Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1285
Revised: January 3, 2010
Accepted: January 10, 2010
Published online: March 14, 2010
AIM: To examine whether heme oxygenase (HO)-1 overexpression would exert direct or indirect effects on Kupffer cells activation, which lead to aggravation of reperfusion injury.
METHODS: Donors were pretreated with cobalt protoporphyrin (CoPP) or zinc protoporphyrin (ZnPP), HO-1 inducer and antagonist, respectively. Livers were stored at 4°C for 24 h before transplantation. Kupffer cells were isolated and cultured for 6 h after liver reperfusion.
RESULTS: Postoperatively, serum transaminases were significantly lower and associated with less liver injury when donors were pretreated with CoPP, as compared with the ZnPP group. Production of the cytokines tumor necrosis factor-α and interleukin-6 generated by Kupffer cells decreased in the CoPP group. The CD14 expression levels (RT-PCR/Western blots) of Kupffer cells from CoPP-pretreated liver grafts reduced.
CONCLUSION: The study suggests that the potential utility of HO-1 overexpression in preventing ischemia/reperfusion injury results from inhibition of Kupffer cells activation.
- Citation: Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: The role of Kupffer cells. World J Gastroenterol 2010; 16(10): 1285-1292
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1285.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1285
Orthotopic liver transplantation (OLT) is an effective treatment for end-stage liver diseases[1]. However, ischemia/reperfusion (I/R) injury of the liver remains a major cause of graft injury, causing liver dysfunction and even failure posttransplantation[2,3]. The destructive effects of I/R arise from the acute generation of reactive oxygen species subsequent to reoxygenation, which inflicts direct tissue damage and initiates a cascade of deleterious cellular responses leading to inflammation, cell death, and organ failure[4]. Endothelial cell damage results from free radicals produced from Kupffer cells and adherent polymorphonuclear leukocytes (PMNs)[5]. Ultimately, this results in loss of microvascular integrity and reduction of blood flow (‘no-reflow phenomenon’)[6]. Methods to protect liver grafts against I/R injury have considerable clinical consequences.
Kupffer cells, the resident macrophages of the liver, are involved in liver I/R injury through the release of cytokines such as tumor necrosis factor-α (TNF-α) and other biologically active mediators[7-9]. Multiple lines of evidence have suggested that Kupffer cells are critical to the onset of liver injury and following secretion, cytokines aggravate hepatocyte damage. The use of in vitro and animal models has shown that inactivation of Kupffer cells prevents liver injury[10,11]. Activation of Kupffer cells directly or indirectly by endotoxin (lipopolysaccharide, LPS) results in the release of an array of inflammatory mediators, growth factors, and reactive oxygen species.
The heme oxygenase (HO) system is the rate-limiting step in the oxidative degradation of heme into biliverdin, carbon monoxide (CO) and free iron[12]. Three HO isoforms have been identified: inducible HO-1, also known as heat shock protein 32; constitutively expressed HO-2; and a related but less well-characterized HO-3. HO-1 is induced in a variety of organs during diverse stress-related conditions and is thought to provide cytoprotection[13,14]. Upregulation of HO-1 is known to be a protective response from cellular stress, following I/R injury, radiation and inflammation[15]. Overexpression of HO-1 exerts a cytoprotective function in a number of I/R injury and liver transplant models[16-18]. Thus, HO-1 is an attractive target for anti-inflammatory therapies and potential candidate responsible for cell injury.
Immunochemical studies with specific monoclonal antibodies have revealed the distribution of HO-1 in the rat liver with distinct topographical patterns[19]. HO-1 has been shown to be expressed principally in Kupffer cells[16,20-22]. However, exact mechanisms by which HO-1 induction may lead to cytoprotection during I/R injury in organ transplantation have not been fully clarified. We designed a study to evaluate the role of HO-1-mediated cytoprotection in rat liver transplantation models. The aim was to demonstrate whether HO-1 plays a critical role in inhibiting Kupffer cells activation.
Male Sprague-Dawley (S-D) Rats (Third Military Medical University Laboratory Animal Center, Chongqing, China) weighing 220-250 g were used. Animals were fed standard rodent chow and water ad libitum and cared for according to the local animal welfare guidelines. All procedures used in this study were approved by the ethics committee for the use of experimental animals at Kunming Medical College.
Metalloporphyrins (CoPP and ZnPP) were purchased from Sigma Chemical inc. USA. They were dissolved in 0.1 mol/L NaOH, subsequently adjusted to pH 7.4 with HCl, and diluted in 0.85% NaCl. The stock concentration was 0.5 mg/mL (CoPP) and 2 mg/mL (ZnPP).
S-D rats underwent ether anesthesia. The basic techniques of liver harvesting and orthotopic transplantation without hepatic arterial reconstruction were performed according to the method described previously by Kamada et al[23]. In the control group (n = 8), no drugs were applied. There were two treatment groups. In ZnPP group (n = 8), donors received ZnPP, an HO-1 inhibitor (20 mg/kg ip) 24 h prior to harvest. CoPP group (n = 8) rats received CoPP, an HO-1 inducer (5 mg/kg ip) 24 h prior to harvest. All liver grafts were harvested and stored with UW solution for 24 h at 4°C, and orthotopically transplanted into syngeneic S-D recipients. All transplant experiments in this study were performed by a single person. The anhepatic phase was 11.3 ± 0.7 min. Separate groups of rats were killed at 6 h after their vessels were unclamped, and liver samples were collected for further analysis.
At 6 h following by liver reperfusion, blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes. After centrifugation of whole blood (2000 g, 15 min), serum was stored at -70°C until analysis. Serum alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were measured using an automated clinical analyzer (7060 automatic analyzer, Hitachi, Japan).
Liver graft tissues were fixed in 10% formalin, embedded in paraffin. Serial sections of 3 μm thickness were stained with hematoxylin and eosin for routine pathological examination.
Kupffer cells were isolated from integral left liver tissue obtained from fresh specimens. The liver was perfused in vitro through the vena cava with 80 mL Hanks’ balanced salt solution (HBSS) Ca2+/Mg2+-free (Hyclone, Germany) at 37°C, and transferred to a 100 mm culture dish and perfusion was continued with complete HBSS containing 0.05% collagenase IV (Sigma, USA) and 3 mmol/L Ca2+ at 37°C. Liver tissue was finely diced into 2 mm3 sized pieces and the suspension incubated under constant agitation at 37°C for 30 min. The liver homogenate was filtered through gauze mesh and the cells suspension was centrifuged at 50 g for 3 min at 4°C to remove hepatocytes. The non-parenchymal cells-enriched supernatant was centrifuged at 400 g for 6 min. The cell pellet was resuspended in 30% Percoll (Pharmacia, Sweden) with a density of 1.040 g/mL, and this was carefully layered onto 60% Percoll with a density of 1.075 g/mL. The double layer discontinuous gradient formed was overlaid with 3 mL of HBSS and centrifuged at 400 g for 15 min at 4°C. The opaque interface was collected, resuspended in HBSS and centrifuged at 400 g for 5 min at 4°C. The cells were seeded onto tissue culture plates at a density of 2 × 106/mL and cultured in RPMI 1640 medium (Gibco, USA) containing 10% heat-inactivated fetal bovine serum (FBS, Gibco, USA), 100 U/mL Penicillin/Streptomycin (Hyclone, Germany) and 10 mmol/L HEPES at 37°C with 5%CO2. Nonadherent cells were removed after 2 h by gentle washing the plated cells and replacing the culture medium. Adherent cells were incubated for 48 h before performing uptake assays. More than 95% of adherent cells were ED2 (Serotec, UK) positive.
Total RNA was extracted from Kupffer cells at 48 h after culture or liver tissues with Trizol (Invitrogen, USA). Reverse transcription was performed on 1 μg RNA using random primers. Reverse-transcription reaction product (PrimeScript™ RT-PCR Kit, TaKaRa) was used for PCR reaction, with initial heating at 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 53-55°C for 30 s, 72°C for 1 min, and finally 72°C for 7 min. The primers are listed in Table 1. The PCR products were subjected to electrophoresis on 1.5% agarose gel containing ethidium bromide and visualized by UV illumination. β-actin was used as an internal control for RNA integrity.
| Gene | Primer sequence | Size of amplified DNA (bp) | Annealing temperature (°C) |
| HO-1 | F 5’ TGGAAGAGGAGATAGAGCGA 3’ | 451 | 53 |
| HO-1 | R 5’ TGTTGAGCAGGAAGGCGGTC 3’ | 451 | |
| CD14 | F 5’ GGACCCGATCTCAACACCT 3’ | 377 | 55 |
| CD14 | R 5’ CCAGCAGTATCCCGCAGT 3’ | 377 | |
| β-actin | F 5’ CGGGAAATCGTGCGTGAC 3’ | 443 | 55 |
| β-actin | R 5’ TGGAAGGTGGACAGCGAGG 3’ | 443 |
Proteins were extracted from Kupffer cells with radioimmunoprecipitation (RIPA) containing phenylmethyl sulfonylfluoride (PMSF). Protein quantification of samples was performed using the BCA assay. Proteins (15 μg/sample) in SDS-loading buffer were heated to 100°C for 5 min, subjected to 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane. The membrane was blocked overnight in 5% nonfat dry milk in TBST buffer at 4°C. Blots were incubated at room temperature (RT) for 2 h with anti-CD14 (dilution, 1:200, Santa Cruz Biotechnology, Inc. USA) in TBST buffer with 5% nonfat dry milk. After washing in TBST buffer three times at RT for 10 min, blots were incubated for 2 h at RT with horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (dilution, 1:50 000, Pierce Biotechnology) in TBST buffer with 5% nonfat dry milk. Finally, membranes were washed three times and developed with ECL (Amersham Pharmacia Biotech, Piscataway, New Jersey).
The medium from Kupffer cells culture was collected and centrifuged at 1000 g for 5 min, and supernatant was kept -70°C until assayed. IL-6 and TNF-α levels in the supernatant were determined with rat-specific enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc. USA). All samples, including standard and control solution, were assayed in duplicate.
Statistical analysis was performed using SPSS Version 13.0 for Windows (SPSS, Inc, Chicago, IL). All data are expressed as mean ± SE. Difference between experimental groups were analyzed using one-way analysis of variance or Student’s t test. All differences were considered statistically significant at the P value of < 0.05.
The isolated Kupffer cells were seeded onto tissue culture plates and incubated for 48 h. Cells attached rapidly to the dish surface, and spread in an irregular outline after 48 h in culture (Figure 1). More than 95% were ED2 positive on immunocytochemistry staining.
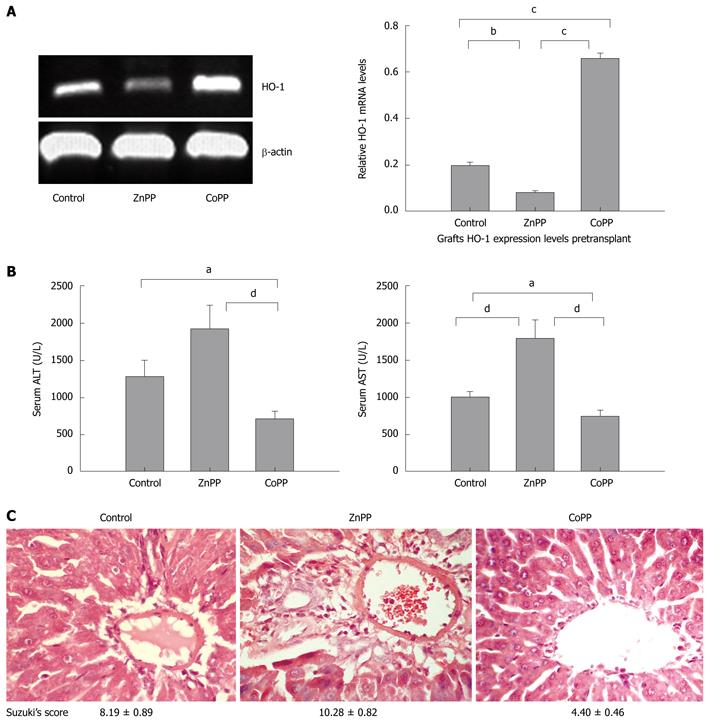
To assess HO-1 expression in experimental livers that were either untreated or pretreated with an HO-1 inducer or inhibitor, we performed RT-PCR analyses. A wide variation in HO-1 gene expression was detected in liver tissues that were collected before transplantation (Figure 2A). Livers harvested from donors that were pretreated with CoPP significantly up-regulated HO-1 mRNA expression levels as compared with control or the ZnPP group (0.658 ± 0.022, 0.198 ± 0.0165, 0.083 ± 0.008, respectively; P < 0.0001). ZnPP treatment decreased HO-1 mRNA as compared with control (P < 0.001).
We analyzed the hepatocellular function in rats that underwent cold ischemia followed by 6 h of reperfusion in three animal groups. As shown in Figure 2B, pretreatment with CoPP significantly decreased serum ALT levels as compared with the control or the ZnPP group (711.37 ± 111.13, 1285.00 ± 219.46, 1932.13 ± 313.41, respectively, P < 0.05 vs control, and P < 0.01 vs ZnPP-treated control). Serum AST levels were also significantly reduced in the CoPP treatment group as compared with both the control and the ZnPP group (740.38 ± 84.71, 998.63 ± 69.45, 1795.50 ± 244.59, respectively, P < 0.05 vs control, and P < 0.01 vs ZnPP-treated control). Both ALT and AST levels were higher in the ZnPP group.
The hepatocellular damage was evaluated by liver histology. Livers in the ZnPP group showed a more severe hepatocyte necrosis, sinusoidal congestion and ballooning, as compared with controls. In contrast, livers in the CoPP group revealed almost complete preservation of lobular architecture without sinusoidal congestion, ballooning, or necrosis (Figure 2C). The hepatocellular damage was graded using a modified Suzuki’s criteria[24].
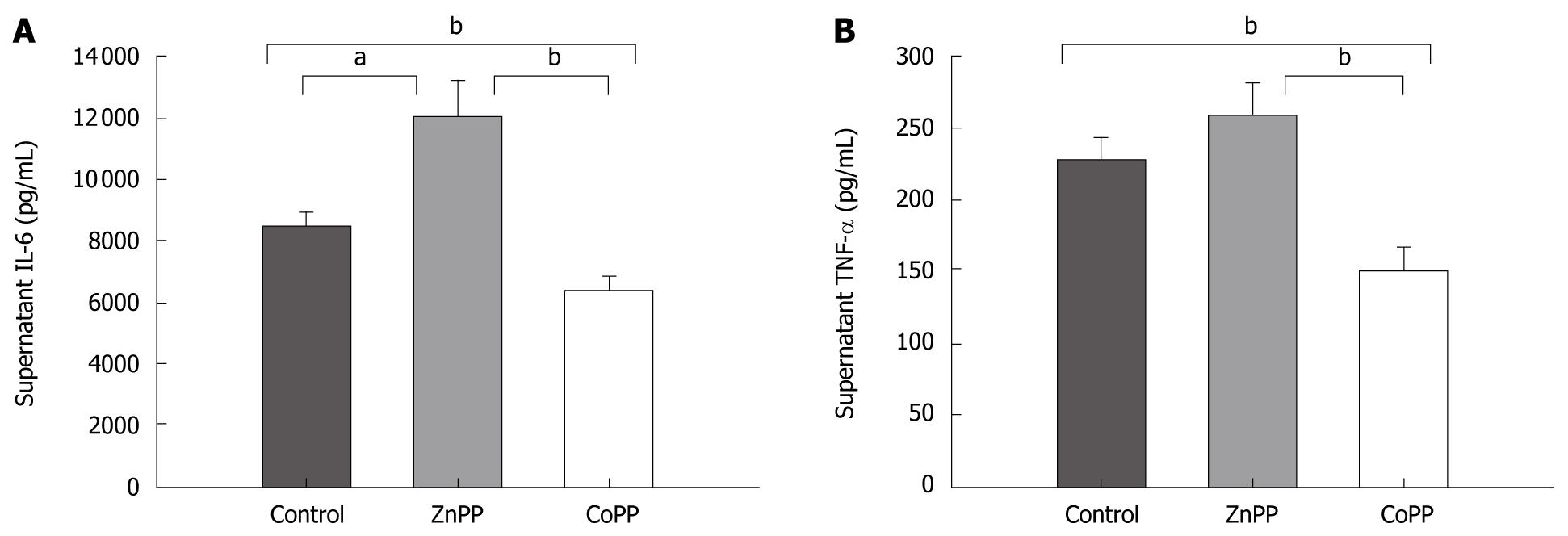
The cytokine levels (IL-6/TNF-α) in supernatant from the release of cultured Kupffer cells revealed a substantial increase in the ZnPP pretreated group as compared with the CoPP group (12019.25 ± 1244.89/257.75 ± 21.56 pg/mL vs 6360.13 ± 522.36/152.00 ± 14.66 pg/mL, respectively; P < 0.01). The IL-6/TNF-α levels of supernatant in CoPP pretreated group were significantly lower, when compared to the control group (8459.86 ± 453.51/228.38 ± 15.04, P < 0.01; Figure 3A and B).
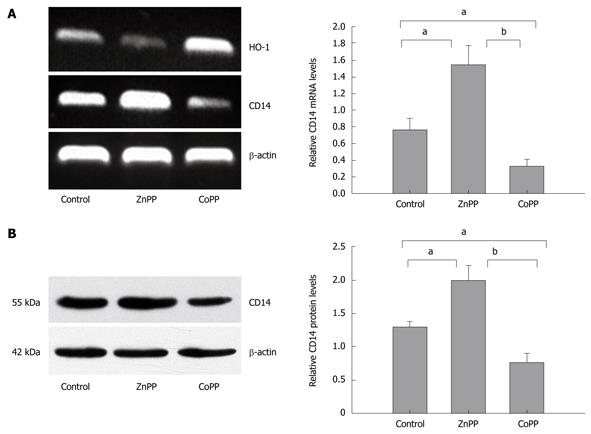
HO-1 mRNA expression levels of Kupffer cells pretreated with CoPP were significantly up-regulated, and those pretreated with ZnPP were down-regulated (Figure 4A). A prominent increase of CD14 mRNA levels in Kupffer cells was detected in the ZnPP group as compared with the control group or the CoPP group (1.547 ± 0.227, 0.772 ± 0.135, 0.325 ± 0.084 pg/mL, respectively; P < 0.05 vs control, and P < 0.01 vs CoPP-pretreated group). CoPP treatment was significantly lower, when compared to the control group (P < 0.05). As shown in Figure 4B and consistent with mRNA data, Western blot-assessed CD14 protein levels of Kupffer cells were also significantly up-regulated in the ZnPP group as compared with the control group (1.996 ± 0.446 pg/mL vs 1.303 ± 0.081 pg/mL, P < 0.05). CoPP treatment reduced CD14 protein levels (0.771 ± 0.136, P < 0.05 vs control).
I/R injury is one of the major obstacles in liver transplantation. HO-1 overexpression exerts cytoprotection and ameliorates hepatocellular damage in a number of liver I/R injury models[25-27], and prolongs cold ischemia followed by ex vivo perfusion or OLT[18]. In our study, CoPP-induced HO-1 overexpression significantly decreased hepatocyte injury posttransplantation. Donor livers with enhanced HO-1 expression reduced serum ALT/AST levels of recipients, ameliorated hepatic injury and suppressed cytokine release. In the transplant model, cultured Kupffer cells from donors pretreated with CoPP down-regulated CD14 mRNA and protein expression levels and reduced cytokine release.
HO-1 has been suggested as a cytoprotective gene during liver transplantation. However, the mechanism of HO-1-cytoprotection against I/R injury remains elusive. Tsuchihashi et al[28] showed that basal HO-1 levels are more critical than the ability to up-regulate HO-1 in response to I/R injury. Generally, the cellular mechanisms of HO-1-derived protection included regulation of the inflammatory response, improvement of microvascular flow and the antiapoptotic effects. The beneficial effects of HO-1 may be the result of the ability of the end-products of heme degradation[18]. Kaizu et al[29] reported that exogenous CO treatment suppressed early proinflammatory gene expression and neutrophil infiltration, and efficiently ameliorated hepatic I/R injury. CO has vasodilatating effects, thereby maintaining microvascular hepatic blood flow[18,30]. Furthermore, Tomiyama et al[31] recently demonstrated that CO ameliorated hepatic I/R injury and in vitro rat primary Kupffer cells culture also showed significant down-regulation of LPS-induced inflammatory responses. Biliverdin and the subsequently formed bilirubin possess potent antioxidant effects.
The mechanism of hepatic I/R injury is largely attributed to Kupffer cell activation and release of proinflammatory mediators such as reactive oxygen species and cytokines. During the initial stages of reperfusion Kupffer cells are activated. Activated Kupffer cells release a large amount of proinflammatory cytokines (TNF-α, IL-6, and IL-1)[32-34], which lead to aggravation of I/R injury. Liver I/R injury can be attenuated by the suppression of Kupffer cells[35]. Therefore modulation of Kupffer cell activity can attenuate I/R injury in liver transplantation models.
In this study, we observed that donor pretreatment with CoPP significantly improved liver function, ameliorated hepatic injury and reduced plasma proinflammatory mediators. The elimination of oxidants from the cell is considered to be an important mechanism of HO-1-mediated protection against oxidative stress. Biliverdin (BV) and bilirubin (BR), which have been regarded as toxic metabolites of heme degradation, may serve as important mediators of nitrosative injury through a similar mechanism[36]. BV and BR were shown to scavenge peroxynitrite. Superinduction of HO-1 leads to BR-mediated reductions in oxidative stress following I/R injury and provides cytoprotection in hepatocytes that are subjected to reperfusion injury[14,18]. CO, another byproduct of HO-1, has been shown to modulate intrahepatic sinusoidal tone and improve microcirculation of the liver[29]. Kupffer cells, liver resident macrophages, are the prime source of HO-1. We monitored the circulating levels of TNF-α and IL-6, both of which are proinflammatory cytokines mainly released by activated Kupffer cells. Kupffer cells overexpressing HO-1 exhibit low levels of cytokines in supernatant. In CoPP treatment, the activation of Kupffer cells was suppressed by lower TNF-α and IL-6 levels compared with ZnPP treatment. In many models of liver injury elevated TNF-α levels are present and correlate with injury, and inhibition of TNF-α activity can decrease liver injury[9]. As potent producers of inflammatory cytokines, Kupffer cells have been implicated in the pathway leading to liver injury[37].
Interestingly, there was an association of Kupffer cell HO-1 levels with lower levels of CD14 mRNA and protein seen in the CoPP treatment group as compared with the ZnPP treatment group. Kupffer cells have relatively low baseline expression of CD14[38]. Some studies showed that activation of Kupffer cells up-regulated expression of CD14 protein and its gene[39-41]. Interpretations of studies in liver injury should take into account the relative contributions of Kupffer cells and hepatocyte CD14[9]. Thus, increased baseline HO-1 levels as seen in CoPP treatment may represent a diminished ability to activate Kupffer cells. Kupffer cells are the major site of expression of hepatic HO-1. It is well known that liver I/R injury results in Kupffer cell activation and subsequent cytokines release, leading to localized hepatic damage. However, Devey et al[42] reported that Kupffer cells depletion resulted in loss of HO-1 expression and increased susceptibility to hepatic I/R injury. Therefore, HO-1 expression levels of Kupffer cells are likely to play a crucial role in liver I/R injury.
In conclusion, CoPP-induced HO-1 overexpression ameliorates liver I/R injury and suppresses cytokine release by inhibiting activation of Kupffer cells. Our data provides evidence for a novel mechanism of HO-1 dependent cytoprotection in liver I/R injury.
Orthotopic liver transplantation (OLT) is an effective treatment for end-stage liver diseases. However, ischemia/reperfusion (I/R) injury of the liver remains a major cause of graft injury, causing liver dysfunction and even failure posttransplantation. Overexpression of heme oxygenase (HO)-1 exerts a cytoprotective function in a number of I/R injury and liver transplant models. Studies of the mechanism by which HO-1 protects donor livers against I/R injury contribute to the development of liver transplantation.
The mechanism of HO-1-mediated cytoprotection is complicated. Kupffer cells are critical to the onset of liver injury and following secretion, cytokines aggravate hepatocyte damage. HO-1 is principally distributed in Kupffer cells. Recently, it has been reported that Kupffer cells depletion resulted in loss of HO-1 expression and increased susceptibility to hepatic I/R injury.
Since the mechanism of HO-1-mediated cytoprotection against I/R injury in organ transplantation remains unclear, upregulation of HO-1 is known to be involved in regulation of inflammatory response, improvement of microvascular flow and antiapoptotic effects. Liver I/R injury can be attenuated by the suppression of Kupffer cells. This study is an attempt to evaluate whether activation of Kupffer cells is related to HO-1 expression levels.
HO-1 can inhibit Kupffer cell activation and reduce release of proinflammatory mediators. This study showed that HO-1 is an attractive target against I/R injury in liver transplantation.
Kupffer cells are the resident macrophages of the liver and involved in I/R injury through the release of cytokines and other biologically active mediators. Modulation of Kupffer cells activity can attenuate I/R injury in liver transplantation models.
The paper deals with the role of heme oxygenase-1 in connection with ischemia/reperfusion injury in liver. It is important to find methods to prevent or reduce the injury that may follow ischemia/reperfusion, and the results presented may be useful for further research in the field. All the different parts of the paper are concise and clear.
Peer reviewers: Dr. Vance Matthews, PhD, BS, Cellular and Molecular Metabolism Laboratory, Baker University of Texas Medical Branch, IDI, PO Box 6492, St Kilda Road Central, VIC 8008, Melbourne, Australia; Trond Berg, Professor, Department of Molecular Biosciences, Universitetet i Oslo, Bolerskrenten 4, 0691 Oslo, Norway
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Starzl TE, Demetris AJ. Liver transplantation: a 31-year perspective. Part III. Curr Probl Surg. 1990;27:181-240. |
| 2. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. |
| 3. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. |
| 4. | Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577-592. |
| 5. | Lemaster JJ, Thurman RG. Hypoxia and reperfusion injury to liver. Prog Liver Dis. 1993;11:85-114. |
| 6. | Lemasters JJ, Bunzendahl H, Thurman RG. Reperfusion injury to donor livers stored for transplantation. Liver Transpl Surg. 1995;1:124-138. |
| 7. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. |
| 8. | Wanner GA, Ertel W, Müller P, Höfer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34-40. |
| 9. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. |
| 10. | Enomoto N, Takei Y, Hirose M, Ikejima K, Kitamura T, Sato N. Thalidomide Prevents Alcoholic Liver Injury in Rats Through Inhibition of Kupffer Cell Sensitization. Comp Hepatol. 2004;3 Suppl 1:S37. |
| 11. | Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ, Collins JL, Thiemermann C, Aasen AO, Wang JE. Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock. 2006;25:141-146. |
| 12. | Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9-19. |
| 13. | Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598-604. |
| 14. | Coito AJ, Buelow R, Shen XD, Amersi F, Moore C, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation. 2002;74:96-102. |
| 15. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. |
| 16. | Kobayashi T, Hirano K, Yamamoto T, Hasegawa G, Hatakeyama K, Suematsu M, Naito M. The protective role of Kupffer cells in the ischemia-reperfused rat liver. Arch Histol Cytol. 2002;65:251-261. |
| 17. | Kobayashi T, Sato Y, Yamamoto S, Takeishi T, Hirano K, Watanabe T, Takano K, Naito M, Hatakeyama K. Augmentation of heme oxygenase-1 expression in the graft immediately after implantation in adult living-donor liver transplantation. Transplantation. 2005;79:977-980. |
| 18. | Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121-128. |
| 19. | Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604-612. |
| 20. | Bauer I, Wanner GA, Rensing H, Alte C, Miescher EA, Wolf B, Pannen BH, Clemens MG, Bauer M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829-838. |
| 21. | Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant. 2005;5:1875-1885. |
| 22. | Kiemer AK, Gerwig T, Gerbes AL, Meissner H, Bilzer M, Vollmar AM. Kupffer-cell specific induction of heme oxygenase 1 (hsp32) by the atrial natriuretic peptide--role of cGMP. J Hepatol. 2003;38:490-498. |
| 23. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. |
| 24. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. |
| 25. | Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation. 2007;83:1628-1634. |
| 26. | McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation. 2006;81:623-626. |
| 27. | Lai IR, Chang KJ, Tsai HW, Chen CF. Pharmacological preconditioning with simvastatin protects liver from ischemia-reperfusion injury by heme oxygenase-1 induction. Transplantation. 2008;85:732-738. |
| 28. | Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol. 2006;177:4749-4757. |
| 29. | Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229-235. |
| 30. | Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: a regulator of hepatobiliary function. Hepatology. 2000;31:3-6. |
| 31. | Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, Gandhi C, Tokita D, Geller DA. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608-1620. |
| 32. | Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200-1208. |
| 33. | Tsukamoto H. Redox regulation of cytokine expression in Kupffer cells. Antioxid Redox Signal. 2002;4:741-748. |
| 34. | Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710-718. |
| 35. | Thomas P, Hayashi H, Lazure D, Burke PA, Bajenova O, Ganguly A, Forse RA. Inhibition of lipopolysaccharide activation of Kupffer cells by transition metals. J Surg Res. 2008;148:116-120. |
| 36. | Kaur H, Hughes MN, Green CJ, Naughton P, Foresti R, Motterlini R. Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett. 2003;543:113-119. |
| 37. | Monbaliu D, van Pelt J, De Vos R, Greenwood J, Parkkinen J, Crabbé T, Zeegers M, Vekemans K, Pincemail J, Defraigne JO. Primary graft nonfunction and Kupffer cell activation after liver transplantation from non-heart-beating donors in pigs. Liver Transpl. 2007;13:239-247. |
| 38. | Antal-Szalmás P. Evaluation of CD14 in host defence. Eur J Clin Invest. 2000;30:167-179. |
| 39. | Li XH, Gong JP, Shi YJ, Liu CA, Peng Y. In vitro expression of CD14 protein and its gene in Kupffer cells induced by lipopolysaccharide. Hepatobiliary Pancreat Dis Int. 2003;2:571-575. |
| 40. | Qiu DK, Hua J, Li JQ, Li EL. CD14 expression on Kupffer cells during the course of carbon tetrachloride-mediated liver injury. Chin J Dig Dis. 2005;6:137-141. |
| 41. | Miyaso H, Morimoto Y, Ozaki M, Haga S, Shinoura S, Choda Y, Murata H, Katsuno G, Huda K, Takahashi H. Protective effects of nafamostat mesilate on liver injury induced by lipopolysaccharide in rats: possible involvement of CD14 and TLR-4 downregulation on Kupffer cells. Dig Dis Sci. 2006;51:2007-2012. |