Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5901
Revised: September 20, 2010
Accepted: September 27, 2010
Published online: December 14, 2010
AIM: To investigate the relationship between human papillomavirus (HPV) infection and concurrent esophagus and gastric cardia cancer from the same patient (CC) and examine the significance of P16INK4A protein expression.
METHODS: Polymerase chain reaction was used to detect the presence of HPV type16 (HPV16). The expression of P16INK4A protein was detected using immunohistochemistry.
RESULTS: Among the CC specimens, HPV16-DNA was found in eight cases of esophageal squamous cell carcinoma (ESCC) and five cases of gastric cardia adenocarcinoma (GCA), respectively (47% vs 29%), and two of both ESCC and GCA. P16INK4A was highly expressed in both ESCC and GCA. In the HPV-associated positive CC, higher P16INK4A expression was observed in the GCA than in the ESCC (75% vs 25%, P < 0.05).
CONCLUSION: HPV16 as a correlated risk factor may play an important role in the development of ESCC and GCA. P16INK4A may be a screening index in the HPV-associated carcinoma of gastric cardia.
- Citation: Ding GC, Ren JL, Chang FB, Li JL, Yuan L, Song X, Zhou SL, Guo T, Fan ZM, Zeng Y, Wang LD. Human papillomavirus DNA and P16INK4A expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol 2010; 16(46): 5901-5906
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5901
Esophageal carcinoma (EC) is one of the most common malignant diseases worldwide. Linzhou (formerly known as Linxian) in Henan Province, northern China, has been recognized as the highest prevalence area of esophageal squamous cell carcinoma (ESCC) in the world[1]. In epidemiology, gastric cardia adenocarcinoma (GCA) in China is characterized by its striking geographic distribution and its concurrence with ESCC[2]. Similar phenomenon can be also observed in other esophageal cancer incidence areas worldwide[3]. At present, ESCC and GCA still are the main cause of tumor-related deaths in this area. Another interesting feature at the high prevalence area is that the primary cancers of the esophagus and gastric cardia are concurrent in the same patient in Henan, we named them concurrent carcinoma of the esophagus and gastric cardia in the same patient (CC), which is not uncommon in this area (0.4%-2.5%)[4]. The special pattern suggested that similar risk factors and mechanism might be involved in these two cancers.
Human papillomavirus (HPV) as one of the important tumor-related viruses has been firmly recognized in cervical cancer with HPV-DNA detected in > 99% specimens[5]. However, its oncogenic role in other tumors still remains controversial. As to its role in ESCC, it was firstly mentioned by Syrijanen twenty years ago[6]. Since then, many reports regarding this topic have been published, but the HPV infection rate in ESCC varied from zero to 90%[7,8] and in GCA from zero to 68%[9,10], depending on the specimens obtained from low- or high-risk areas and the methods used in each study[11]. In order to further investigate the prevalence of HPV infection in upper digestive tract tumor, the samples with concurrent ESCC and GCA from the same patient were tested for the existence of HPV type16 (HPV16)-DNA.
HPVs are small DNA viruses that can be classified as either high-risk or low-risk types. HPV-16 and 18 is most common in high-risk group, especially type 16 which is considered a risk factor for EC at a high prevalence area in Henan[8,12]. The E6 and E7 oncoproteins of the high-risk HPV types can efficiently destroy the cell cycle regulation and apoptotic pathways by binding to a number of host cell proteins, such as P16INK4A protein, which is an inhibitor of cyclin-dependent kinase. The HPV oncoproteins are able to alter the cell cycle and leaves them vulnerable to other genetic changes, ultimately resulting in malignant transformation[13,14]. However, the reports about HPV infection involved in the carcinogenesis of gastric cardia are very limited, and there has been no related study comparing the HPV detection rate and expression of P16INK4A protein in CC tissues. In our study, we investigated the HPV infection and changes of P16INK4A protein in the same concurrent cancer patient in the high-risk area of EC in Henan to understand the mechanism of esophagus/cardia carcinogenesis in this area, and further illustrate whether the two carcinomas have the similar pathogenesis.
A total of 23 cases of concurrent ESCC and GCA were obtained. Among them, DNAs from 17 ESCC and their corresponding GCA tissues were extracted from paraffin-embedded samples by conventional phenol-chloroform procedure. Six cases were from Linzhou Center Hospital, eight from Linzhou Yaocun Esophageal Cancer Hospital and three from Anyang City Cancer Hospital from September 2005 to June 2008. All the hospitals are located in the high incidence region of ESCC in Henan. There were twelve men and five women with an average age of 58 years. None of the patients received chemo- and radiotherapy before surgery.
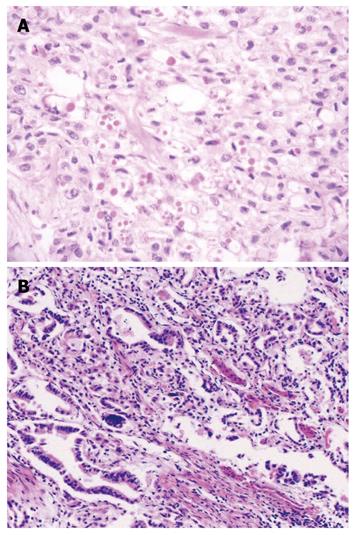
The samples were immediately fixed by 10% formalin, dehydrated and paraffin-embedded, followed by pathological diagnosis and immunochemical analysis. The diagnosis of the CC was based on the following criteria: (1) All the tumors in the esophagus and cardia in the same patient are malignant; (2) All the tumors have defined pathological modality, i.e. concurrent esophageal squamous cell carcinomas and gastric cardia adenocarcinomas; and (3) None the tumors are metastatic (Figure 1). This study was approved by the Institutional Review Board of the School of Medicine, Zhengzhou University, China.
The methods were used as described by Greer et al[15]. Briefly, each formalin-fixed and paraffin-embedded sample was cut into 10 μm thick sections, 5-10 slides were deparaffinized in xylene and graded alcohol, then the lysis buffer (300 mmol/L NaCl; 50 mmol/L Tris HCl pH 8.0; 0.2% SDS) was added into the tube with proteinase K (200 mg/L), and the solution was incubated at 55°C overnight until it became clear. DNA was then extracted using conventional phenol-chloroform procedure, precipitated with cold alcohol and dissolved in ion-free water, and the concentration was determined based on its optical density. Quality of the extracted DNA was tested by polymerase chain reaction (PCR) with β-actin primer: 5'-TCACCCACACTGTGCCCATC-3' and 5'-GAACCGCTCATTGCCAATGG-3'. The DNA which was β-actin gene amplification positive was used to detect the presence of HPV16-DNA.
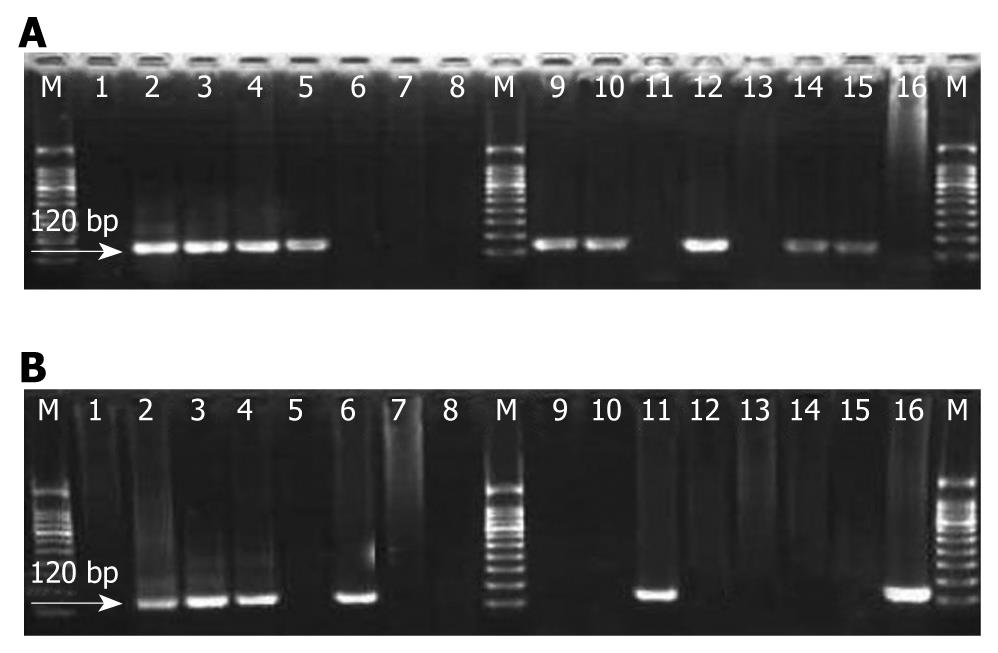
The usable DNA went through PCR amplification using type-specific primer: 5'-TCAAAAGCCACTGTGTCCTG-3' and 5'-CGTGTTCTTGATGATCTGCA-3' targeting HPV16-E6 gene. Recombinant plasmid DNA HPV16-pBR322 as positive control was obtained from Professor You-Lin Qiao, the Cancer Research Institute, Chinese Academy of Medical Sciences and double water was used as negative control in PCR. Reactions were set in 25 μL 1 × PCR buffer containing 10 mmol/L Tris HCl, pH8.4, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 4 mmol/L dNTP and 1.5 U Taq DNA polymerase (Promega). PCR conditions were as follows: an initial denaturation at 95°C for 5 min followed by 5 cycles with cycling profile at 95°C denaturing for 1 min, at 55°C annealing for 1 min, and at 72°C prolonging for 1 min, and then at 95°C denaturing for 20 s, at 55°C annealing for 30 s and at 72°C prolonging for 30 s with 45 cycles[8]. The amplified products were revealed by electrophoresis on 3.0% agarose gels (Promega) containing 0.5% μg/mL of ethidium bromide in 1 × TAE buffer at 100 volts for 30 min. Samples were considered positive if a band of 120 bp was observed under the ultraviolet light (Figure 2). All the experiments from DNA extraction to HPV gene amplification were carried out at the Virus Research Institute, Chinese Academy of Preventive Medicine in Beijing, China by the same technician with the same protocol as established previously in Dr. Yi Zeng’s laboratory. Positive control (plasmid with HPV16-pBR322 DNA fragment) and negative control (double water) were set in each HPV-DNA amplification process. Each experimental step was taken following the previously established rules.
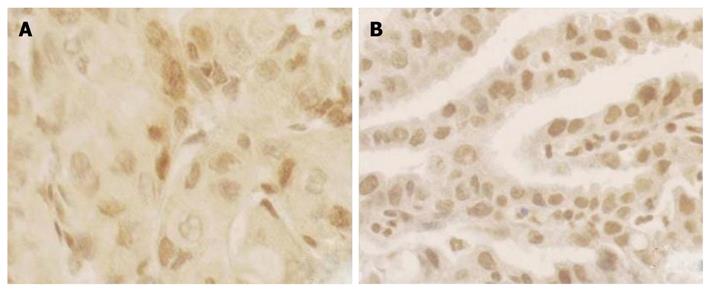
For immunohistochemistry, sections of paraffin-embedded tissue with a thickness of 3 μm were deparaffinized by passage through xylene. After the endogenous peroxidase activity was blocked with 0.3% H2O2, the slides were then rehydrated with 0.01 mol/L sodium phosphate/citrate buffer at pH 8.0 and heated in 0.01 mol/L-citrate buffer at pH 6.0, 95°C for 30 min to retrieve the antigen. After rinsed in 0.01 mol/L phosphate-buffered saline (PBS) at pH 7.4, nonspecific antibody binding was reduced by incubating the sections with 10% fetal bovine serum in PBS for 30 min. The sections were incubated overnight at 4°C with a mouse monoclonal antibody of p16INK4A protein (1:200 dilution, PharMingen International). After washing thoroughly with PBS, the slides were incubated with biotinylated horse anti-mouse IgG for 30 min followed by 1:100 dilution of the Avidin-Biotin-Peroxidase Complex (Vectastain elite ABC kit, Vector Laboratories, Burlingame, CA) for an additional 30 min. The peroxidase signal was visualized by treatment with DAB substrate-chromogen system (DAKO) for 8 min. Finally, the sections were stained lightly with hematoxylin. In statistical analysis, those having less than 10% cells stained positive were classified as negative and the others were regarded as positive cases[16] (Figure 3).
Fisher’s exact test was used to examine the association between HPV status and each clinicopathological factor including p16 expression. All the P values presented in the present study were two-sided. Experimental data were analyzed by statistical software SPSS 13.0.
The results of the PCR showed that, in the 17 CC, the detection rate of HPV16 in the ESCC was 47% (8/17), and 29% (5/17) in GCA. There was no significant difference between the two groups (P > 0.05). Two positive and six negative cases of ESCC and GCA were found in the HPV16-DNA simultaneously, illustrating the high consistency of HPV involved in the two kinds of tumors (47%) (Table 1).
| Sample No. | Age (yr) | Gender | ESCC | GCA | ||||||
| Differentiation | Stage | HPV | P16 | Differentiation | Stage | HPV | P16 | |||
| 1 | 57 | Male | M | T1N0M0 | + | - | M | T3N0M0 | + | + |
| 2 | 60 | Male | M | T1N0M0 | + | + | L | T2N0M0 | + | + |
| 3 | 62 | Female | H | T3N0M0 | + | - | M | T2N0M0 | - | + |
| 4 | 62 | Male | M | T2N0M0 | - | + | H | T2N0M0 | + | - |
| 5 | 50 | Male | M | T1N0M0 | - | + | M | T3N0M0 | - | + |
| 6 | 68 | Male | M | T1N0M0 | - | - | H | T3N0M0 | - | - |
| 7 | 71 | Male | M | T3N0M0 | + | - | L | T3N0M0 | - | - |
| 8 | 61 | Female | M | T2N0M0 | + | - | M | T1N1M0 | - | + |
| 9 | 51 | Male | H | T3N0M0 | - | + | L | T3N0M0 | + | + |
| 10 | 48 | Male | M | T1N0M0 | + | - | M | T3N0M0 | - | - |
| 11 | 57 | Female | M | T3N0M0 | - | - | L | T3N0M0 | - | - |
| 12 | 55 | Male | H | T2N0M1 | + | + | M | T3N1M0 | - | + |
| 13 | 57 | Female | L | T3N0M0 | + | - | M | T1N0M0 | - | + |
| 14 | 67 | Male | M | T2N0M0 | - | + | L | T1N0M0 | + | + |
| 15 | 64 | Male | M | T3N0M0 | - | + | L | T2N0M0 | - | + |
| 16 | 51 | Male | M | T1N0M0 | - | - | L | T3N1M0 | - | + |
| 17 | 52 | Female | H | T1N0M0 | - | - | L | T3N0M0 | - | - |
In the 17 cases with CC, the positive ratio of P16INK4A protein expression was 41% (7/17) in ESCC and 59% (10/17) in GCA, respectively. Twelve patients showed P16INK4A protein changes (71%), among them, six patients were P16INK4A immunoreaction positive (35%) in ESCC and GCA simultaneously.
According to the Fisher’s Exact test, there was no significant difference between HPV infection and P16INK4A protein expression in the ESCC and GCA specimens (P > 0.05). However, among the eight HPV positive ESCC specimens, only two expressed P16INK4A, while four expressed P16INK4A in the HPV positive GCA tissues.
The present study demonstrates that both ESCC and GCA tissues from the same patient had HPV16-DNA infection, with an incidence of 47% and 29%, respectively. And HPV16 E6-DNA was observed in both ESCC and GCA tissues in two patients simultaneously. These results suggest that HPV16 might participate in the carcinogenesis of the ESCC and GCA.
In the Linzhou area in Henan, almost half of the ESCC patients have HPV16 infection, with a higher incidence than in GCA (47% vs 29%), but without a significant difference (P > 0.05). However, there were no correlations between HPV16 infection and gender, age, tumor size, depth of penetration, differentiation, lymph node metastasis and TNM stage (all P > 0.05). The difference and the mechanism of the affinity specificity of HPV16 to squamous epithelium and styloid glandular epithelium are still not clear[17]. Esophageal epithelium and cardia epithelium are under the same internal environment and heredity of the same organism, as well as exposure to the same environment and carcinogenic agents, which may be associated with the co-infection of the two different epithelial tissues for HPV. Recently, it has been reported that colonic epithelium and colon carcinoma have HPV infections[18-20], and further studies are still needed to confirm the biological significance and mechanisms for HPV invading the body.
Over the past 20 years, many reports regarding HPV infection in EC have been published, and the reported HPV detection rate in the literature varied largely. To explain these marked differences, different region, sampling methods, demographic and ethnic factor, disease status, and sensitivity of detection methods have been cited as potential causes of this inconsistency[11,21]. In the present study, the same method was used to analyze the two kinds of neoplasms with different histological type in the same patient, which greatly reduced the disparity of the methodology and/or population.
CC is an ideal model for illustrating the environmental influence on both ESCC and GCA with the similar geno-background and comparability of environmental agents. The present study will deepen the understanding on the mechanism of esophageal/cardia carcinoma in this area. Although these results indicating the presence of HPV-DNA in esophagus and gastric cardia carcinoma tissues, suggest a possible role for HPV in upper digestive tract tumors, further studies are necessary for establishing a definite causative role.
P16INK4A gene is an important member of P53-Rb system, and its product P16INK4A protein can prevent the cell to enter S-phase from G1-phase, and suppress cell proliferation, through inhibition of the phosphorylation of the retinoblastoma (pRb)[22]. In this cascade regulation, pRb could negatively inhibit the expression of P16INK4A protein.
The studies of cervical cancer showed that the HPV-E6/E7 protein combined with pRb is deactivated, and removes the negative inhibition of the expression of P16INK4A protein, which causes the over-expression of P16INK4A. In the present study, 7 (41%) cases showed the expression of P16INK4A protein in ESCC, and 10 (59%) in GCA, which is similar to the results in the adenocarcinoma of the uterine cervix by Ansari-Lari et al[23]. In the study of HPV-associated cervical cancer, expression of P16INK4A protein is higher in the adenocarcinoma than in the squamous cell carcinoma, and combined detection of HPV infection and P16INK4A protein expression would be helpful to the diagnosis of the primary adenocarcinoma of the uterine cervix[24]. In the present study, 2 of the 8 HPV-positive ESCC cases expressed P16INK4A, while 4 of 5 HPV-positive GCA cases expressed P16INK4A. These results suggested that in the HPV- associated CC, expression of P16INK4A protein in GCA is higher than in ESCC. It is obvious that it would be of great significance to further understand the molecular discrepancy of the HPV16 positive and negative patients, and explore the exact mechanisms of the role of HPV in the carcinogenesis of esophagus and cardia.
Esophageal cancer (EC) is one of the most common malignant diseases, with a remarkable geographical distribution and poor prognosis. The five-year survival rate is only 10%. However, the five-year survival rate for the patients with the early EC is more than 90%. More than 85% of the EC patients are diagnosed at the late stage due to lack of early specific symptoms and unknown etiological factors, and the carcinogenesis remains the leading cause of late diagnosis for EC. Therefore, the current challenges in EC research are to obtain a better understanding of the exact etiological factors and molecular alteration in the esophageal carcinogenesis process to establish the strategies for prevention and early diagnosis of those with high risks.
It has been well recognized that esophageal carcinogenesis is a progressive process involving multi-factors and multistage: tobacco, alcohol, fungal toxins, nutritional deficiencies, as well as infectious agents, are related to esophageal carcinogenesis. Among the infectious agents, human papillomavirus (HPV), a major cause of carcinoma of the cervix uteri throughout the world, is strongly implicated in the etiology of EC. HPV-16 and-18 are the most frequent genotypes, especially type-16 which is considered to be a risk factor for EC in the high prevalence area in Henan. On the other hand, oncoproteins of the high-risk HPV types can efficiently destroy the cell cycle and apoptotic pathways by binding to a number of host cell proteins, ultimately resulting in malignant transformation.
In this study, the authors found the HPV-16 DNA in 47% of esophageal squamous cell carcinomas (ESCC) and 29% of gastric cardiac adenocarcinomas (GCA) in concurrent cancers in the same patients (CC). Interestingly, HPV-16 DNA was detected in two cases of ESCC and GCA tissues simultaneously. These results suggested that HPV16 might participate in the carcinogenesis of the ESCC and GCA, and the two carcinomas might have similar risk of carcinogenesis. P16INK4A was highly expressed in both ESCC and GCA tissues. In the HPV-associated positive CC, higher P16INK4A expression was found in GCA than in ESCC (75% vs 25%, P < 0.05).
By knowing the prevalence and molecular alteration of high-risk HPV-associated EC, this study may contribute to the future strategies for prevention and early diagnosis of HPV-related malignancies, through the development of effective vaccines and biomarkers.
HPVs are DNA viruses that infect basal skin and mucosal cells, and categorized according to their cervical oncogenicity-based risks.
The manuscript is interesting, presenting data of HPV DNA detection in the concurrent esophageal squamous cell carcinoma and cardia adenocarcinoma and expression of p16 protein. Given the truth of these data, this would be a piece of evidence for involvement of HPV in esophageal carcinogenesis in man.
Peer reviewer: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Shi ZF L- Editor Ma JY E- Editor Lin YP
| 1. | Ferlay J, Bray F, Pisani P, Parkin DM. Globocan 2000: Cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press 2001; . |
| 2. | Li B, Taylor PR, Li JY, Dawsey SM, Wang W, Tangrea JA, Liu BQ, Ershow AG, Zheng SF, Fraumeni JF Jr. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3:577-585. |
| 3. | Tytgat GN, Bartelink H, Bernards R, Giaccone G, van Lanschot JJ, Offerhaus GJ, Peters GJ. Cancer of the esophagus and gastric cardia: recent advances. Dis Esophagus. 2004;17:10-26. |
| 4. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. |
| 5. | Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796-802. |
| 6. | Syrjänen K, Pyrhönen S, Aukee S, Koskela E. Squamous cell papilloma of the esophagus: a tumour probably caused by human papilloma virus (HPV). Diagn Histopathol. 1982;5:291-296. |
| 7. | Talamini G, Capelli P, Zamboni G, Mastromauro M, Pasetto M, Castagnini A, Angelini G, Bassi C, Scarpa A. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. Int J Cancer. 2000;86:874-878. |
| 8. | Li SY, Li Y, Wang LD, Wu XZ, Zhou L, Zhao XY, Liu HT, Zeng Y. [Detection of human papillomavirus in tissues of esophageal carcinomas by polymerase chain reaction]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2008;22:251-253. |
| 9. | Koshiol J, Wei WQ, Kreimer AR, Ren JS, Gravitt P, Chen W, Kim E, Abnet CC, Zhang Y, Kamangar F. The gastric cardia is not a target for human papillomavirus-induced carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2010;19:1137-1139. |
| 10. | Xu WG, Zhang LJ, Lu ZM, Li JY, Ke Y, Xu GW. [Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract]. Zhonghua Yixue Zazhi. 2003;83:1910-1914. |
| 11. | Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-728. |
| 12. | Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929-934. |
| 13. | zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342-350. |
| 14. | Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741-1748. |
| 15. | Greer CE, Whee Le CM, Manos MM. PCR primer a laboratory manual. New York: Cold Spring Harbor Laboratory Press 1995; 64-69. |
| 16. | Ralhan R, Mathew R, Arora S, Bahl R, Shukla NK, Mathur M. Frequent alterations in the expression of tumor suppressor genes p16INK4A and pRb in esophageal squamous cell carcinoma in the Indian population. J Cancer Res Clin Oncol. 2000;126:655-660. |
| 17. | Zhou XB, Guo M, Quan LP, Zhang W, Lu ZM, Wang QH, Ke Y, Xu NZ. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World J Gastroenterol. 2003;9:1170-1173. |
| 18. | Salepci T, Yazici H, Dane F, Topuz E, Dalay N, Onat H, Aykan F, Seker M, Aydiner A. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. J BUON. 2009;14:495-499. |
| 19. | Bognár G, István G, Ledniczky G, Ondrejka P. [Detection of human papilloma virus type 16 in squamous cell carcinoma of colon and its lymph node metastases]. Magy Seb. 2008;61:225-229. |
| 20. | Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039-1048. |
| 21. | Shuyama K, Castillo A, Aguayo F, Sun Q, Khan N, Koriyama C, Akiba S. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. Br J Cancer. 2007;96:1554-1559. |
| 22. | Yang W, Zhang Y, Tian X, Ning T, Ke Y. p53 Codon 72 polymorphism and the risk of esophageal squamous cell carcinoma. Mol Carcinog. 2008;47:100-104. |
| 23. | Ansari-Lari MA, Staebler A, Zaino RJ, Shah KV, Ronnett BM. Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol. 2004;28:160-167. |
| 24. | Murphy N, Ring M, Killalea AG, Uhlmann V, O'Donovan M, Mulcahy F, Turner M, McGuinness E, Griffin M, Martin C. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56-63. |