h, which indicated that the growth inhibition by SSE might be, in part, due to cell cycle arrest. Because cell cycle arrest is always accompanied with cell apoptosis, we analysed apoptosis in the SSE-treated cells. The data showed that SSE treatment induced cell apoptosis, and the apoptosis rates increased with increasing SSE concentrations[11]. More significantly, the induction of apoptosis by SSE in HepG-2 cells was observed at an initial concentration of 30 mg/L within 24 h, further suggesting the safety of SSE for systemic use in the treatment of HCC. It is known that apoptosis is regulated by two main pathways: the extrinsic pathway, which is initiated by the binding of ligands to specific death receptors on the cell surface, and the intrinsic pathway, which is initiated in mitochondria[12,13]. To understand the major in vivo pathway through which SSE induces HepG-2 cell growth suppression and apoptosis, the expression of caspase-8 and caspase-9, which play important roles in apoptosis triggered by various proapoptotic signals, were studied[14]. The results showed that 45 mg/L SSE treatment for 24 h significantly increased caspase-9 but not caspase-8 expression, indicating that SSE-induced apoptosis may be through the intrinsic pathway.
The intrinsic pathway for apoptosis involves several steps including mitochondrial membrane permeabilization and release of cytochrome C, followed by caspase-9 activation[15,16]. Our results showed that treatment with SSE at 45 mg/L for 24 h led to loss of Δψm as indicated by a decrease in Rh-123 fluorescence. SSE also induced cytochrome C release from the mitochondria to cytosol in HepG-2 cells. Taken together, these results further confirmed that SSE may directly trigger the intrinsic pathway to induce the mitochondrial pathway for apoptosis in HCC cells.
Previous studies have shown that in apoptotic cells, anti-apoptotic bcl-2 members are often inactivated whereas pro-apoptotic members, such as bax and bak, are activated and oligomerized in the mitochondria outer membrane. This triggers mitochondrial membrane permeabilization and release of soluble apoptogenic factors such as cytochrome C into the cytosol, which results in caspase activation[17,18]. Further studies revealed that 45 mg/L SSE treatment significantly decreased Bcl-xl and Mcl-1 expression and increased Bak expression and led to mitochondria endomembrane action. Finally, it was confirmed that SSE-induced HepG-2 cell apoptosis is mediated through the bcl-2 pathway. These results demonstrated that SSE-induced apoptosis is mediated primarily by down-regulated expression of Bcl-xl and Mcl-1, which led to the release of Bak and ultimately activated the intrinsic apoptosis pathway.
In addition to inducing tumor cell apoptosis, systemic injection of SSE into HepG-2 xenografted mice inhibited tumor growth and significantly minimised tumor size but caused no obvious pathological changes in the heart, lungs, liver, spleen or kidneys. The levels of TNFα in the tumor tissue and liver function tests suggest that SEE treatment is relatively safe for the mice.
In conclusion, our research shows that the extract of the Chinese herb sargentgloryvine stem has in vitro anti-cancer effects including inhibition of proliferation and induction of apoptosis in the hepatoma cell line HepG-2 by mechanisms involving expression of Bcl-2 family proteins activating the intrinsic mitochondria apoptosis pathway. Moreover, an in vivo solid tumor growth assay further confirmed that systemic administration of the extract could inhibit tumor growth with little cytotoxicity to normal tissues. These in vitro and in vivo studies provide evidence urging the development of SSE as a novel regimen for human HCC.
Plant-derived natural products have become available such as anticancer derivatives of vincristine, vinblastine, paclitaxel and camptothecin.
Sargentgloryvine stem extract (SSE) as a chemotherapeutic adjuvant can enhance the efficacy and ameliorate the side effects of cancer chemo- or radio-therapy. However, the effect of SSE on the human hepatocellular carcinoma (HCC) cells remains unknown.
This study showed that SSE treatment was not only able to inhibit the proliferation of human HCC cell HepG-2 cells in a dose and time dependent manner, but also induce apoptosis and cell cycle arrest at S phase.
SEE is able to inhibit proliferation of human HCC cells and is relatively safe for the mice, and therefore it has a great potential to be a therapeutic agent in the treatment of HCC.
SSE: Sargentgloryvine stem is the dried vine stem of Sargentodoxa cuneata (Oliv.) and has been widely used as an ingredient in formulated Chinese medicine for thousands of years in the treatment of diseases such as chronic pelvic cavity inflammation, rheumatism and appendicitis.
The paper by Wang et al addresses an important issue, i.e. novel options for systemic therapy of HCC. In general, the paper is well written, the methods used are sound and the described approach is of potential interest.
Peer reviewer: Fritz von Weizsäcker, Professor, Department of Medicine Schlosspark-Klinik, Humboldt University, Heubnerweg 2, Berlin D-14059, Germany
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Li X, Xu W. Recent patents therapeutic agents for cancer. Recent Pat Anticancer Drug Discov. 2006;1:255-284. |
| 2. | Tuma MC, Malikzay A, Ouyang X, Surguladze D, Fleming J, Mitelman S, Camara M, Finnerty B, Doody J, Chekler EL. Antitumor Activity of IMC-038525, a Novel Oral Tubulin Polymerization Inhibitor. Transl Oncol. 2010;3:318-325. |
| 3. | Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72-79. |
| 4. | Kim BK, Ko YG, Oh S, Kim JS, Kang WC, Jeon DW, Yang JY, Choi D, Hong MK, Ahn T. Comparisons of the effects of stent eccentricity on the neointimal hyperplasia between sirolimus-eluting stent versus paclitaxel-eluting stent. Yonsei Med J. 2010;51:823-831. |
| 5. | Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, Capaccioli S. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365-378. |
| 6. | Hong YH, Chao WW, Chen ML, Lin BF. Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. J Biomed Sci. 2009;16:64. |
| 7. | Wang X, Wang R, Hao MW, Dong K, Wei SH, Lin F, Ren JH, Zhang HZ. The BH3-only protein PUMA is involved in green tea polyphenol-induced apoptosis in colorectal cancer cell lines. Cancer Biol Ther. 2008;7:902-908. |
| 8. | Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020-2033. |
| 9. | Relja B, Meder F, Wilhelm K, Henrich D, Marzi I, Lehnert M. Simvastatin inhibits cell growth and induces apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int J Mol Med. 2010;26:735-741. |
| 10. | Kang JX, Liu J, Wang J, He C, Li FP. The extract of huanglian, a medicinal herb, induces cell growth arrest and apoptosis by upregulation of interferon-beta and TNF-alpha in human breast cancer cells. Carcinogenesis. 2005;26:1934-1939. |
| 11. | Wu WY, Guo HZ, Qu GQ, Han J, Guo DA. Mechanisms of pseudolaric acid B-induced apoptosis in Bel-7402 cell lines. Am J Chin Med. 2006;34:887-899. |
| 12. | You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657-1663. |
| 13. | Soriano ME, Scorrano L. The interplay between BCL-2 family proteins and mitochondrial morphology in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:97-114. |
| 14. | Alenzi FQ, Lotfy M, Wyse R. Swords of cell death: caspase activation and regulation. Asian Pac J Cancer Prev. 2010;11:271-280. |
| 15. | Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423-1433. |
| 16. | Gao J, Jia WD, Li JS, Wang W, Xu GL, Ma JL, Ge YS, Yu JH, Ren WH, Liu WB. Combined inhibitory effects of celecoxib and fluvastatin on the growth of human hepatocellular carcinoma xenografts in nude mice. J Int Med Res. 2010;38:1413-1427. |
| 17. | Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577-1590. |
| 18. | Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396-1402. |