Published online Dec 28, 2009. doi: 10.3748/wjg.15.6034
Revised: October 16, 2009
Accepted: October 23, 2009
Published online: December 28, 2009
AIM: To characterize the gastric myoelectric activity (GMA) and intra-abdominal pressure changes induced by emetic stimuli (apomorphine and cisplatin) in the ferret.
METHODS: GMA and intra-abdominal pressure were recorded in conscious, unrestrained ferrets surgically implanted with radiotelemetry transmitters. Animals were challenged with apomorphine (0.25 mg/kg sc) and cisplatin (10 mg/kg ip), and the emetic response was quantified via direct observation and intra-abdominal pressure recording for 1 and 4 h, respectively. The GMA was analyzed by spectral analysis; the parameters used to characterize the GMA were the dominant frequency (DF) and the repartition of spectral power in the bradygastric, normogastric and tachygastric frequency ranges.
RESULTS: Retches were identified on the intra-abdominal pressure trace as peaks 0.30 ± 1.01 s in duration and 59.57 ± 2.74 mmHg in amplitude, vomit peaks were longer (0.82 ± 0.06 s, P < 0.01) and reached a higher pressure (87.73 ± 8.12 mmHg, P < 0.001). The number of retches and vomits quantified via direct observation [apomorphine: 65.5 ± 11.8 retches + vomits (R+V), cisplatin: 202.6 ± 64.1 R+V] and intra-abdominal pressure (apomorphine: 68.3 ± 13.7 R+V, n = 8; cisplatin: 219.0 ± 69.2 R+V, n = 8) were correlated (r = 0.97, P < 0.0001) and the timing of emesis was consistent between the 2 methods. Apomorphine induced a decrease in normogastria from 45.48% ± 4.35% to 36.70 ± 4.34% (n = 8, P < 0.05) but the DF of the slow waves was not changed [8.95 ± 0.25 counts/min (cpm) vs 8.68 ± 0.35 cpm, n = 8, P > 0.05]. Cisplatin induced a decrease in normogastria from 55.83% ± 4.30% to 29.22% ± 5.16% and an increase in bradygastria from 14.28% ± 2.32% to 31.19% ± 8.33% (n = 8, P < 0.001) but the DF (9.14 ± 0.13 cpm) remained unchanged (P > 0.05). The GMA changes induced by cisplatin preceded the emetic response as normogastria was reduced for 1 h before the onset of emesis (57.61% ± 5.66% to 39.91% ± 5.74%, n = 6, P < 0.05). Peri-emesis analysis revealed that the GMA was significantly disturbed during and immediately after, but not immediately before, the emetic episodes.
CONCLUSION: The induction of emesis is reliably associated with a disrupted GMA, but changes may also occur prior to and following the emetic response.
- Citation: Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Reduced normogastric electrical activity associated with emesis: A telemetric study in ferrets. World J Gastroenterol 2009; 15(48): 6034-6043
- URL: https://www.wjgnet.com/1007-9327/full/v15/i48/6034.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.6034
Nausea and vomiting are components of the body’s defense response against toxins either ingested (e.g. plant alkaloids: nicotine, veratridine, emetine), or released following bacterial (e.g. Staphylococcus aureus, Salmonella enterica, Vibrio cholerae) or viral infection (e.g. norovirus, rotavirus)[1]. However, in the clinic, the emetic response can also be triggered inappropriately, for example, as a sequelae of anesthesia with surgery (postoperative nausea and vomiting)[2], or as a side effect of cancer chemotherapy (e.g. cisplatin)[3]. In addition, nausea and vomiting are also commonly encountered during the pre-clinical and early clinical development of novel chemical entities (NCE) for a variety of therapeutic indications. Indeed, nausea and vomiting has been ranked second, after abuse liability, in the side effects negatively impacting on the development of NCE[4].
Pre-clinical studies using species capable of emesis such as the ferret (Mustela putorius furo L.) or the least shrew (Cryptotis parva) correctly identified an emetic liability for several NCE. For example, emetic side effects were observed for phosphodiesterase-IV inhibitors[5], a nicotinic receptor agonist[6], and a cannabinoid CB1 receptor antagonist[7], considered for the treatment of asthma, pain and obesity , respectively. Studies in the ferret also identified the anti-emetic potential of 5-hydroxytryptamine-3 (5-HT3) and tachykinin NK1 receptor antagonists against cancer chemotherapy agents (see[8] for review), however, their more limited efficacy against nausea[9] could not be predicted from pre-clinical studies. Emesis can be divided into 2 components: the pre-ejection (or prodromal) phase and the ejection phase. The prodromal phase is characterized by sympathetic outputs such as cold sweating, skin vasoconstriction and pupil dilation. Additionally, under vagal control, the proximal stomach relaxes, delaying gastric emptying, and the retrograde giant contraction carries to the stomach intestinal contents. This phase is usually temporally correlated with the sensation of nausea[10]. Retching and emesis (vomiting), which constitute the ejection phase, are therefore end-stages in activation of the emetic reflex and there would be considerable utility in the identification of biomarkers induced at sub-emetic doses of a compound, which could be used to better identify the separation between a therapeutic dose and one with emetic liability (therapeutic index). Such biomarkers may provide insights into the potential for induction or detection and reduction of nausea, which remains controversial in studies using laboratory animals[4].
In humans, correlates of nausea include an increase in plasma levels of vasopressin and changes in the electrogastrogram (EGG) frequency, rhythm and power[11]. The EGG reflects gastric myoelectric activity (GMA), or gastric slow waves; it is usually recorded from cutaneous abdominal electrodes to investigate the frequency of pacemaker activity, which underlies the genesis of gastric contractile activity[12]. However, the precise relationship of the EGG to motility itself is unclear[13] but tachygastria has been linked to gastric motor quiescence[14]. Of the 2 markers, only the recording of the gastric slow waves provides a method amenable to telemetry with an adequate temporal resolution to examine relationships to emesis in unrestrained animals.
This paper reports the use of radiotelemetry in the conscious unrestrained ferret to record gastric slow wave activity and investigate the effect of the emetic challenges, apomorphine and cisplatin. Data are also included on telemetric recordings of intra-abdominal pressure following administration of emetic agents in this species. The results show that both apomorphine and cisplatin are associated with a reduction of normal gastric rhythm, and demonstrate the potential applicability of telemetric recording techniques to the study of emetic mechanisms and the identification and understanding of emetic liability of NCE. This provides insights into the changes in gastric function occurring prior to the onset of emesis and which, in humans, have been associated with the occurrence of nausea.
Castrated male fitch (pigmented) ferrets (Mustela putorius furo L.) (1.17-1.65 kg) were obtained from Southland Ferrets (Invercargill, New Zealand). Prior to the experiments, they were housed communally in a temperature-controlled room at 24 ± 1°C, under artificial lighting with lights on between 06:00 am and 18:00 pm. Water and pelleted cat food (Tri Pro Feline Formula Cat Food, American Nutrition®, Utah, USA) were available ad libitum until the start of the experiments. All animals were then housed individually from the day of surgery to the end of the experiment. The experiments were conducted under the authority of a license provided by the Government of the Hong Kong SAR and approval from the Animal Experimentation Ethics Committee, The Chinese University of Hong Kong.
Telemetry transmitter implantation for GMA and abdominal pressure recording: Anesthesia was induced with ketamine (20 mg/kg im; Alfasan, Holland) and maintained with isoflurane (Halocarbon Products Corporation, USA) about 1.5%, in a 3:1 O2 to N2O ratio using a custom-made face mask and an anesthetic machine (Narkomed 2C, Drager, USA). Animals were placed on a heating pad (UCI#390 Analog moist heating pad, Rebirth Medical & Design, Inc., Taiwan) and the level of anesthesia was assessed and monitored throughout the surgery by the pedal withdrawal reflex. Following a midline abdominal incision, the antrum was exposed and the biopotential wires of the telemetry transmitter (C50-PTX, DSI, USA) were inserted in the muscle and secured in place by suturing the serosa. The body of the transmitter (with the pressure catheter) was inserted in the peritoneal cavity and sutured to the muscle layer on the side. The abdominal cavity was treated with antibiotic (Nebacetin®, Altana Pharma, Germany), sutured closed in layers and covered with a permeable spray dressing (Opsite®, Smith and Nephew, UK).
Analgesia and post-operative recovery: Buprenorphine (0.05 mg/kg sc; Temgesic®, Schering Plough, UK) was given as a preoperative analgesic 15 min before the induction of anesthesia, and 12 h post surgery. Recovery was unremarkable and the wound healed within a week.
Following surgery, animals were housed individually in observation cages (W49 cm × L61 cm × H49.5 cm). They were allowed to recover for at least 7 d prior to further experimentation. Some ferrets were administered apomorphine (0.25 mg/kg sc) at least 7 d prior to the administration of cisplatin (10 mg/kg ip). These doses of apomorphine and cisplatin have been shown to induce a reliable emetic response in the ferret[15,16]. At the end of the observation period, animals were killed with an overdose (> 100 mg/kg ip) of pentobarbital sodium (Dorminal®, Alfasan, Woerden, Holland).
Baseline telemetry recordings (GMA and abdominal pressure) were made for a least 1 h prior to presenting the animals with food, or an emetic challenge. Recordings then continued for 1 h in studies assessing the effect of food alone or apomorphine (0.25 mg/kg sc), or for 4 h in experiments assessing the action of cisplatin (10 mg/kg ip). Emesis was characterized by rhythmic abdominal contractions that were either associated with the oral expulsion of solid or liquid material from the gastrointestinal tract (i.e. vomiting), or not associated with the passage of material (i.e. retching movements). An episode of retching and/or vomiting was considered separate when the animal changed its location in the observation cage, or when the interval between retches and/or vomits exceeded 5 s[17]. The latency was defined as the time between the administration of the drug and the first emetic episode.
Effect of feeding on GMA: Food was withdrawn for 12-14 h before the start of the studies. The ferrets were then presented with 20 g of pelleted cat food, and all uneaten food was withdrawn 10 min later. This design was chosen to mimic human studies describing the effect of a meal on GMA in humans[12]. GMA data were analyzed during the 5 min period prior to presentation of food, and during a 5 min post-prandial period, starting 5 min after the uneaten food was withdrawn.
Effect of apomorphine (0.25 mg/kg sc) and cisplatin (10 mg/kg ip) on GMA and abdominal pressure: On the day of the experiment water was freely available but food was withdrawn for approximate 2 h before the start of the studies. Ferrets were presented with pelleted cat food and 30 min later animals were injected subcutaneously with apomorphine (0.25 mg/kg) or saline (0.5 mL/kg NaCl 154 mmol/L); or injected intraperitoneally with cisplatin (10 mg/kg) or saline (10 mL/kg NaCl 154 mmol/L).
Drugs: Cisplatin [cis-diamminedichloroplatinum(II), David Bull Laboratories, Victoria, Australia] was purchased as a sterile saline solution at an active concentration of 1 mg/mL. Apomorphine hydrochloride (Sigma-Aldrich, St. Louis, USA) was dissolved in sodium metabisulphite (526 μmol/L, Riedel-de Haën, Germany) and injected at a concentration of 0.5 mg/mL and a volume of 0.5 mL/kg sc. Doses are expressed as the free base.
A DSI Dataquest® A.R.T. telemetry system (Data Science International, Minnesota, USA) was used. The GMA and intra-abdominal pressure were recorded using PhysioTel® C50-PXT Small Animal Transmitters. Telemetric signals were recorded via 2 receiver plates (PhysioTel® RPC-1) placed under the cages. The receivers were connected to a PC desktop computer via a matrix (Dataquest ART Data Exchange Matrix). An ambient pressure reference monitor (APR-1) was connected to the exchange matrix. Data was recorded with the Dataquest Acquisition software (DQ ART 4.0). Analysis of telemetry recordings was carried out using Spike2® (version 6.06, Cambridge Electronic Design, UK).
Quantification of the retches and vomits via intra-abdominal pressure: The abdominal pressure signal was recorded with a sampling frequency of 500 Hz. Retches and vomits were quantified from the intra-abdominal pressure recordings in a semi-automated manner. Thus, the traces of each ferret were inspected visually and then a detection threshold was set and pressure profiles corresponding to retches and vomits were isolated manually. To test the validity of this method, the number of retches + vomits (R+V) detected was compared to the number obtained via direct observation using a Spearman test for non-parametric correlation.
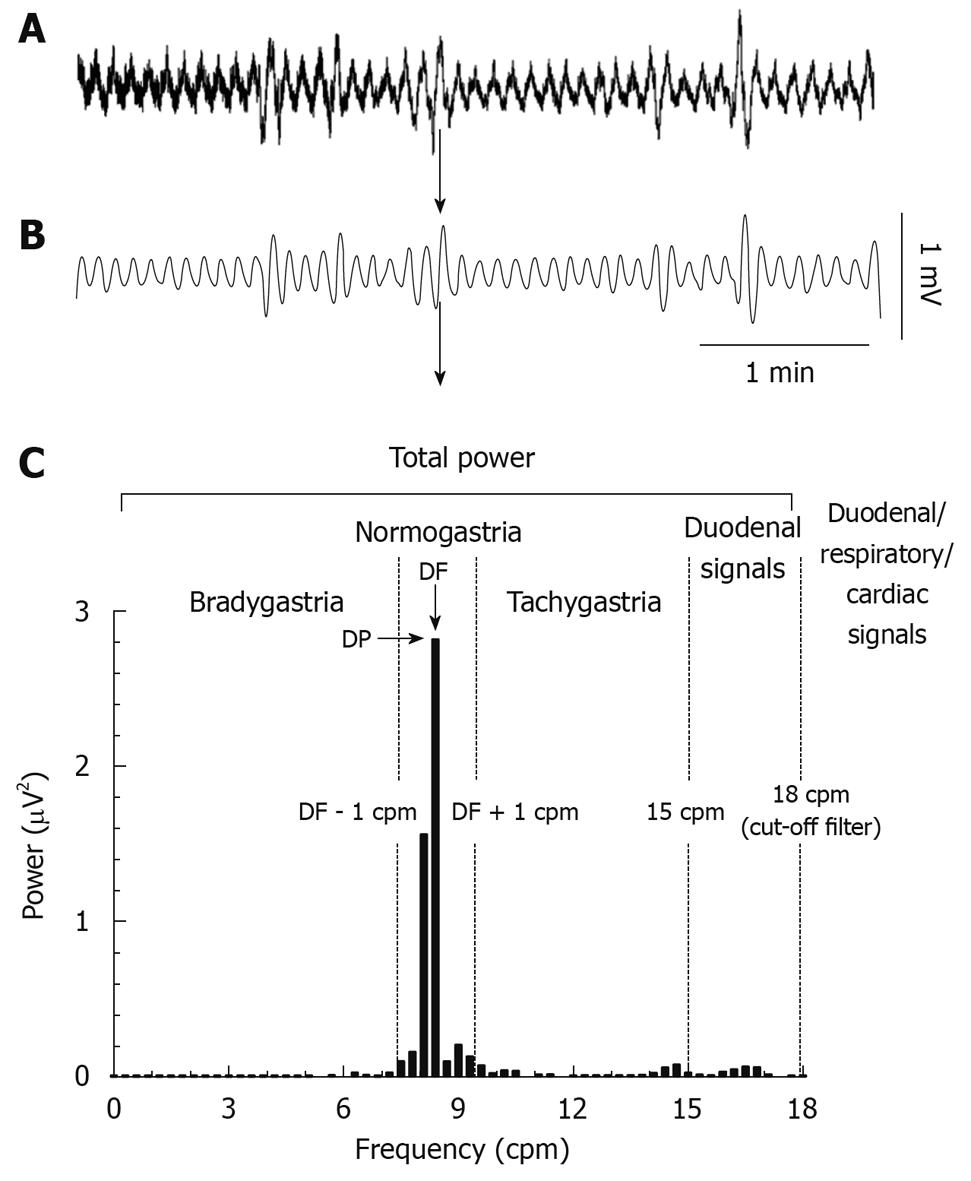
GMA recordings: The GMA signal was recorded with a sampling frequency of 1000 Hz; selected steps of the analysis procedure are presented in Figure 1. Briefly, a low pass finite impulse response (FIR) filter with a cut-off frequency of 2.5 Hz (transition gap: 10 Hz) was used to remove any signal with a frequency higher than 150 counts/min (cpm). The traces were then interpolated to a sampling frequency of 10.24 Hz and a second low pass FIR filter with a cut-off frequency of 0.3 Hz (18 cpm, transition gap: 0.1 Hz) was applied. This cut-off was chosen to filter out signals of probable cardiac (about 200 cpm in the ferret) and respiratory (33-36 cpm) origins[18].
The following parameters were used to characterize the GMA (Figure 1): (1) the dominant power (DP, the highest power in the 0 to 15 cpm range); (2) the dominant frequency (DF, frequency bin with the highest power in the 0 to 15 cpm range); (3) the repartition of power in the bradygastric, normal and tachygastric ranges (i.e. bradygastria, normogastria and tachygastria). The DF during a 1 h baseline was used to define the normal range in each animal, the limits of each range were then defined as follow: bradygastria: 0 to (DF - 1) cpm, normogastria: DF ± 1 cpm, tachygastria: (DF + 1) to 15 cpm. To investigate the general effect of apomorphine and cisplatin on the GMA, fast Fourier transforms (FFT, bin size: 0.3 cpm) were computed on successive 10 min epochs to construct the profiles of GMA repartition and the data were averaged in 1 h blocks for statistical analysis.
Peri-emesis analyses were carried out following cisplatin; FFTs (bin size: 0.6 cpm) were used on 2 min sections of data. Percentages of bradygastria, normogastria and tachygastria were computed from 2 min long sections divided as follows: (1) before cisplatin (mean of 5 successive 2 min sections immediately before the drug was injected); (2) before episodes (mean of all 2 min sections, free of emesis, immediately preceding an emetic episode); (3) during episodes (mean of all the 2 min sections containing emetic episodes); (4) after episodes (mean of all the 2 min sections, free of emesis, immediately after an episode).
Prior to any statistical comparisons, the normality of the data was assessed with a Kolmogorov-Smirnov test. Differences between the abdominal pressure correlates of retches and vomits were assessed with unpaired t-tests, the number of retches and vomits quantified by direct observation and abdominal pressure recordings were compared with paired t-tests and the correlation between the 2 methods was assessed with a Spearman test. For the overall effect of apomorphine and cisplatin on the GMA, differences between treatment groups were compared using repeated measures two-way ANOVAs (factors: treatment and time) followed by Bonferroni post-tests. In the peri-emetic analysis, the differences in GMA repartition between the different time-points were computed using repeated measures one-way ANOVA followed by Bonferroni post-tests.
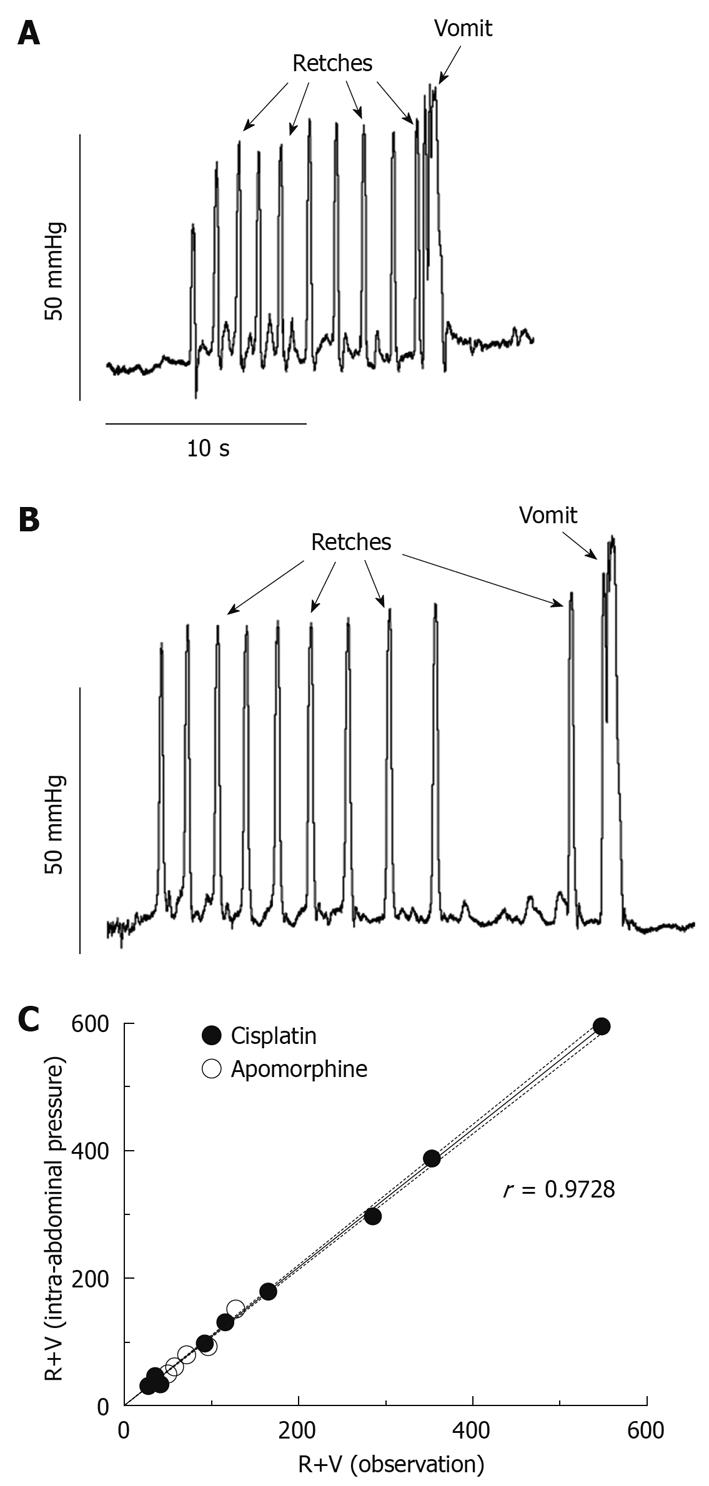
Figure 2 shows specific patterns on the intra-abdominal pressure recordings that are correlates of retching and vomiting. Retches were identified as round-ended peaks, 0.30 ± 0.01 s in duration and reaching a pressure of 59.57 ± 2.74 mmHg (mean ± SE of 40 measures, 5 retches × 8 animals). Vomits reached a higher pressure than retches (87.73 ± 8.12 mmHg; mean ± SE of 8 measures, 1 vomit × 8 animals, P = 0.0002, unpaired t-test) and they lasted longer (0.82 ± 0.06 s; mean ± SE of 8 measures, P = 0.0056, unpaired t-test); typically, an oscillation in pressure was observed during the peak (Figure 2).
Apomorphine (0.25 mg/kg sc) induced emesis with a latency of 7.17 ± 0.74 min (n = 8), 65.5 ± 11.8 R+V (59.6 ± 11.1 retches and 5.8 ± 0.8 vomits) and 68.3 ± 13.7 R+V (61.6 ± 12.9 retches and 6.6 ± 0.9 vomits) were quantified via observation and pressure, respectively; these values were not different (P = 0.34, paired t-test).
Cisplatin (10 mg/kg ip) induced emesis with a latency of 1.70 ± 0.23 h (n = 8). One ferret had an episode of retching immediately (20 s) after the intraperitoneal injection of cisplatin. This case seems more likely to be a result of the effect of the injection/handling rather than an effect of cisplatin itself; for this ferret, the latency of its second episode (46 min and 50 s), after which a sustained emetic response was initiated, was taken as the latency. Overall, 202.6 ± 64.1 R+V (185.0 ± 60.1 retches and 17. 6 ± 4.6 vomits) were calculated by direct observation and this number was increased by 8.1% ± 1.0% to 219.0 ± 69.2 R+V (199.1 ± 64.5 retches and 19.9 ± 5.2 vomits) using the pressure traces, a statistically significant difference (P = 0.0189, paired t-test).
The linear correlation (r = 0.9728) between the values obtained via observation and pressure was extremely significant (Figure 2C, P < 0.0001). The time of occurrence of emetic episodes was consistent between the 2 methods.
After being deprived of food overnight, the animals displayed a GMA characterized by a DF of 9.63 ± 0.23 cpm and a DP of 4.80 × 10-4± 1.15 × 10-4 mV2 (n = 10). Five minutes after food ingestion, there was a trend for the DF to be reduced to 9.24 ± 0.34 cpm and the DP was increased by a ratio of 6.80 ± 3.61, to 9.45 × 10-4± 2.84 × 10-4 mV2. These changes were not statistically significant, P = 0.38 and P = 0.13 for the DF and DP respectively (paired t-tests, n = 10).
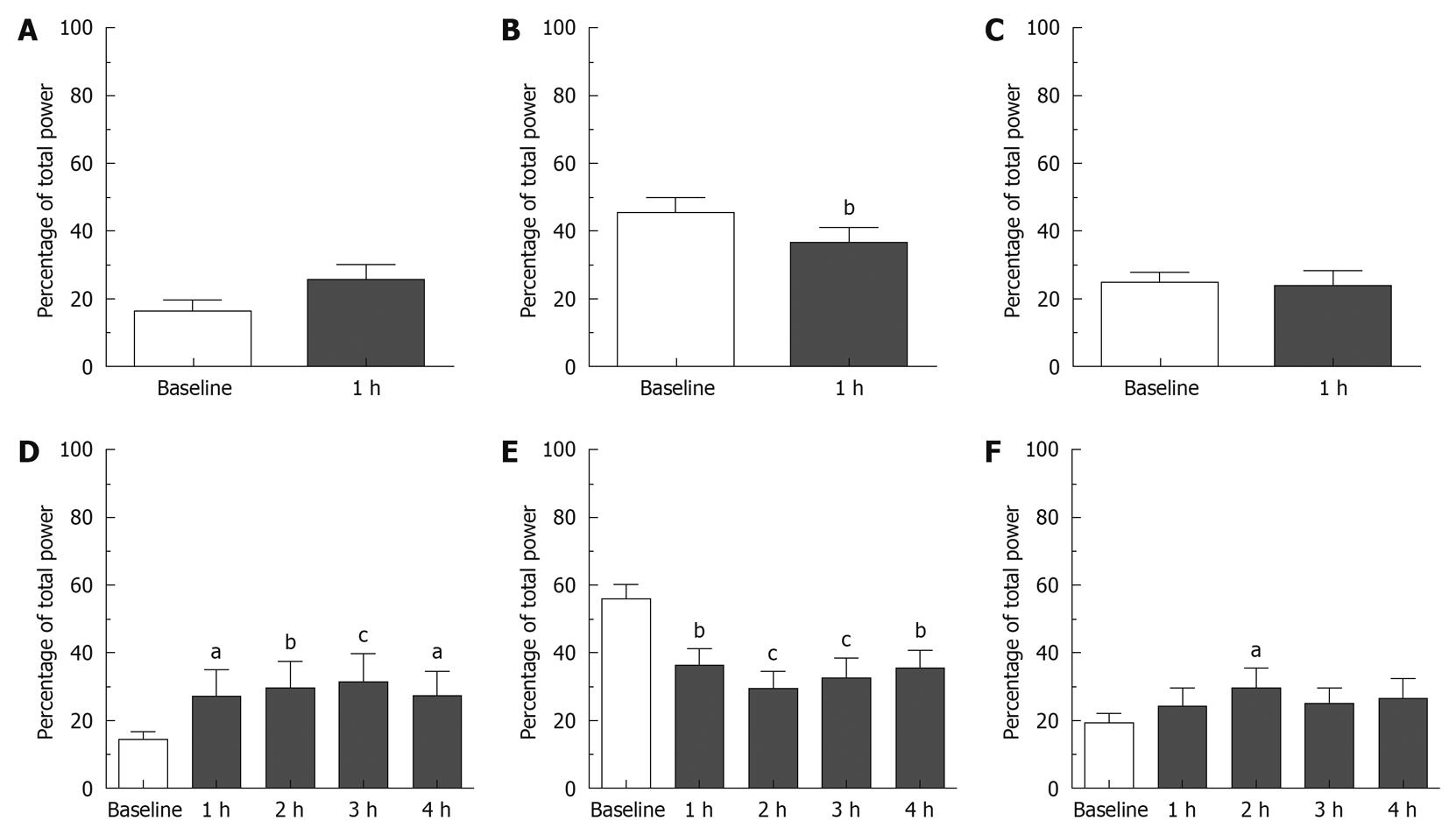
Effect of apomorphine: During the 1 h that preceded the injection of saline (0.5 mL/kg sc), baseline bradygastria, normogastria and tachygastria were 13.57% ± 3.53%, 63.31% ± 9.99% and 16.26% ± 5.82% of the total power, respectively. Baseline DF was 9.54 ± 0.26 cpm (n = 4). Following saline injection, the GMA repartition was 15.14% ± 1.28%, 57.40% ± 8.03% and 18.46% ± 6.13% in the bradygastric, normal and tachygastric ranges respectively; the DF was 9.73 ± 0.24 cpm. None of these values were significantly different from the baseline and no differences were detected between the saline and apomorphine groups during baseline (P > 0.05). During the 1 h that preceded the injection of apomorphine, the GMA was repartitioned as follows: bradygastria: 16.57% ± 3.17%, normogastria: 45.48% ± 4.35% and tachygastria: 25.66% ± 2.72%; DF: 8.95 ± 0.25 cpm (n = 8). As shown in Figures 3 and 4, following the administration of apomorphine, the percentage of power in the normal range decreased to 36.70% ± 4.34% (P < 0.01), whereas the percentage of power in the bradygastric and the tachygastric ranges was not significantly altered: 25.76% ± 4.65% and 22.77% ± 4.67%, respectively (P > 0.05). The DF did not change, and was 8.68 ± 0.35 cpm following apomorphine [P > 0.05, two-way ANOVAs followed by Bonferroni post-tests, n = 8 (apomorphine) and n = 4 (saline)].
Effect of cisplatin: During the 1 h baseline recordings prior to the injection of saline (10 mL/kg ip), baseline bradygastria, normogastria and tachygastria were 17.11% ± 5.35%, 43.32% ± 6.37% and 25.85% ± 3.83%, respectively; baseline DF was 8.44 ± 0.61 cpm (n = 4). The saline treatment had no significant effect on the GMA up to 3 h following intraperitoneal injection, however 4 h post injection the percentage of power in the tachygastric range was significantly increased compared to baseline to 40.40% ± 8.31% (P < 0.05). During the 1 h baseline prior to the injection of cisplatin, the percentages in the bradygastric, normogastric and tachygastric ranges were 14.28% ± 2.32%, 55.83% ± 4.30% and 19.17% ± 3.08%, respectively. The DF was 9.14 ± 0.13 cpm (n = 8). As shown in Figures 3 and 4, following the administration of cisplatin the percentage of power in the normogastric range decreased and reached a nadir in the second hour post-injection (29.22% ± 5.16%), whereas the percentage of bradygastria and tachygastria increased. Bradygastria reached a peak during the third hour after the injection (31.19% ± 8.33%) and tachygastria reached a peak during the second hour post-injection (29.56% ± 6.01%); the effects on normogastria and bradygastria were statistically significant during the entire observation period whereas the increase in tachygastria were only statistically significant 2 h post-injection (P < 0.05, Bonferroni post-tests compared to the 1 h baseline). The DF was not significantly altered after cisplatin administration (P < 0.05). No differences could be detected at any time points between saline and cisplatin [P > 0.05, repeated measures two-way ANOVA followed by Bonferroni post-tests, n = 8 (cisplatin), n = 4 (saline)].
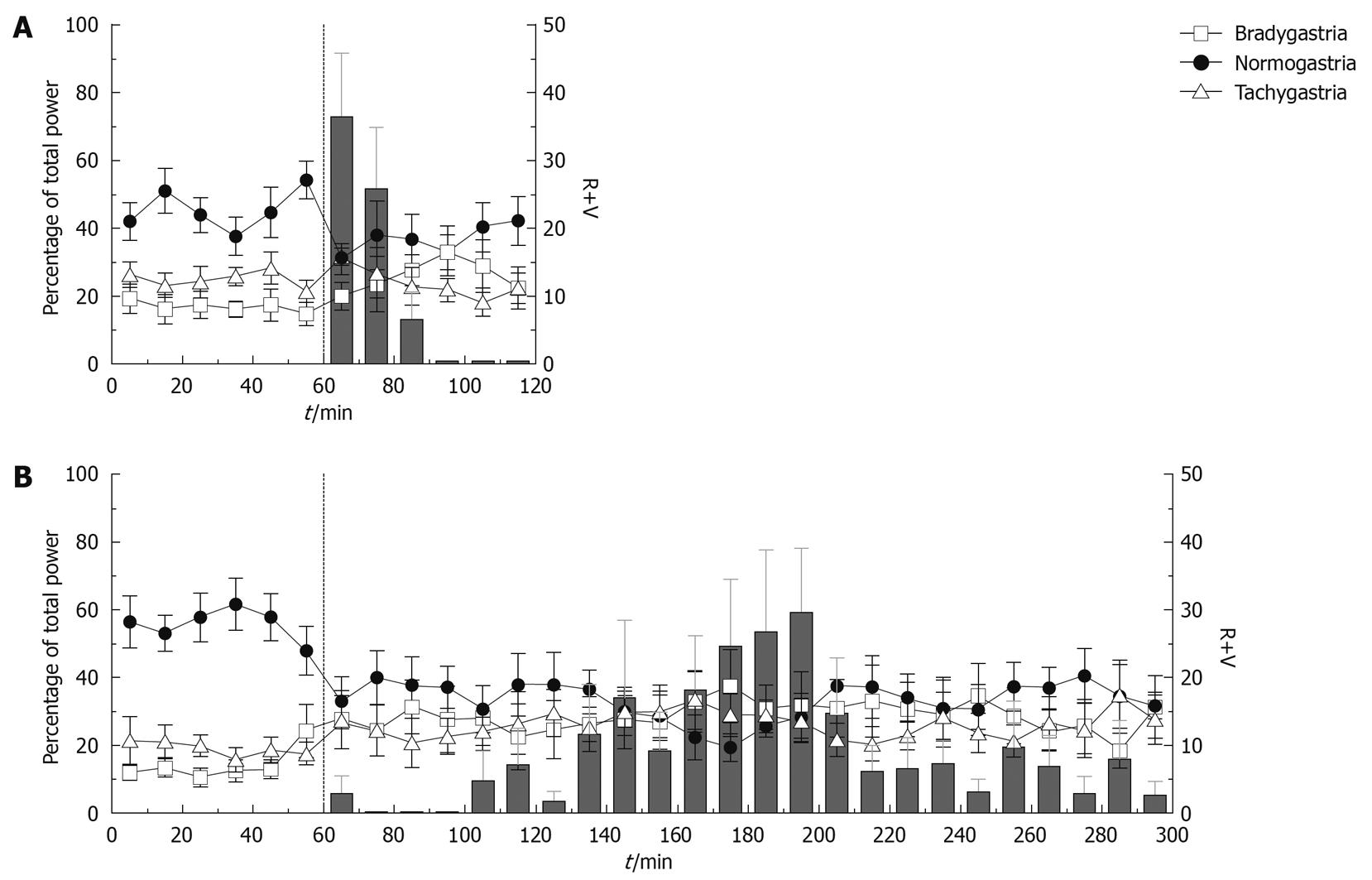
A secondary analysis was carried out only on the animals with a latency to the onset of emesis greater than 1 h (n = 6). In these animals, the percentage of power in the normogastric range was significantly reduced in the first hour post-cisplatin injection from 57.61% ± 5.66% to 39.91% ± 5.74% (P < 0.05) even though no emesis was observed during that period. Percentages of power in the bradygastric and tachygastric ranges were unchanged [P > 0.05, repeated measures two-way ANOVA followed by Bonferroni post-tests, n = 6 (cisplatin), n = 4 (saline)].
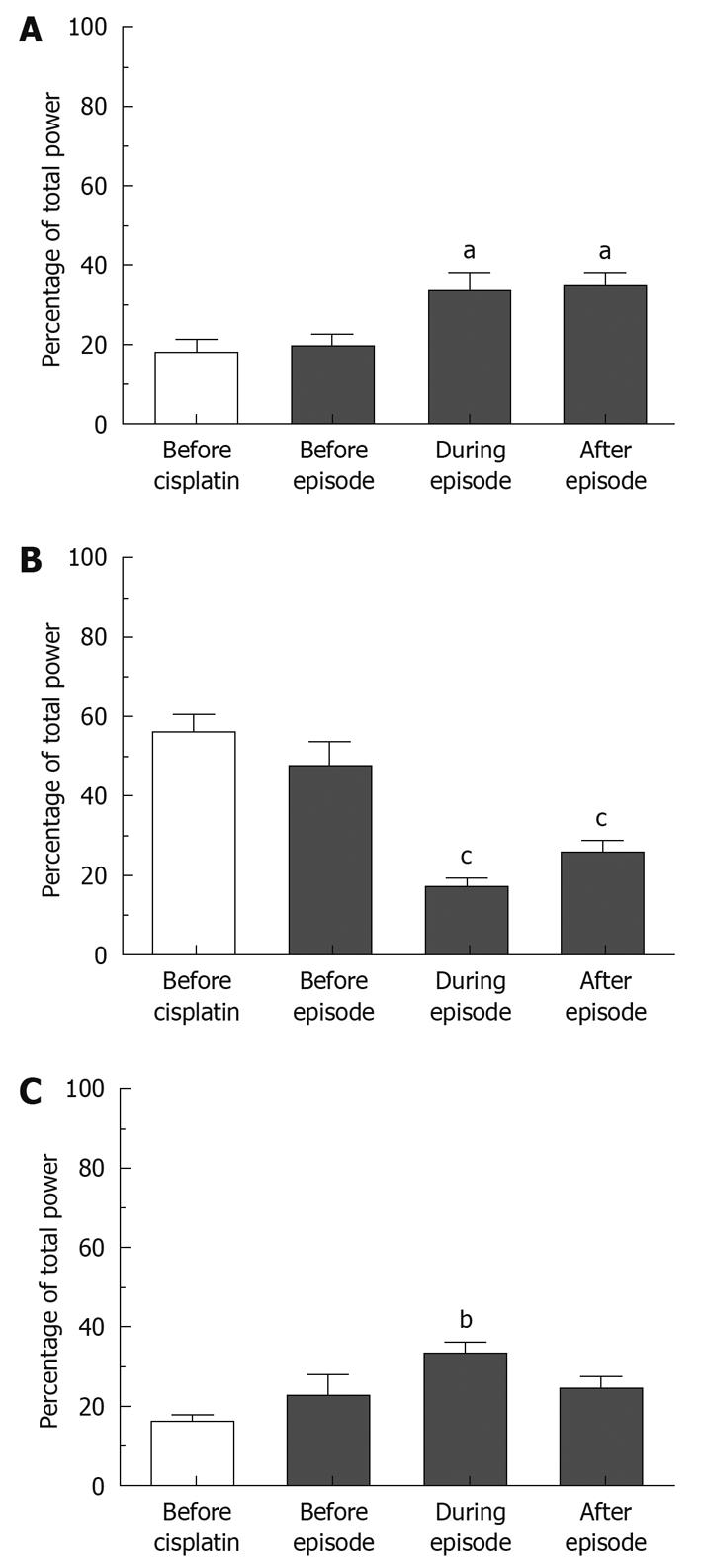
We investigated the GMA repartition surrounding and during emetic episodes; altogether, 40, 41, 62 and 41 determinations were used to characterize the GMA repartition before cisplatin and before, during and after emetic episodes, respectively. Bradygastria, normogastria and tachygastria values during the 10 min preceding the injection of cisplatin were 17.67% ± 3.50%, 56.00% ± 4.41% and 16.15% ± 1.66% (mean ± SE for 8 animals), respectively. Peri-emesis, the percentage repartition in all 3 ranges was significantly altered (P = 0.0085, P < 0.0001 and P = 0.0261 for bradygastria, normogastria and tachygastria respectively, repeated measures one-way ANOVAs, Figure 5). Bonferroni post-tests revealed that these values did not change significantly immediately before an episode (bradygastria: 19.36% ± 3.02%, normogastria: 47.56% ± 6.18 % and tachygastria: 22.87% ± 5.20%, P > 0.05), but were altered during (bradygastria: 33.08% ± 4.90%, normogastria: 17.23% ± 2.06% and tachygastria: 33.27% ± 3.00%, P < 0.05) and immediately after emetic episodes (bradygastria: 34.68% ± 3.33%, normogastria: 25.57% ± 3.27% and tachygastria: 24.38% ± 3.12%, P < 0.05).
The present study is the first report of ambulatory gastric myoelectric recordings in conscious unrestrained ferrets. The frequency of the gastric slow waves was 9.12 ± 0.23 cpm (mean ± SE of 12 animals), which is in accordance with post-prandial frequency of antral contractions (8.8 ± 0.5 cpm)[19], and the frequency of the antral slow waves (9.5 cpm) reported by Diamant et al[20] in abstract form; both were measured in conscious but restrained ferrets. In contrast to common protocols used to investigate the GMA in humans[21-23] and other animals (dog)[24,25], which used a fixed normogastric range (typically 2.5-3 to 3.7-4 cpm in humans and 4-6 cpm in dogs)[12,26,27], normogastria was defined according to each animal’s intrinsic slow waves frequency during baseline. This was done for 2 reasons: (1) because of a relative paucity of literature, not enough data on the ferret’s GMA was available to determine with confidence what the normogastric range should be (DF range 7.8-9.2 cpm in 12 animals in the present study) or how wide it should be; (2) the purpose of the present study was to focus on the changes induced by an experimental stimulus; setting the normogastric range relative to each individual animal reduced the influence of inter-animal variability and rendered the analysis more powerful to detect the effect of an emetic stimulus on the GMA in a limited number of animals.
Regarding the GMA analysis parameters, we chose to report the DF and compute the repartition of power in the normogastric, bradygastric and tachygastric ranges. An alternative analysis, which has been used in most preclinical studies[27-29] and some studies in humans[30], consists of calculating the percentage of time during which the DF falls within each frequency range. The analysis parameters used in the present study were chosen as the power repartition encompass all the data present in the GMA signal and does not solely focus on the time at which the DF is included within a range of interest[12].
Similar GMA changes were observed following apomorphine and cisplatin; both stimuli induced a decrease in the percentage of power in the normogastric range, which was accounted for in the case of cisplatin by a clear increase in power in the bradygastric range, without changing the DF. Our findings on the effects of apomorphine are partially supported by a number of studies in conscious, restrained dogs, which also reported a transient disruption of the gastric antral rhythm following the administration of apomorphine[31-33]. To the best of our knowledge, the present study represents the first clear evidence that apomorphine is associated with a reduction of normogastria for up to 1 h, which outlasts the emetic response as the last emetic episode was observed 21.12 ± 1.76 min (n = 8) post-apomorphine.
Regarding the effect of cisplatin, GMA changes were temporally correlated with the occurrence of emesis and maximal changes in the GMA repartition were observed when the emetic response was the most intense (2-3 h post cisplatin). However, GMA changes preceded the emetic response as evidenced by a decrease in normogastria during the first hour post-cisplatin in animals, which had not yet developed emesis during that period. Consistent with our findings, a recent study in the dog showed that cisplatin reduced the percentage of normal gastric slow waves in the hours preceding the onset of emesis and during the emetic response[34]. In the present study, a peri-emesis analysis revealed that normogastria was decreased and bradygastria and tachygastria were increased during and immediately after-but not immediately before-an emetic episode, which is in accordance with the short periods of dysrhythmia associated with emetic episodes have been reported in human patients during the administration of cisplatin[35]. Our findings are partly supported by human studies, which reported dysrhythmias in a few patients treated with anti-cancer chemotherapy. However, such events appeared to be transient rather than an overall change in slow wave activity[30,35]. The apparent differences in the effect of chemotherapy in human patients and in the ferret model could have four explanations:
(1) In the present study, the ferret model of acute cisplatin-induced emesis was used. This model uses a high bolus dose of cisplatin (10 mg/kg ip)-a highly emetic chemotherapeutic agent-to provoke an intense, reliable emetic response in all the animals, typically quantifiable over 4 h[36]. In human patients however, chemotherapy (platinum-based or not) is infused over hours and the emetic response is less reliable. Correspondingly, the GMA disturbance may be more severe in the ferret model than it is in human patients.
(2) Additionally, cancer patients treated with chemotherapy are not healthy subjects and their GMA may already be altered before they receive chemotherapy, rendering it less likely to detect an additional effect of the anti-cancer treatment. It is interesting that following a chemotherapy session, Riezzo et al[30] reported a higher percentage of tachygastria in cancer patients compared to healthy volunteers. However, they did not compare the EGG repartition in the cancer patients prior to chemotherapy, the measurements were collected 7 d post-chemotherapy and the chemotherapy regimens were not reported, precluding any direct comparison with the present study.
(3) The use of anti-emetic prophylaxis may also have an influence on GMA, and 5-HT3 receptor antagonists-commonly administered with chemotherapy and used in the 2 above-mentioned human studies-have been reported to reduce vection-induced dysrhythmias[37].
(4) The analysis of the data is an important factor to consider; as discussed above, in the present study, the DF and the percentage repartition of the power in the bradygastric, tachygastric and normogastric ranges were computed. In contrast, Samsom et al[35] identified bradygastria and tachygastria as intervals of at least 2 min, during which the DF was < 2.4 cpm or > 3.6 cpm and no overall assessment of normogastria was made, apart from the mean DF. Also, Riezzo et al[30] calculated the percentage of successive spectra in which the DF falls within one of the 3 ranges, or used visual inspection of the waveform traces or the regular spiking activity (RSA).
On the intra-abdominal pressure traces, retches were identified as brief peaks whereas vomits were more prolonged. These findings in the freely moving, conscious ferret are consistent with what McCarthy et al[38] described in the decerebrate cat. Our technique represents a great advantage in that quantifying the retches and vomits from the intra-abdominal pressure traces is more accurate than from a video or even direct observation; the distinction between retches and vomits is unequivocal and abdominal pressure recordings enable precise analysis regarding the timing and the frequency of the retches. Further information regarding the central neuronal circuitry involved in the mechanism of various emetics and anti-emetics can be gained from, for example, the interval between retches, the pattern of retching preceding a vomit or the characteristics of episodes including a vomit and those of episodes of retches. The major disadvantage is that this is an invasive technique, which requires abdominal surgery.
The use of telemetry enables the recording of gastric slow waves in freely moving, conscious animals; additionally, analysis of the emetic response via intra-abdominal pressure permits a more rigorous data collection and the investigation of the precise temporal correlation between gastric changes and emesis. Using an EGG-like analysis of GMA recordings, disruption of the gastric rhythm was detected in ferrets challenged with apomorphine and cisplatin. Both emetic stimuli were associated with a reduction of normogastria, and cisplatin increased bradygastria. The GMA changes were subtle and not detectable by a simple DF analysis but comparison of the power repartition of the gastric signal, before and after the emetic challenges, indicated a clear change, which was temporally correlated with the development of the emetic response but not restricted to the immediate peri-emetic period. In the ferret, recording and analysis of the GMA, which is a physiological correlate of nausea in humans, will improve the understanding of the changes in gastric function associated with emesis.
Nausea is a subjective sensation, therefore impossible to study directly in animals. However physiological correlates of nausea have been identified in humans and include disruption of the gastric electrical rhythm. As nausea usually precedes the onset of emesis, recording the gastric electrical rhythm in animals potentially enables the detection of emetic pathway activation before the emetic threshold is reached.
The application of human biomarkers of nausea to an animal model enables the refinement of this model as it improves its translational potential. The research hotspot is the correlation of physiological events occurring around the time of vomiting, which may give insight into nausea.
Recent in vivo research studies investigating the relationship between emesis and gastric myoelectric activity (GMA) have used either restrained or anesthetized animals (mainly dogs) with wires connected to the serosa and externalized through the skin. The use of telemetry represents a novel approach and the present study is the first report of GMA recording in freely moving animals, which represents an indisputable advantage in terms of animal welfare.
The ferret is a species commonly used in emesis research and our approach could be integrated to standard study designs, therefore refining this animal model by enabling the detection of emetic pathway(s) activation before the emetic threshold is reached.
The GMA consists of electrical pacemaker signals, which trigger contractions of the stomach. Gastric dysrhythmia refers to a departure from the normal gastric electrical rhythm, the term encompasses bradygastria, tachygastria and gastric arrhythmia.
This is a well written manuscript from an established group expert in this field using a novel relevant approach to assess GMA and intra-abdominal pressure in the conscious ferret implanted with radiotelemetry transmitters.
Peer reviewer: Yvette Taché, PhD, Professor of Medicine, David Geffen School of Medicine at UCLA, Director, CURE: Digestive Diseases Research Center - Animal Core, Co-Director, Center for Neurovisceral Sciences & Women’s Health, VA Greater Los Angeles Healthcare System, 11301 Wilshire Boulevard, CURE Building 115, Room 117, Los Angeles, CA 90073, United States
S- Editor Wang YR L- Editor Cant MR E- Editor Zheng XM
| 1. | Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100-115. [Cited in This Article: ] |
| 2. | Apfel CC, Malhotra A, Leslie JB. The role of neurokinin-1 receptor antagonists for the management of postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2008;21:427-432. [Cited in This Article: ] |
| 3. | Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482-2494. [Cited in This Article: ] |
| 4. | Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PL. Opportunities for the replacement of animals in the study of nausea and vomiting. Br J Pharmacol. 2009;157:865-880. [Cited in This Article: ] |
| 5. | Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262-269. [Cited in This Article: ] |
| 6. | Chin CL, Fox GB, Hradil VP, Osinski MA, McGaraughty SP, Skoubis PD, Cox BF, Luo Y. Pharmacological MRI in awake rats reveals neural activity in area postrema and nucleus tractus solitarius: relevance as a potential biomarker for detecting drug-induced emesis. Neuroimage. 2006;33:1152-1160. [Cited in This Article: ] |
| 7. | Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24:198-203. [Cited in This Article: ] |
| 8. | Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. Management of nausea and vomiting in cancer and cancer treatment. Boston: Jones and Bartlett 2005; 15-66. [Cited in This Article: ] |
| 9. | Jordan K, Schmoll HJ, Aapro MS. Comparative activity of antiemetic drugs. Crit Rev Oncol Hematol. 2007;61:162-175. [Cited in This Article: ] |
| 10. | Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992;69:2S-19S. [Cited in This Article: ] |
| 11. | Koch KL. A noxious trio: nausea, gastric dysrhythmias and vasopressin. Neurogastroenterol Motil. 1997;9:141-142. [Cited in This Article: ] |
| 12. | Koch KL, Stern R. Handbook of Electrogastrography. New York: Oxford University Press 2004; . [Cited in This Article: ] |
| 13. | Abid S, Lindberg G. Electrogastrography: poor correlation with antro-duodenal manometry and doubtful clinical usefulness in adults. World J Gastroenterol. 2007;13:5101-5107. [Cited in This Article: ] |
| 14. | Chen JD, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol. 1993;88:1324-1336. [Cited in This Article: ] |
| 15. | Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol. 1986;88:497-499. [Cited in This Article: ] |
| 16. | Lau AH, Ngan MP, Rudd JA, Yew DT. Differential action of domperidone to modify emesis and behaviour induced by apomorphine in the ferret. Eur J Pharmacol. 2005;516:247-252. [Cited in This Article: ] |
| 17. | Rudd JA, Bunce KT, Naylor RJ. The interaction of dexamethasone with ondansetron on drug-induced emesis in the ferret. Neuropharmacology. 1996;35:91-97. [Cited in This Article: ] |
| 18. | Wolfenshn S, Lloyd M. Carnivores. 2nd ed. Oxford: Blackwell Science 1998; 218-238. [Cited in This Article: ] |
| 19. | Grundy D. The effect of surgical anaesthesia on antral motility in the ferret. Exp Physiol. 1990;75:701-708. [Cited in This Article: ] |
| 20. | Diamant SC, Scott RB, Davison JS. The effects of anaesthesia and surgery on the migrating myoelectric complex (MMC) in the ferret. Gastroenterology. 1985;88:1365. [Cited in This Article: ] |
| 21. | Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987;92:92-97. [Cited in This Article: ] |
| 22. | Koch KL, Summy-Long J, Bingaman S, Sperry N, Stern RM. Vasopressin and oxytocin responses to illusory self-motion and nausea in man. J Clin Endocrinol Metab. 1990;71:1269-1275. [Cited in This Article: ] |
| 23. | Uijtdehaage SH, Stern RM, Koch KL. Effects of eating on vection-induced motion sickness, cardiac vagal tone, and gastric myoelectric activity. Psychophysiology. 1992;29:193-201. [Cited in This Article: ] |
| 24. | Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401-409. [Cited in This Article: ] |
| 25. | Liu J, Qiao X, Chen JD. Therapeutic potentials of a novel method of dual-pulse gastric electrical stimulation for gastric dysrhythmia and symptoms of nausea and vomiting. Am J Surg. 2006;191:255-261. [Cited in This Article: ] |
| 26. | Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15:89-102. [Cited in This Article: ] |
| 27. | Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17:236-244. [Cited in This Article: ] |
| 28. | Qian LW, Pasricha PJ, Chen JD. Origins and patterns of spontaneous and drug-induced canine gastric myoelectrical dysrhythmia. Dig Dis Sci. 2003;48:508-515. [Cited in This Article: ] |
| 29. | Ueno T, Chen JD. Vomiting and gastric electrical dysrhythmia in dogs. Scand J Gastroenterol. 2004;39:344-352. [Cited in This Article: ] |
| 30. | Riezzo G, Clemente C, Leo S, Russo F. The role of electrogastrography and gastrointestinal hormones in chemotherapy-related dyspeptic symptoms. J Gastroenterol. 2005;40:1107-1115. [Cited in This Article: ] |
| 31. | Akwari OE. The gastrointestinal tract in chemotherapy-induced emesis. A final common pathway. Drugs. 1983;25 Suppl 1:18-34. [Cited in This Article: ] |
| 32. | Lang IM, Marvig J, Sarna SK. Comparison of gastrointestinal responses to CCK-8 and associated with vomiting. Am J Physiol. 1988;254:G254-G263. [Cited in This Article: ] |
| 33. | Lang IM, Marvig J, Sarna SK, Condon RE. Gastrointestinal myoelectric correlates of vomiting in the dog. Am J Physiol. 1986;251:G830-G838. [Cited in This Article: ] |
| 34. | Yu X, Yang J, Hou X, Zhang K, Qian W, Chen JD. Cisplatin-induced gastric dysrhythmia and emesis in dogs and possible role of gastric electrical stimulation. Dig Dis Sci. 2009;54:922-927. [Cited in This Article: ] |
| 35. | Samsom M, Akkermans LMA, Neijt J, van Berge-Henegouwen GP, Smout AJPM. Gastric myoelectrical activity in patient treated with Cisplatin. An electrographic study. Electrogastrography: Principles and applications. New York: Raven Press 1994; 345-355. [Cited in This Article: ] |
| 36. | Florczyk AP, Schurig JE, Bradner WT. Cisplatin-induced emesis in the Ferret: a new animal model. Cancer Treat Rep. 1982;66:187-189. [Cited in This Article: ] |
| 37. | Levine ME, Chillas JC, Stern RM, Knox GW. The effects of serotonin (5-HT3) receptor antagonists on gastric tachyarrhythmia and the symptoms of motion sickness. Aviat Space Environ Med. 2000;71:1111-1114. [Cited in This Article: ] |
| 38. | McCarthy LE, Borison HL. Respiratory mechanics of vomiting in decerebrate cats. Am J Physiol. 1974;226:738-743. [Cited in This Article: ] |