Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4575
Revised: June 23, 2010
Accepted: June 30, 2010
Published online: September 28, 2010
AIM: To prove that the protein expression level of thymidylate synthase is a predictive factor for the response to S-1/cisplatin (CDDP) chemotherapy in gastric cancer.
METHODS: We measured the protein expression levels of thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD), and orotate phosphoribosyltransferase (OPRT) in advanced gastric cancer. Before S-1/CDDP chemotherapy, tumor specimens from primary sites were obtained by endoscopic biopsy and analyzed by enzyme-linked immunosorbent assay. The chemotherapeutic effects on the primary sites were evaluated by endoscopic biopsy performed more than once after S-1/CDDP chemotherapy. The effects are a predictive factor for the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer, as evaluated by endoscopic biopsy over time.
RESULTS: The protein expression level of TS was significantly higher (P < 0.05) in the tumor than in the normal tissue, and significantly lower (P < 0.05) in the responders than in the non-responders. We were able to evaluate the correlation between changes in the protein expression levels of TS, DPD and OPRT and chemotherapeutic responses in 7 patients by assessing tumor tissues more than twice. In the responders, the protein expression level of TS was < 40 ng/mg protein. However, there were significant increases in the protein expression levels of TS (P < 0.01) and DPD (P < 0.05) after chemotherapy in 3 patients. In these cases, the patient assessment changed from “responder” to “non-responder”. In the non-responders, the protein expression level of TS was > 40 ng/mg protein.
CONCLUSION: We have confirmed that the protein expression level of TS is a predictive factor for the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer.
- Citation: Miyazaki I, Kawai T, Harada Y, Moriyasu F. A predictive factor for the response to S-1 plus cisplatin in gastric cancer. World J Gastroenterol 2010; 16(36): 4575-4582
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4575.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4575
In Japan, S-1 plus cisplatin (S-1/CDDP) chemotherapy is currently the most commonly used first-line chemotherapeutic regimen in patients with advanced gastric cancer. This is attributed to the high response rate (76%)[1] and significantly longer survival of patients administered with S-1/CDDP chemotherapy than with S-1 alone, as demonstrated in a randomized phase III study[2]. However, it has also been reported that about 25% of patients treated with S-1/CDDP chemotherapy failed to show a significant response. Therefore, accurate prediction of the response to chemotherapy is essential for identifying the most effective drug and form of chemotherapy.
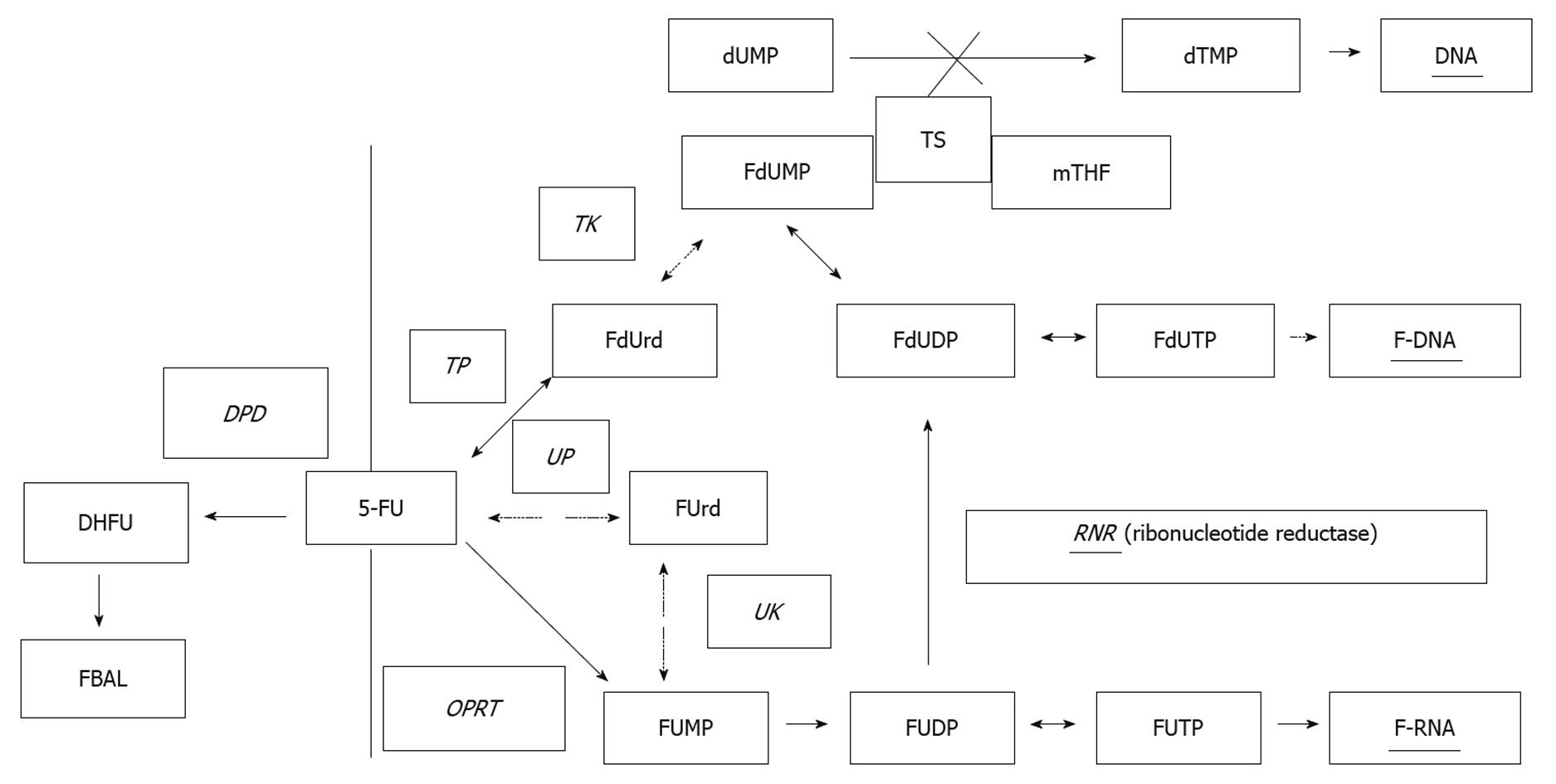
For predicting the response to chemotherapy, metabolic enzymes have recently received considerable attention as possible predictors. While the mechanism of metabolism of 5-fluorouracil (5-FU), a principal fluoropyrimidine used against colorectal cancer, has been clarified by many researchers, there have also been reports on the relationship between various metabolic enzymes and drug sensitivity, as well as between enzymes and clinical response. Recent studies have focused on the relationship between thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD) or orotate phosphoribosyltransferase (OPRT) and prediction of response to fluoropyrimidines[3-7] (Figure 1).
The aim of this study was to confirm whether TS, DPD and OPRT can be used as predictors of the response of patients with advanced gastric cancer to S-1/CDDP chemotherapy by measuring their expression level from biopsy specimens over time. Measurements over time were carried out in biopsy specimens sampled using an endoscope. Endoscopic biopsy specimens have conventionally been considered to pose difficulties in the measurement of enzyme expression level owing to their small size. Nevertheless, we succeeded in measuring enzyme expression level in small specimens by enzyme-linked immunosorbent assay (ELISA)[8]. Effect of treatment on advanced gastric cancer was then examined by endoscopy. Endoscopic examination made it possible to confirm precisely whether TS, DPD and OPRT can be used as effective predictors of the response to S-1/CDDP chemotherapy using gastric cancer specimens. In addition, we also assessed whether the commonly used tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) can also be used as effective predictors of the response to S-1/CDDP chemotherapy.
Patients with locally advanced or metastatic gastric cancer with primary sites were considered eligible. Further eligibility criteria were as follows: (1) histologically confirmed gastric adenocarcinoma; (2) an Eastern Cooperative Oncology Group performance status of 0-2; (3) over 20 years of age; (4) no prior chemotherapy or radiotherapy; (5) sufficient hematological, renal and hepatic functions; and (6) life expectancy over 12 wk. Written informed consent was obtained from all patients. This study was approved by the institutional ethical review board of Tokyo Medical University Hospital.
Patients received S-1 orally twice daily at least 1 h after breakfast and supper on days 1 to 21, followed by a 14-d recovery period. S-1 dosage according to the body surface area (BSA) of a patient was as follows: BSA < 1.25 m2, 40 mg twice daily (80 mg day 1); BSA ≥ 1.25 m2 but < 1.5 m2, 50 mg twice daily (100 mg day 1); BSA ≥ 1.5 m2, 60 mg twice daily (120 mg day 1). CDDP was administered intravenously over 2 h at 60 mg/m2 on day 8. Chemotherapy cycles were repeated every 35 d.
Chemotherapeutic effects on primary sites were evaluated endoscopically by response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria (Japanese Classification of Gastric Carcinoma-2nd English Edition)[9]. Patients were classified into 2 groups: “responders” and “non-responders”. “Responders” was defined as patients with complete response (CR: disappearance of all tumoral lesions and no diagnosis of any cancers) or partial response (PR: dramatic regression, flattening on endoscopic examination roughly corresponding to at least a 50% decrease in tumor size). In this study, many metastatic lesions were presented in the form of lymph node or peritoneal dissemination. The shrinkage or growth of these lesions was difficult to evaluate only through the imaging procedure, and therefore only the primary lesions were evaluated. We accordingly added tumor markers for evaluation.
Biopsy specimens were obtained from 2 sites of the tumor and 2 sites of the normal stomach area before the first chemotherapy and after every 2 cycles of chemotherapy. The forceps used were FB-25K-1 (Olympus Corp., Tokyo, Japan) and Radial Jaw3 1534 (Boston Scientific Corp., MA, USA). All specimens were immediately frozen and stored at -80°C. The specimens were assigned anonymous marks and enzyme expression level was measured by the Pharmacokinetic Research Laboratory of Taiho Pharmaceutical Co., Ltd. Specifically, the protein expression levels of TS, DPD and OPRT were determined by ELISA[10].
To evaluate the correlation between the protein expression levels of TS, DPD and OPRT and the anti-tumor effects of S-1/CDDP chemotherapy, the protein expression level before chemotherapy and the endoscopic assessment of chemotherapeutic effects after S-1/CDDP chemotherapy were ascertained. The Student’s t-test was used to compare protein expression level and various factors. The paired t-test was used to compare tumor and normal areas, responders and non-responders, and the first and last measurements in each patient. The JMP software program (version 7.0) was used in all analyses, and P < 0.05 was considered significant.
Fourteen patients were enrolled in this study between December 2005 and July 2007 conducted at the Department of Gastroenterology and Hepatology, Tokyo Medical University Hospital, Japan. Patient characteristics are shown in Table 1. All of the patients (9 males and 5 females; median age, 64 years) had primary sites of advanced gastric cancer. The primary site was the gastric body in 9 patients, gastric fundus in 2, and vestibular area in 3. The size of the primary tumor was < 5 cm in 3 patients, < 10 cm in 7, and at least 10 cm in 4. The histological type was highly differentiated adenocarcinoma in 6 patients and poorly differentiated adenocarcinoma in 8. The metastatic site was the lymph node in 3 patients, peritoneum in 5, liver in 5, and ovary in 1.
| Sex | Age (yr) | Performance status | Site | Size (cm) | Pathology | Metastasis |
| Male | 62 | 1 | Antrum | 5 | Diffuse | Peritoneum |
| Female | 52 | 0 | Fundus | 4 | Diffuse | Ovary |
| Female | 67 | 2 | Corpus | 10 | Diffuse | Lymph node |
| Male | 68 | 2 | Corpus | 8 | Intestinal | Lymph node |
| Male | 78 | 2 | Corpus | 15 | Intestinal | Lymph node |
| Male | 64 | 2 | Corpus | 8 | Intestinal | Liver |
| Male | 67 | 2 | Fundus | 5 | Intestinal | Liver |
| Female | 70 | 2 | Corpus | 5 | Diffuse | Peritoneum |
| Male | 65 | 1 | Corpus | 4 | Diffuse | Peritoneum |
| Female | 59 | 2 | Corpus | 15 | Diffuse | Peritoneum |
| Male | 67 | 2 | Corpus | 5 | Diffuse | Liver |
| Male | 39 | 2 | Corpus | 20 | Diffuse | Peritoneum |
| Female | 62 | 0 | Antrum | 7 | Intestinal | Liver |
| Male | 77 | 1 | Antrum | 3 | Intestinal | Liver |
The protein expression levels of TS, DPD and OPRT were measured in all 14 patients by ELISA before the first S-1/CDDP chemotherapy. The tumors and normal areas could also be measured in all 14 patients for DPD but in only 12 patients for TS and 12 patients for OPRT. The protein expression levels of TS, DPD and OPRT were 42.9 ± 19.1 ng/mg protein, 156.6 ± 63.3 ng/mg protein, and 9.3 ± 5.8 ng/mg protein in the tumors, and 21.5 ± 13.7 ng/mg protein, 168.1 ± 36.5 ng/mg protein, and 10.1 ± 6.1 ng/mg protein in the normal areas, respectively. The protein expression level of TS, but not of DPD and OPRT, in the tumors was significantly higher than that in the normal areas (P < 0.001) (Table 2). There was no difference in the protein expression levels of TS, DPD and OPRT in the tumors with regard to patient characteristics, gender, tumor size and pathological type.
| No. of cases | Tumour tissue | Normal tissue | Paired t-test | |
| TS | 12 | 42.9 ± 19.9 (median 40.5) | 21.5 ± 13.7 (median 19.9) | P < 0.001 |
| DPD | 14 | 156.6 ± 63.3 (median 143.3) | 168.1 ± 36.5 (median 170.3) | NS |
| OPRT | 12 | 9.3 ± 5.8 (median 8.7) | 10.1 ± 6.1 (median 8.2) | NS |
The relationship between the protein expression levels of TS, DPD and OPRT in the tumors and the endoscopic assessment of the first chemotherapeutic response was investigated. Measurement of expression levels from biopsy specimens, administration of S-1/CDDP chemotherapy, and endoscopic assessment of the primary tumor could be carried out in 10 of the 14 patients. The protein expression level of TS in responders was significantly lower (27.4 ± 9.4 ng/mg protein) than that in non-responders (56.9 ± 19.9 ng/mg protein) (P < 0.05, Table 3). There was no marked difference in the protein expression levels of DPD and OPRT between responders and non-responders.
| Responder (n = 6) | Non-reponder (n = 4) | t-test | |
| TS | 27.4 ± 9.4 (median 29.2) | 56.9 ± 19.9 (median 53.6) | P < 0.05 |
| DPD | 170.9 ± 91.4 (median 135.7) | 148.9 ± 33.5 (median 155.6) | NS |
| OPRT | 6.1 ± 3.7 (median 6.5) | 9.6 ± 9.7 (median 5.8) | NS |
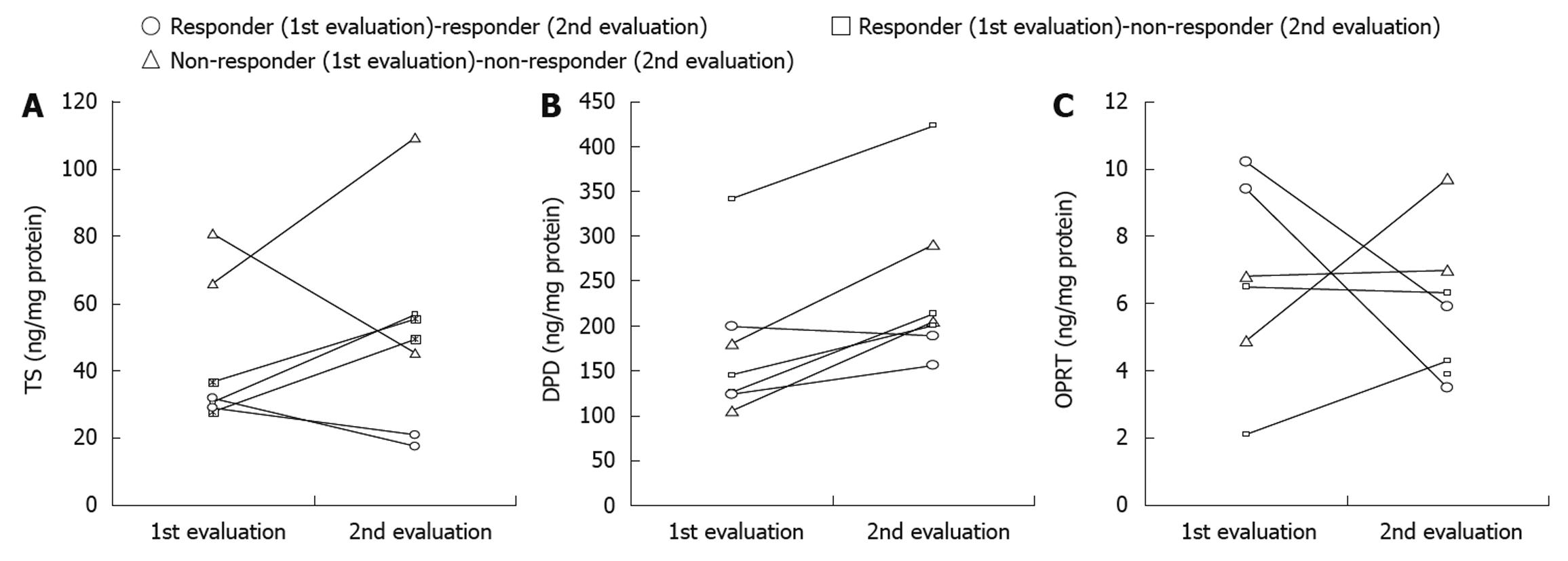
The correlation between the changes in the protein expression levels of TS, DPD and OPRT and the chemotherapeutic responses could be evaluated more than twice in each of 7 patients. After the first S-1/CDDP chemotherapy, 5 of the 7 patients showed a response and the remaining 2 showed no response. Of the 5 responders, 2 showed a continuous response and the remaining 3 started to show a worse response after several courses. Two non-responders in the first chemotherapy underwent a second S-1/CDDP chemotherapy but showed no response.
The protein expression levels of TS, DPD and OPRT before the first chemotherapy were compared with those after tumor progression or with the latest measurement in each patient. In 2 patients, a continuous but nonsignificant response was observed from the initial response to the last response with regard to the protein expression levels of TS, DPD and OPRT. On the other hand, in 3 patients who showed tumor progression after the initial response, a significant increase in the protein expression levels of TS (P < 0.01) and DPD (P < 0.05) was observed at the time of non-response compared with the time of the initial response. In addition, in the 2 non-responders after the second chemotherapy, a significant increase in the protein expression level of DPD (P < 0.05) was observed. These results clearly show that the changes in the protein expression level of TS in the primary tumors correlated with the changes in the response to S-1/CDDP chemotherapy in these patients (Table 4). The changes in the individual protein expression levels of TS, DPD and OPRT in the 7 patients are shown in Figure 2. In these 7 patients, the protein expression level of TS was 27.6 ± 6.5 ng/mg protein in the responders and 66.1 ± 22.4 ng/mg protein in the non-responders (P < 0.05). The protein expression levels of DPD and OPRT were 181.1 ± 75.7 ng/mg protein and 6.2 ± 3.2 ng/mg protein, respectively, in the responders and 231.0 ± 100.6 ng/mg protein and 6.1 ± 2.0 ng/mg protein, respectively, in the non-responders.
| 1st evaluation | 2nd evaluation | Paired t-test | |
| Responder(1st evaluation) → responder (2nd evaluation) (n = 2) | |||
| TS | 30.0 ± 2.8 | 19.2 ± 2.3 | NS |
| DPD | 161.7 ± 53.3 | 172.6 ± 22.8 | NS |
| OPRT | 9.8 ± 0.6 | 4.7 ± 1.7 | NS |
| Responder(1st evaluation) → non-responder(2nd evaluation) (n = 3) | |||
| TS | 31.7 ± 4.6 | 53.9 ± 3.9 | P < 0.01 |
| DPD | 204.4 ± 119.5 | 279.1 ± 124.7 | P < 0.05 |
| OPRT | 4.3 ± 3.1 | 5.3 ± 1.3 | NS |
| Non-responder(1st evaluation) → non-responder (2nd evaluation) (n = 2) | |||
| TS | 73.4 ± 10.7 | 77.3 ± 45.4 | NS |
| DPD | 142.3 ± 52.6 | 247.5 ± 60.5 | P < 0.05 |
| OPRT | 5.9 ± 1.3 | 8.4 ± 1.9 | NS |
Correlations between CEA or CA19-9 level and the endoscopy-assessed chemotherapeutic response of the primary tumor were also studied. We could evaluate the correlation between CEA or CA19-9 level and the endoscopy-assessed response of the primary tumor after the first S-1/CDDP chemotherapy in 10 of the 14 patients. The CEA and CA19-9 levels in 6 responders were 6.0 ± 4.2 mg/dL and 110.4 ± 116.8 mg/dL, and 51.4 ± 86.7 mg/dL and 7168.9 ± 14 268.8 mg/dL in 4 non-responders, respectively. In 7 patients, although the correlation between the changes in the CEA and CA19-9 levels and the responses could be evaluated more than twice in each patient, no correlations were found.
This study examined whether the protein expression level of TS is a predictive factor for the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer. Patients with a low protein expression level of TS showed a response to S-1/CDDP chemotherapy, whereas those with a high protein expression level of TS showed no response to S-1/CDDP chemotherapy. Furthermore, in 3 patients who showed tumor progression after the initial response, a significant increase in the protein expression level of TS (P < 0.01) was observed at the time of tumor progression compared with the time of the initial response. On the other hand, although the protein expression level of DPD had not been expected to be correlated with the response to S-1/CDDP chemotherapy, the results indicate a possible association.
S-1 is a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, which has been shown to produce a high response rate against advanced gastrointestinal cancer in phase II studies[11,12]. S-1 is an oral anticancer agent consisting of tegafur, the prodrug of 5-FU, 5-chloro-2,4-dihydroxypyridine, a strong DPD inhibitor, and potassium oxonate, which inhibits OPRT in the gastrointestinal tract, resulting in the suppression of gastrointestinal toxicity caused by the phosphoribosylation of 5-FU[13]. Considering that cancer has a high proliferative ability, nucleic acid metabolism is more active and the nucleic acid metabolic enzyme normally shows increased levels in cancer[14].
Amatori et al[15] reported that high sensitivity to 5-FU was associated with a low expression level of TS in vitro. Salonga et al[16] described that the intratumoral gene expression level of DPD was associated with tumor response to 5-FU. Meropol et al[17] conducted a biomarker analysis and provided preliminary evidence that the expression level of TP (thymidine phosphorylase) may be a predictive marker for treatment response in patients with metastatic colorectal cancer. In addition, Honda et al[18] reported that TP and DPD are predictive factors for the therapeutic efficacy of capecitabine monotherapy for breast cancer. On the other hand, Harada et al[19] reported that the expression of TS in biopsy samples before S-1 chemotherapy was significantly lower in responders than in non-responders with oral squamous cell carcinoma (P = 0.0001). Ichikawa et al[20] investigated simple combinations of 2 genes, namely, OPRT and TS, which may allow the identification of gastric cancer patients who will benefit from S-1 chemotherapy. Shimizu et al[21] reported that S-1 may be effective even in gastric scirrhous carcinoma with a high level of DPD activity.
These previous results are consistent with ours. However, the specimens used for assessment in most of these past studies had been resected during surgery. In contrast, our assessment is based on endoscopic biopsy specimens. Since the amount of specimens that were endoscopically collected by biopsy in our study was very small, we used ELISA for all measurements to confirm whether the protein expression levels of TS, DPD and OPRT may be a predictive factor for the response to S-1/CDDP chemotherapy. Fukui et al[22] investigated the differences in the protein expression levels of TS, DPD and OPRT by ELISA in various tumor tissue specimens. They reported that comparison of the protein expression levels of these enzymes among matched tumor and non-tumor tissue specimens revealed significantly higher expression levels of TS (25.3 ng/mg protein) and DPD (150.3 ng/mg protein) in gastric cancer. This suggests that measurements of even small amounts of endoscopic biopsy materials would not be different from those of large-scale studies. Further, Koga et al[23] collected biopsy materials from the oral cavity of a great number of patients and tried to determine TS and DPD with ELISA. Their published report also showed that a small amount of specimens could produce correct measurements.
The present study indicated that the protein expression level of TS was significantly lower (P < 0.05) in the responders than in the non-responders. This result is consistent with the findings of previous reports which showed fluorinated pyrimidine to be effective in the case of a low expression level of TS. In 2 patients who continued to show low protein expression levels of TS, the responses to S-1/CDDP chemotherapy continued for more than 5 and 8 mo during the study period. In addition, 2 non-responders after the first course of S-1/CDDP chemotherapy demonstrated continuously high protein expression levels of TS. As expected, the second course of S-1/CDDP chemotherapy was also not effective. Because 2 responders continued to show both low protein expression level of TS and a high chemotherapeutic response, unfortunately we could not identify 5 patients whose chemotherapeutic evaluations changed from “responder” to “non-responder” during the study period. However, these results clearly proved that the protein expression level of TS in gastric cancer is a predictive factor for the response to S-1/CDDP chemotherapy. In all responders, the protein expression level of TS was ≤ 36.7 ng/mg protein, whereas it was ≥ 45.2 ng/mg protein in all non-responders. It was considered that 40 ng/mg protein was therefore the approximate cutoff value between responders and non-responders.
We suspected that the protein expression level of DPD may not be related to response to S-1/CDDP chemotherapy, because S-1 is a strong DPD-inhibitory fluoropyrimidine drug. In the present study, there was no difference in the protein expression level of DPD between the responders and the non-responders after the first course of S-1/CDDP chemotherapy. Therefore, it is speculated that the protein expression level of DPD is not a predictive factor for response to S-1/CDDP chemotherapy in gastric cancer. However, in this study on the relationship between the changes in the protein expression level of DPD and the endoscopy-assessed response at the individual level, a significant increase in the protein expression level of DPD (P < 0.05) was observed in the patients with proceeding tumor progression.
The results indicate that a significant increase in the protein expression level of DPD can be considered a predictive factor for the progression of S-1/CDDP chemotherapy at the individual level. The protein expression level of DPD was increased in 6 of 7 patients (Figure 2); it will be necessary to investigate a large number of patients in the future before any definitive conclusion can be made.
Regarding the relationship between the changes in the protein expression level of OPRT and the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer, no data indicating any type of relationship was obtained.
How to select an effective chemotherapeutic agent to avoid unnecessary treatment in patients with advanced gastric cancer, specifically in terms of how comfortably patients can spend their survival time, in addition to the problem of how to improve patient survival whenever possible, are highly important questions that must be taken into consideration. Presently in Japan, S-1/CDDP chemotherapy is the most popular regimen as first-line chemotherapy in patients with advanced gastric cancer. Therefore, one alternative method is to attempt a tailor-made treatment to predict the effectiveness of S-1/CDDP chemotherapy for advanced gastric cancer by monitoring the protein expression level of TS in endoscopic biopsy specimens.
Based on the results of this study, it has been confirmed that the response to S-1/CDDP chemotherapy in patients with a low protein expression level of TS should be determined, and that the same S-1/CDDP chemotherapy should be continuously administered as the first-line treatment. However, the first-line treatment should be changed to the second-line treatment in patients whose protein expression level of TS tends to increase, considering that S-1/CDDP chemotherapy will become ineffective. In gastric cancer, specimens cannot easily be collected from the oral cavity or other parts of the body surface. Unlike surgical samples, many specimens cannot be obtained from different sites. Therefore, the number of samples was small in our study. While this was a weakness, the advantage was that the specimens could repeatedly be collected. Further investigation of a larger number of patients is required.
There is as yet no report in which the correlation between the protein expression levels of TS, DPD and OPRT and the effects of chemotherapy was studied more than twice in each patient. This study strongly suggested that the protein expression level of TS is a predictive factor for the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer, because changes in the protein expression level of TS correlated with changes in the response to S-1/CDDP chemotherapy after evaluating biopsy specimens more than twice in all patients.
In Japan, S-1 plus cisplatin (S-1/CDDP) chemotherapy is currently the most commonly used first-line chemotherapeutic regimen in patients with advanced gastric cancer. Medical doctors usually rely on their experience in deciding the best timing for changing first-line chemotherapy to second-line chemotherapy. Determination of the optimum timing for changing the treatment modality from first-line to second-line chemotherapy, together with the precise prediction of response to chemotherapy, is therefore expected to improve clinical outcome.
The specimens used for assessment in most past studies had been resected during surgery. In contrast, the authors’ assessment is based on endoscopic biopsy specimens. They measured the protein expression levels of thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD) and orotate phosphoribosyltransferase (OPRT). Before S-1/CDDP chemotherapy, tumor specimens from primary sites were obtained by endoscopic biopsy. Since the amount of specimens that were endoscopically collected by biopsy in their study was very small, they used enzyme-linked immunosorbent assay for all measurements to confirm whether the protein expression levels of TS, DPD and OPRT may be a predictive factor for the response to S-1/CDDP chemotherapy.
The protein expression level of TS was significantly higher in tumors than in normal tissue, and significantly lower in the responders than in the non-responders. There is as yet no report in which the correlation between the protein expression levels of TS, DPD and OPRT and the effects of chemotherapy was studied more than twice in each patient.
The authors have confirmed that the protein expression level of TS is a predictive factor for the response to S-1/CDDP chemotherapy in patients with advanced gastric cancer. This will assist medical doctors in avoiding chemotherapeutic regimens with strong side effects and thus prevent a decrease in the quality of life of patients.
“S-1” is an oral anticancer agent consisting of tegafur, the prodrug of 5-fluorouracil (5-FU), 5-chloro-2,4-dihydroxypyridine, a strong DPD inhibitor, and potassium oxonate, which inhibits orotate phosphoribosyltransferase in the gastrointestinal tract which results in the suppression of gastrointestinal toxicity caused by the phosphoribosylation of 5-FU.
This is an original article which discussed whether the quantity of the expression of TS proteins could be a predictive factor for the response of S-1/CDDP chemotherapy. This is a very significant and interesting topic, and also clinically very important and estimable.
Peer reviewer: Dr. Takuya Watanabe, Department of Intern Medicine and Gastroenterology, The Nippon Dental University School of Life Dentistry at Niigata, 1-8 Hamauracho, Chu-o-ku, Niigata 951-8580, Japan
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
| 1. | Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207-2212. |
| 2. | Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191-197. |
| 3. | Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215-237. |
| 4. | Beck A, Etienne MC, Chéradame S, Fischel JL, Formento P, Renée N, Milano G. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer. 1994;30A:1517-1522. |
| 5. | Etienne MC, Chéradame S, Fischel JL, Formento P, Dassonville O, Renée N, Schneider M, Thyss A, Demard F, Milano G. Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol. 1995;13:1663-1670. |
| 6. | Ishikawa Y, Kubota T, Otani Y, Watanabe M, Teramoto T, Kumai K, Kitajima M, Takechi T, Okabe H, Fukushima M. Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluorouracil on human tumor xenografts in nude mice. Clin Cancer Res. 1999;5:883-889. |
| 7. | Ususyo N, Yoshida M, Akimoto S, Yano K, Fujiwara R, Yautida S, Hagiike M, Deishi K, Aida F, Okada S. Prediction of effects and side effects of fluoropyrimidine administered for gastric cancers and criteria of drug selection:. Jpn J Cancer Clin. 2004;50:473-479. |
| 8. | Kurebayashi J, Yamamoto Y, Udagawa K, Okubo S, Fukushima M, Sonoo H. Establishment of enzyme-linked immunosorbent assays for thymidylate synthase and dihydropyriminide dehydrogenase in cancer tissues. Oncol Rep. 2004;11:973-979. |
| 9. | Japanese classification of gastric carcinoma--2nd English edition--response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer. 2001;4:1-8. |
| 10. | Ishida H, Shirakawa K, Ohsawa T, Sobajima J, Hayashi Y, Nakada H, Yokoyama M, Hashimoto D. [Expression of mRNA levels of thymidylate synthase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase of colorectal cancer--relationships among mRNA levels in association with response to 5-FU based treatment]. Gan To Kagaku Ryoho. 2005;32:1929-1934. |
| 11. | Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191-197. |
| 12. | Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T. Phase II study of S-1, a novel oral fluorophyrimidine derivative, in patients with metastatic colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer. 2000;83:141-145. |
| 13. | Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602-2606. |
| 14. | Ichikawa W, Takahashi T, Sudo K, Mituyoshi S, Yamashita T, Sirota Y, Nihei Y, Hirayama K. Predictive values of thymidine synthase and dehydropyrimidine dehydrogenase gene expression in gastric cancer patients treated with S-1 based chemotherapy. Jpn J Cancer Clin. 2003;49:599-560. |
| 15. | Amatori F, Di Paolo A, Del Tacca M, Fontanini G, Vannozzi F, Boldrini L, Bocci G, Lastella M, Danesi R. Thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer and normal mucosa in patients. Pharmacogenet Genomics. 2006;16:809-816. |
| 16. | Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman L, Diasio RB. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322-1327. |
| 17. | Meropol NJ, Gold PJ, Diasio RB, Andria M, Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E, Schwarting R. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:4069-4077. |
| 18. | Honda J, Sasa M, Moriya T, Bando Y, Hirose T, Takahashi M, Nagao T, Tangoku A. Thymidine phosphorylase and dihydropyrimidine dehydrogenase are predictive factors of therapeutic efficacy of capecitabine monotherapy for breast cancer-preliminary results. J Med Invest. 2008;55:54-60. |
| 19. | Harada K, Kawashima Y, Yoshida H, Sato M. Thymidylate synthase expression in oral squamous cell carcinoma predicts response to S-1. Oncol Rep. 2006;15:1417-1423. |
| 20. | Ichikawa W, Takahashi T, Suto K, Shirota Y, Nihei Z, Shimizu M, Sasaki Y, Hirayama R. Simple combinations of 5-FU pathway genes predict the outcome of metastatic gastric cancer patients treated by S-1. Int J Cancer. 2006;119:1927-1933. |
| 21. | Shimizu T, Yamada Y, Yasui H, Shirao K, Fukuoka M. Clinical application of immunoreactivity of dihydropyrimidine dehydrogenase (DPD) in gastric scirrhous carcinoma treated with S-1, a new DPD inhibitory fluoropyrimidine. Anticancer Res. 2005;25:2997-3001. |
| 22. | Fukui Y, Oka T, Nagayama S, Danenberg PV, Danenberg KD, Fukushima M. Thymidylate synthase, dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase mRNA and protein expression levels in solid tumors in large scale population analysis. Int J Mol Med. 2008;22:709-716. |
| 23. | Koga M, Anegawa E, Yoh J, Tsuyama H, Sakaino H, Iwamoto O, Koga C, Kusukawa J. Clinical relevance of thymidylate synthase (TS) activity for S-1-based chemotherapy in squamous cell carcinoma of the oral cavity. Br J Oral Maxillofac Surg. 2010;48:88-93. |