INTRODUCTION
A gastrointestinal duplication is defined as a spherical hollow structure with a smooth muscle coat, lined by a mucous membrane, and attached to any part of the gastrointestinal tract, from the base of the tongue to the anus. However, foregut duplication cyst of the stomach is rare[1,2]. Foregut duplications may or may not communicate with the gastrointestinal tract, and are usually diagnosed at a young age[2]. There have been relatively few case reports describing this entity[3-12] Adenocarcinoma has been reported in four cases of gastric duplication cyst, but not in cysts that have a ciliated epithelium[13-16]. Controversy exists concerning the embryological origin of these anomalies[11,17,18]. Here, we present two cases of gastric ciliated duplication cyst with emphasis on immunophenotype and embryogenesis.
CASE REPORT
Case 1
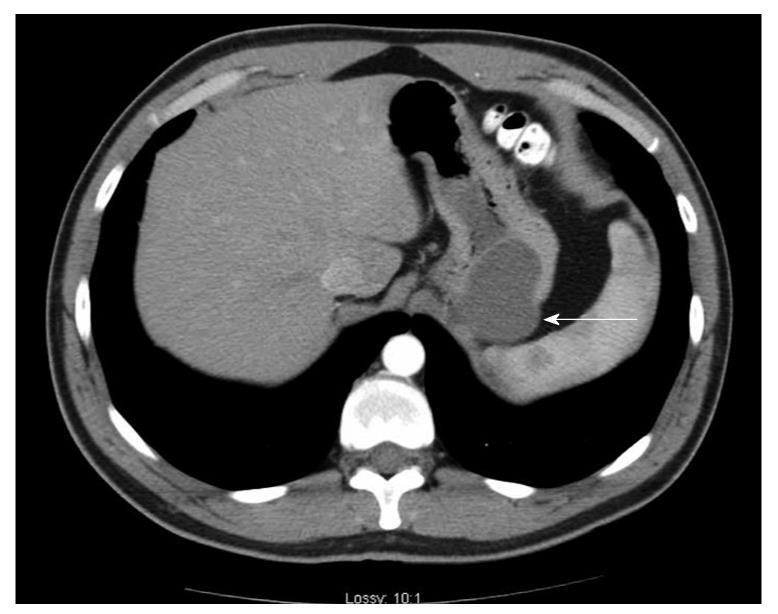
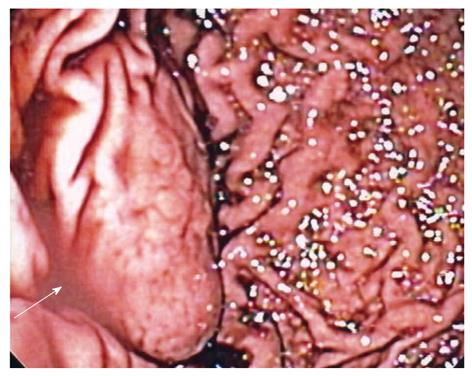
Case history: The patient was a 29-year-old Caucasian man who presented to his local emergency department for evaluation of acute abdominal pain. The pain started in the lower abdomen and then localized to the left lower quadrant. He had mild nausea, but no vomiting. He had a history of gastroesophageal reflux disease. Physical examination was notable only for left lower quadrant tenderness to palpation. A computed tomography scan (CT-scan) of the abdomen and pelvis was performed and revealed a mass at the greater curvature of the stomach (Figure 1). An esophagogastroduodenscopy was performed, revealing a submucosal mass in the fundus of the stomach, approximately 2 cm from the gastroesophageal junction (Figure 2), which was soft on compression. Endoscopic ultrasound revealed a cystic mass in the submucosa of the fundus of the stomach. The imaging and endoscopic findings were most consistent with a gastric duplication cyst. To secure the diagnosis and prevent possible malignant degeneration, the patient was advised to undergo a partial gastrectomy.
Figure 1 A computed tomography scan of the abdomen and pelvis was performed utilizing intravenous and oral contrast.
A 4 cm × 5 cm cystic mass (arrow) appears along the greater curvature of the stomach adjacent to the spleen.
Figure 2 Retroflexed endoscopic view of a gastric mass arising in the fundus (arrow).
Overlying rugae are effaced, suggestive of a submucosal process.
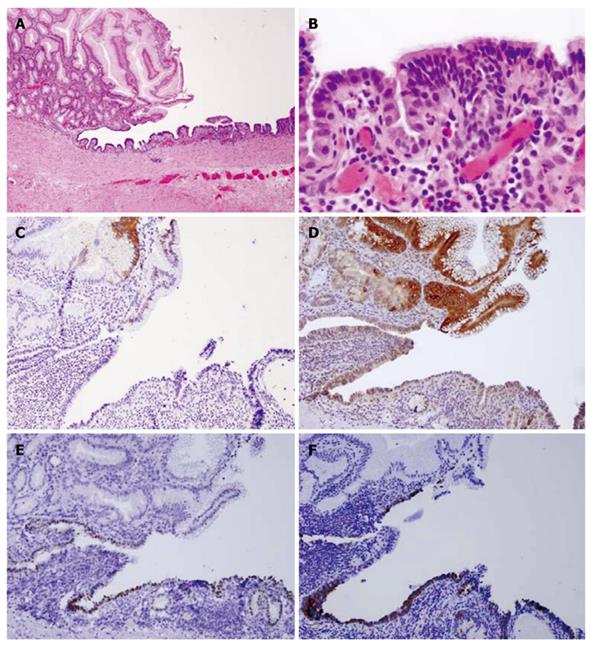
Pathological findings: Macroscopically, a sack-like lesion (8.5 cm × 5.5 cm × 4.8 cm) was found with a smooth capsulated wall. The inner wall was also smooth. Microscopically, the cyst was lined by an antrum-type gastric mucosa and a respiratory epithelium with ciliated cells (Figure 3A and B). No intestinal-type epithelium was present. The outside layer consisted of a circular and a longitudinal muscle wall, with a myenteric plexus. The gastric epithelium immunophenotype was cytokeratin 20+, MUC5a/c+, cytokeratin 7+, MUC-1-, MUC-2-, CDX-2-, TTF-1-, and surfactant-, while the ciliated epithelium had the following immunophenotype: cytokeratin 20-, MUC5a/c-, cytokeratin 7+, MUC1-, MUC2-, CDX-2-, TTF-1+, and surfactant+ (Figure 3C-F).
Figure 3 Case 1.
A: Hematoxylin and eosin satin of gastric/ciliated epithelium (4 ×); B: High power view of the ciliated epithelium (40 ×); C: CK20 showing staining in the surface gastric epithelium, but not in the ciliated epithelium (10 ×); D: MUC5a/c staining showing positive expression in the gastric epithelium but not in the ciliated epithelium (10 ×); E: Thyroid transcription factor-1 nuclear staining in the ciliated epithelium only (10 ×); F: Surfactant staining in the ciliated epithelium only (10 ×).
Case 2
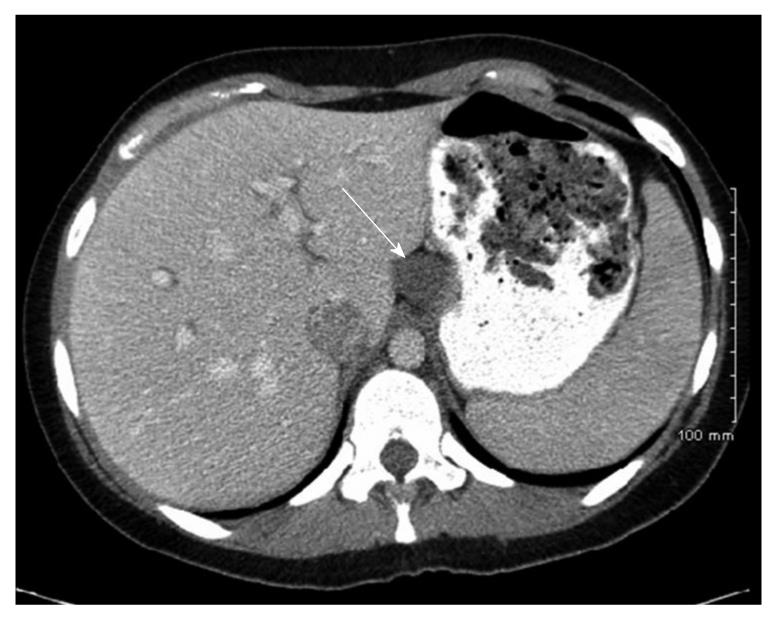
Case history: The patient was a 26-year-old woman with history of chronic epigastric pain for eight years following her first childbirth. The pain was intermittent, and colicky, without identifiable aggravating factors. More recently, the pain had become severe in intensity and required narcotics for relief. The pain was not associated with nausea, vomiting, abdominal distention, fevers, or chills. She admitted to a normal appetite, but had had an unintentional 50-pound weight loss over the last year. She underwent an esophagogastroduodenoscopy, which revealed a normal esophagus, a small hiatal hernia, and diffuse moderate inflammation of the stomach. A CT-scan of the abdomen revealed a cystic mass near the gastroesophageal junction along the lesser curvature of the stomach, which extended to the superior margin of the pancreas and abutted the liver and inferior vena cava (Figure 4). The mass was nonenhancing and cystic in appearance. The primary suspicion was that this represented a congenital gastrointestinal duplication cyst. A partial gastrectomy was performed for further evaluation and treatment.
Figure 4 Case 2.
Computed tomography-scan of the abdomen and pelvis with intravenous and oral contrast showing a non-enhancing cystic structure (arrow) abutting the lesser curvature of the stomach and liver along the ligamentum venosum.
Pathological findings: Macroscopically, the cyst was an ovoid-shaped structure (5 cm × 2.2 cm × 2 cm). The external surface was covered with tan, shiny serosa. The cystic cavity was filled with a tan-white mucoid material. The cyst inner surface was unremarkable, tan, and smooth. Microscopically, the cyst was lined by a respiratory epithelium with ciliated cells (Figure 5A and B). No intestinal-type epithelium was present. The outside layer consisted of a circular and a longitudinal muscle wall, with a myenteric plexus. The epithelium had the following immunophenotype: cytokeratin 20-, MUC5a/c-, cytokeratin 7+, MUC1-, MUC2-, CDX-2, TTF-1+, and surfactant+ (Figure 5C and D).
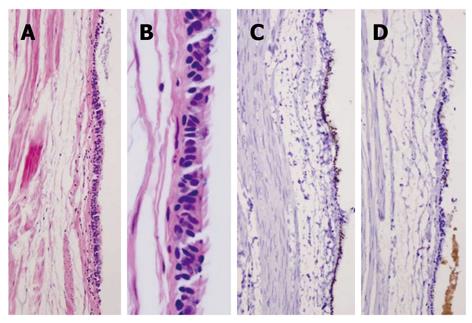
Figure 5 Case 2.
A, B: Hematoxylin and eosin staining of pseudostratified ciliated epithelium (A: 4 ×, B: 40 ×); C: Thyroid transcription factor-1 nuclear staining in the ciliated epithelium (10 ×); D: Surfactant staining in the ciliated epithelium (10 ×).
DISCUSSION
Gastrointestinal duplication can occur in any region of the gastrointestinal tract; however, a foregut duplication cyst of the stomach is rare. The stomach is the site of only 24% of all alimentary tract duplication cysts, which is the least common site after the ileum, esophagus, and colon. Of reported gastric duplication cysts, most occur in females, with 80% presenting in patients younger than 12 years of age. They are characteristically located on the distal greater curvature, and the majority are cystic and non-communicating. Histologically, they are usually lined with a typical gastric mucosa[15]. As in the present two cases, a ciliated pseudostratified epithelium has been reported[3-12].
We present two foregut duplication cysts of the stomach, one connected and one separated, in a 29-year old woman and a 26-year old man, respectively. These two cases showed distinct morphological and immunoprofile findings. Both cysts were lined with pseudostratified ciliated epithelia, and expressed TTF-1 and surfactant.
The pathogenesis of alimentary tract duplications is controversial. There has been no suggestion of an explanation based on embryological development[17]. However, others have suggested theory of embryological origin from supernumerary foregut buds[11]. Others have proposed that abnormal recanalization after the solid epithelial stage of embryonic bowel development is the underlying cause of these lesions[18].
We found that these cysts are lined with a ciliated pseudostratified epithelium and express TTF-1 and surfactant. These two factors are involved in lung embryogenesis. These findings might partially explain the embryogenesis of these lesions. When the embryo is approximately four weeks old, the respiratory diverticulum appears along the ventral wall of the pharyngeal gut. The esophagotracheal septum gradually partitions this diverticulum from the dorsal part of the foregut. In this manner, the foregut divides into the ventral portion, the respiratory primordium, and a dorsal portion, which becomes the esophagus and stomach[19]. The middle branch of the tracheal trifurcation is grossly and histologically identical to the other two branches of the trifurcation, including expression of TTF1, which become the lungs[20]. Early in the development of the esophagus, it is lined by a pseudostratified columnar epithelium. This is a characteristic of the respiratory epithelium, but the mucosa of the esophagus apparently undergoes subsequent metaplasia and squamous differentiation[1,8]. Based on the presence of the ciliated epithelium, we propose that a branch coming off the trifurcation gives rise to the duplication cyst. Later, the cyst either remains connected to the esophagus/stomach or becomes separated.
TTF-1 is a homeobox transcription factor whose expression in the developing foregut is specifically limited to the respiratory tract[21-23]. It is particularly important for normal lung development[24-28]. TTF-1 enhances branching morphogenesis of embryonic lung explants in vitro[29]. A TTF-1 knockout mouse developed no distal lung parenchyma[30]. Furthermore, TTF-1 is thought to be important for terminal respiratory epithelial cytodifferentiation. It has the capacity to regulate expression of surfactant proteins, A, B, and C, as well as the Clara cell secretary protein, all markers of respiratory cell specific differentiation[25,26,28,31-33]. Thus, the presence of TTF-1 and surfactant expression is a function of lung differentiation. The presence of these two factors in the described cysts might explain why these cysts maintain their phenotype of a respiratory epithelium with no squamous metaplasia. As with tracheo-esophageal fistulae[20], TTF-1 might have lost its normal patterning role in the developing lung, preventing induction of branching morphogenesis in the duplication cyst. The nonbranching pattern of growth of the duplication cyst might be attributable to local mesenchymal-epithelial interactions that override TTF-1 patterning activity.
In conclusion, gastric duplication cysts are rare, and their origin remains uncertain. The two cases presented here,and the resulting histological findings, suggest a novel origin. In both cases, the cysts are lined with a pseudostratified respiratory epithelium with ciliated cells, which express TTF-1 and surfactant. This suggests an origin from the respiratory diverticulum, which arises from the ventral foregut and could also explain why these cysts do or do not maintain their connection to the gastrointestinal tract.