Published online Dec 14, 2009. doi: 10.3748/wjg.15.5843
Revised: October 29, 2009
Accepted: November 5, 2009
Published online: December 14, 2009
AIM: To investigate the dysfunction of the immunological barrier of the intestinal mucosa during endotoxemia and to elucidate the potential mechanism of this dysfunction.
METHODS: Male Wistar rats were randomly distributed into two groups: control group and lipopolysaccharide (LPS) group. Endotoxemia was induced by a single caudal venous injection of LPS. Animals were sacrificed in batches 2, 6, 12 and 24 h after LPS infusion. The number of microfold (M)-cells, dendritic cells (DCs), CD4+ T cells, CD8+ T cells, regulatory T (Tr) cells and IgA+ B cells in the intestinal mucosa were counted after immunohistochemical staining. Apoptotic lymphocytes were counted after TUNEL staining. The levels of interleukin (IL)-4, interferon (IFN)-γ and forkhead box P3 (Foxp3) in mucosal homogenates were measured by ELISA. The secretory IgA (sIgA) content in the total protein of one milligram of small intestinal mucus was detected using a radioimmunological assay.
RESULTS: This research demonstrated that LPS-induced endotoxemia results in small intestinal mucosa injury. The number of M-cells, DCs, CD8+ T cells, and IgA+ B cells were decreased while Tr cell and apoptotic lymphocyte numbers were increased significantly. The number of CD4+ T cells increased in the early stages and then slightly decreased by 24 h. The level of IL-4 significantly increased in the early stages and then reversed by the end of the study period. The level of IFN-γ increased slightly in the early stages and then decreased markedly by the 24 h time point. Level of Foxp3 increased whereas sIgA level decreased.
CONCLUSION: Mucosal immune dysfunction forms part of the intestinal barrier injury during endotoxemia. The increased number and function of Tr cells as well as lymphocyte apoptosis result in mucosal immunodeficiency.
- Citation: Liu C, Li A, Weng YB, Duan ML, Wang BE, Zhang SW. Changes in intestinal mucosal immune barrier in rats with endotoxemia. World J Gastroenterol 2009; 15(46): 5843-5850
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5843.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5843
Endotoxemia can induce sepsis which is among the leading causes of death in noncardiac intensive care units (ICUs) in the US, with approximately 750 000 cases and up to 200 000 deaths per year[1]. An epidemiological investigation in 3665 ICUs in China showed that the overall hospital mortality of severe sepsis is 48.7%; the mean hospital cost is $11 390 per patient at a mean cost of $502 per patient per day[2]. Despite hospital mortality of 20% for simple sepsis and 40% or higher for severe sepsis or septic shock, there has nonetheless been improvement over the past decade[3].
Lipopolysaccharide (LPS) is a component of the outer cell wall of gram-negative bacteria, which gives rise to various manifestations of gram-negative endotoxemia and septic shock[4]. Endotoxemia-induced sepsis has been associated with deleterious functional and structural changes in many organs, such as the gastrointestinal tract[5], lungs, and other organs. During sepsis, the most frequent complications within the gastrointestinal tract are mucosal barrier dysfunction and ileus[6]. One of the most important functions of the gastrointestinal tract is the ability to act as a mucosal barrier to infections. Mucosal barrier dysfunction plays an important role in the pathophysiology of sepsis by promoting bacterial stasis, bacterial overgrowth, and bacterial translocation, which can lead to the development of secondary infections and multiple organ failure[7]. Intestinal mucosal barriers consist of a mechanical barrier, a chemical barrier, an immunological barrier, and a biological barrier[8]. Damage to any one of these components causes mucosal barrier dysfunction. The immunological barrier is considered as the first line of defense of the intestinal mucosa from bacterial invasion[9] and plays an important role in the overall defense. It consists of Peyer’s patches, which are the induction sites of the immune response, and diffused lymphoid tissue which are the effector sites. Intestinal mucosa immune responses rely largely on humoral immunity. The primary functions of the immunological barrier include[10-12] inhibiting bacterial adhesion to the mucosa so that they can be eliminated, neutralizing viruses and toxins, enclosing some antigens of acquired extraneous material to prevent systemic reactions, activating the complement 3 (C3) pathway, participating in the anti-infection effect, and protecting probiotics. Therefore, damage to the intestinal immune barrier will result in bacterial translocation and gut-derived endotoxemia.
Previous studies have discussed the changes to immunity during sepsis, but what happens to immunity, specifically the gut immunity, during endotoxemia before sepsis is not clear. Thus in the present study, changes to the number and function of intestinal mucosal immune cells in rats with endotoxemia were observed to investigate whether dysfunction of immunological barrier occurred during endotoxemia and to elucidate the potential mechanism of this dysfunction.
Male Wistar rats weighing 200.5 ± 12.3 g were purchased from Vital River Laboratories (Beijing, China). The rats were fed a standard laboratory chow diet (Vital River Laboratories) for 72 h before the experiment and maintained at 24 ± 1°C, at a relative humidity of 50% ± 1% with a 12/12-h light/dark cycle. The animals were fasted for 12 h before the experiment, allowing free access to water. All animals were handled according to the institutional criteria for the care and use of laboratory animals in research.
Establishment of animal model: A total of 80 animals were included and randomly distributed into the control group (40 rats) and LPS (Escherichia coli, O55: B5, Sigma, St Louis, MO, USA) group (40 rats). In accordance with the study of Cheng et al[13], endotoxemia was induced by a single caudal venous injection of LPS at 10 mg/kg, while control animals received caudal venous injections of saline. Ten animals were sacrificed in each group at each time point (2, 6, 12 and 24 h) after LPS infusion.
Histological Testing: Small intestinal tissue was obtained from the middle part of the ileum. Samples were fixed in 4% paraformaldehyde and then observed by hematoxylin and eosin (HE) staining to explore the histopathological changes in the intestinal mucosa.
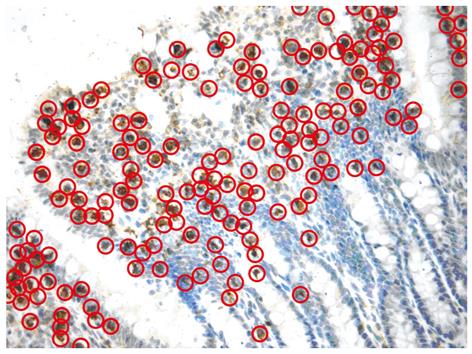
Cytological Testing: A 5-cm-long tissue section with Peyer’s patches cut off was obtained from the ileum near the cecum and observed by immunohistochemical staining after fixation in 4% paraformaldehyde to investigate immune cell changes in the mucosa. The fixed tissue was transferred to phosphate buffer solution (PBS) overnight at 4°C and then transferred to 30% sucrose for 2 h at 4°C. Then the tissue was mounted in optimum cutting temperature (OCT) embedding medium and placed on dry ice until frozen before cryosectioning of 4 μm thick continuous slides. The slides were washed 3 × 5’ (3 times, 5 min each) with PBT (Phosphate Buffer Saline with 0.02% Tween 20). Next, 500 μL of diluted primary antibody in blocking buffer (containing 40 mL PBT, 400 μL heat-inactivated goat serum, 400 μL heat-inactivated donkey serum, 400 μL 10% Triton X-100) was added to each slide before they were covered and stored at 4°C overnight. The slides were then washed 6 × 5’ with PBT before the addition of 500 μL of diluted secondary antibody in blocking buffer to each slide. The slides were then covered and incubated at room temperature for 90 min before being washed 6 × 5’ with PBT. The slides were then mounted with Vectashield. After staining, six clear-viewed slides were selected from each group and two viewing fields for each slide at high magnification (400 ×) were randomly selected for counting immune cells. Brownish-yellow stained lymphocytes were an indication of positive cells (Figure 1). Rabbit anti-rat cytokeratin 8[14], integrin αE2[15], cluster of differentiation (CD) 4, CD8, neuropilin-1 (NRP-1)[16] and immunoglobulin A (IgA) polyclonal antibodies were purchased from Biosynthesis Biotechnology (Beijing, China) to indicate microfold cells (M-cells), dendritic cells (DC), CD4+ thymus dependent lymphocytes (T cells), CD8+ T cells, CD4+CD25+ T cells (regulatory T cell, Tr) and IgA+ bursa dependent lymphocytes (B cells), respectively.
The terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling (TUNEL) assay was used to observe apoptotic lymphocytes in the small intestinal mucosa. The TUNEL kit was obtained from Roche Diagnostics (Indianapolis, IN, USA) to label apoptotic lymphocytes. The slides were deparaffinized in two changes of xylene for 5 min each, hydrated with two changes of 100% ethanol for 3 min each and 95% ethanol for 1 min, and then rinsed in distilled water. Slides were then incubated in TdT reaction buffer for 10 min before incubation in TdT reaction mixture for 1-2 h at 37-40°C in a humidified chamber. The reaction was stopped by rinsing the slides in stop wash buffer for 10 min. The slides were then rinsed 3 × 2’ in PBT before incubation with FITC-Avidin D in PBS for 30 min at room temperature. Slides were then rinsed 3 × 2’ in PBT, counterstained with PI or DAPI for 20 min and rinsed in PBS for 5 min. Slides were then mounted with Vectashield. Brownish-yellow stained lymphocytes were an indication of positive cells. The intraepithelial apoptotic lymphocytes were identified and counted.
Detection of IL-4, IFN-γ and Foxp3: The small intestinal mucosa was stripped off by circumferentially pushing the muscularis with a moist cotton applicator, as described previously[17] and then weighed to prepare a 10% homogenate by adding an appropriate amount of normal saline. The homogenate was then centrifuged at 3000 ×g for 10 min at 0°C. The supernatant was harvested and diluted with normal saline to make a 1% homogenate. The levels of interferon-γ (IFN-γ), interleukin-4 (IL-4) and forkhead box P3 (Foxp3) in the homogenate were measured by enzyme-linked immunosorbent assays (ELISA) to evaluate the function of Helper T-cell (TH) 1, TH2 and Tr cells. The IFN-γ and IL-4 ELISA kits were obtained from R&D Systems (Minneapolis, MN, USA). The Foxp3 ELISA kit was obtained from Adlitteram Diagnostic Laboratories (San Diego, CA, USA). The ELISA assays were performed according to the manufacturer’s instructions.
Detection of sIgA: Secretory IgA (sIgA) was detected using a radioimmunological assay (RIA). A 10-cm-long tissue section was obtained from the small intestine, dissected and carefully washed with normal saline. Small intestinal mucus was collected into an Eppendorf tube, and 1 mL of 0.01 mol/L PBS was added into the Eppendorf tube. The solution was then centrifuged at 3000 ×g for 10 min at 0°C. The supernatant was then harvested and the level of sIgA was measured by a double antibody sandwich RIA purchased from Beijing Nuclear Research Center (Beijing, China). The total protein level of the intestinal mucus was assayed by the Bradford brilliant blue method simultaneously. The sIgA content in 1 mg of total protein from small intestinal mucus was detected.
All statistical analyses were performed using the SPSS 15.0 software package. All data were expressed as the mean ± SD. Group comparisons were carried out using a one-factor analysis of variance. P < 0.05 was considered statistically significant.
During the course of the study there were no rats that died in the control group. A total of 5 rats died in the LPS group; 3 at 12 h and 2 at 24 h after LPS injection.
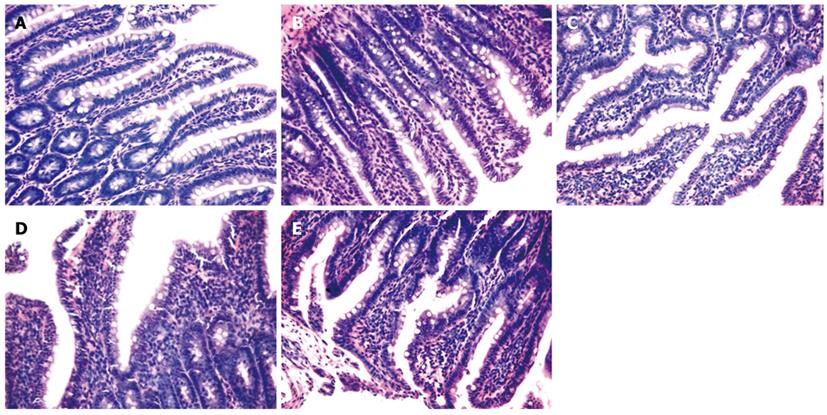
As shown in Figure 2, the intestinal mucosa of saline-treated rats was complete and the villi were presented in an orderly fashion. Samples displayed no abnormal epithelial cell morphology and there was no evidence of congestion, edema or infiltration of inflammatory cells (Figure 2A). In contrast, the intestinal mucosal villi of rats with endotoxemia were loosened and atrophic where the epithelial cells were necrotic. The mucosa was edematous and infiltrated with inflammatory cells (Figure 2B-E). These abnormal changes to the intestinal mucosa were most obvious 12 h after LPS injection (Figure 2D).
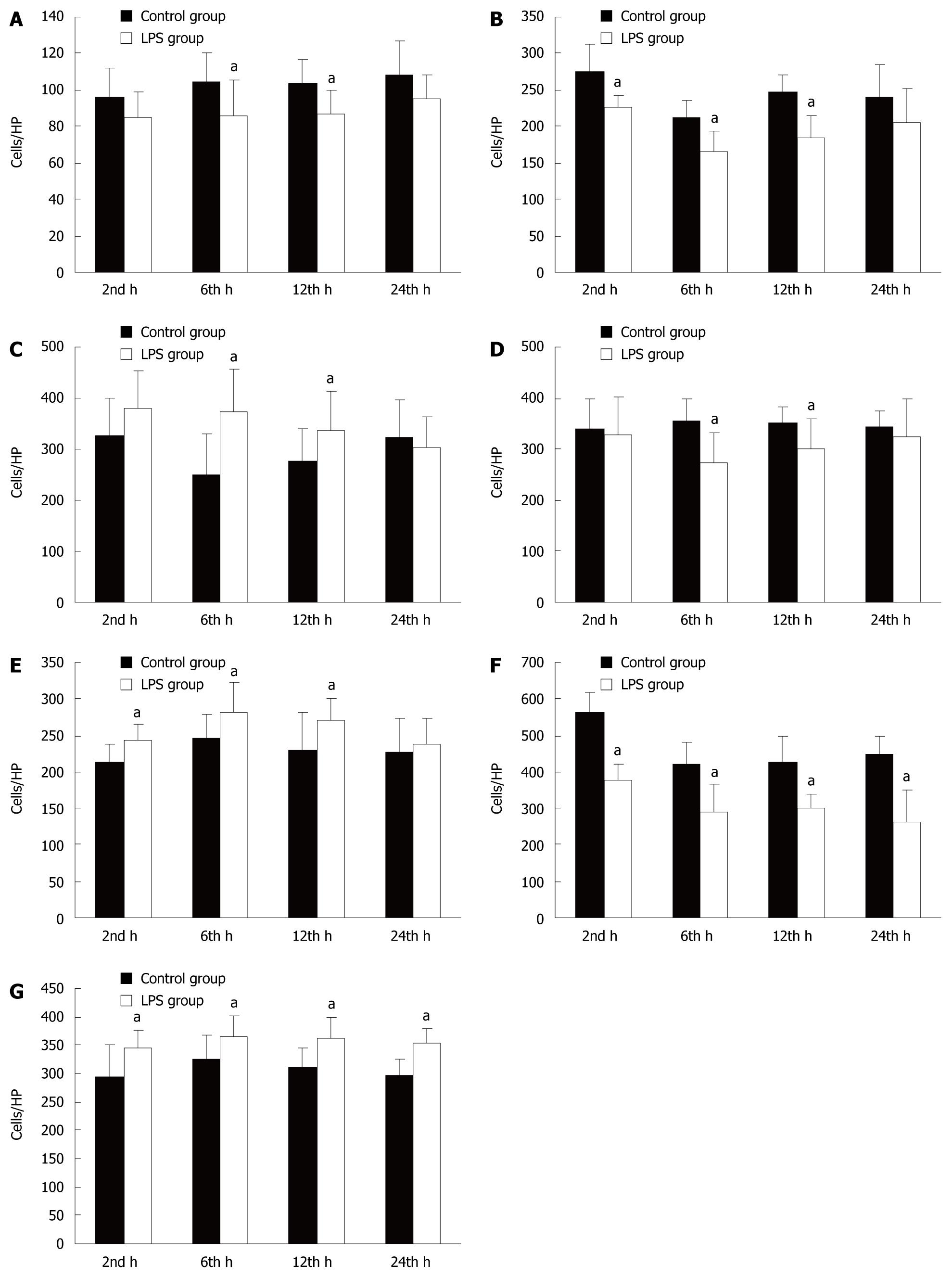
The effects on the immune system induced by LPS were assessed in the rat intestinal mucosa (Figure 3). We found that M-cell and CD8+ T cell numbers in the small intestinal mucosa were significantly decreased 6 and 12 h after LPS challenge compared to controls. Furthermore, the number of DCs was significantly decreased after 2, 6 and 12 h. In contrast, the number of CD4+ T cells was significantly increased after 6 and 12 h, before decreasing slightly by 24 h, although this decrease was not statistically significant. The number of Tr cells was significantly increased at 2, 6 and 12 h. The number of IgA+ B cells and apoptotic lymphocytes were significantly increased at all time points.
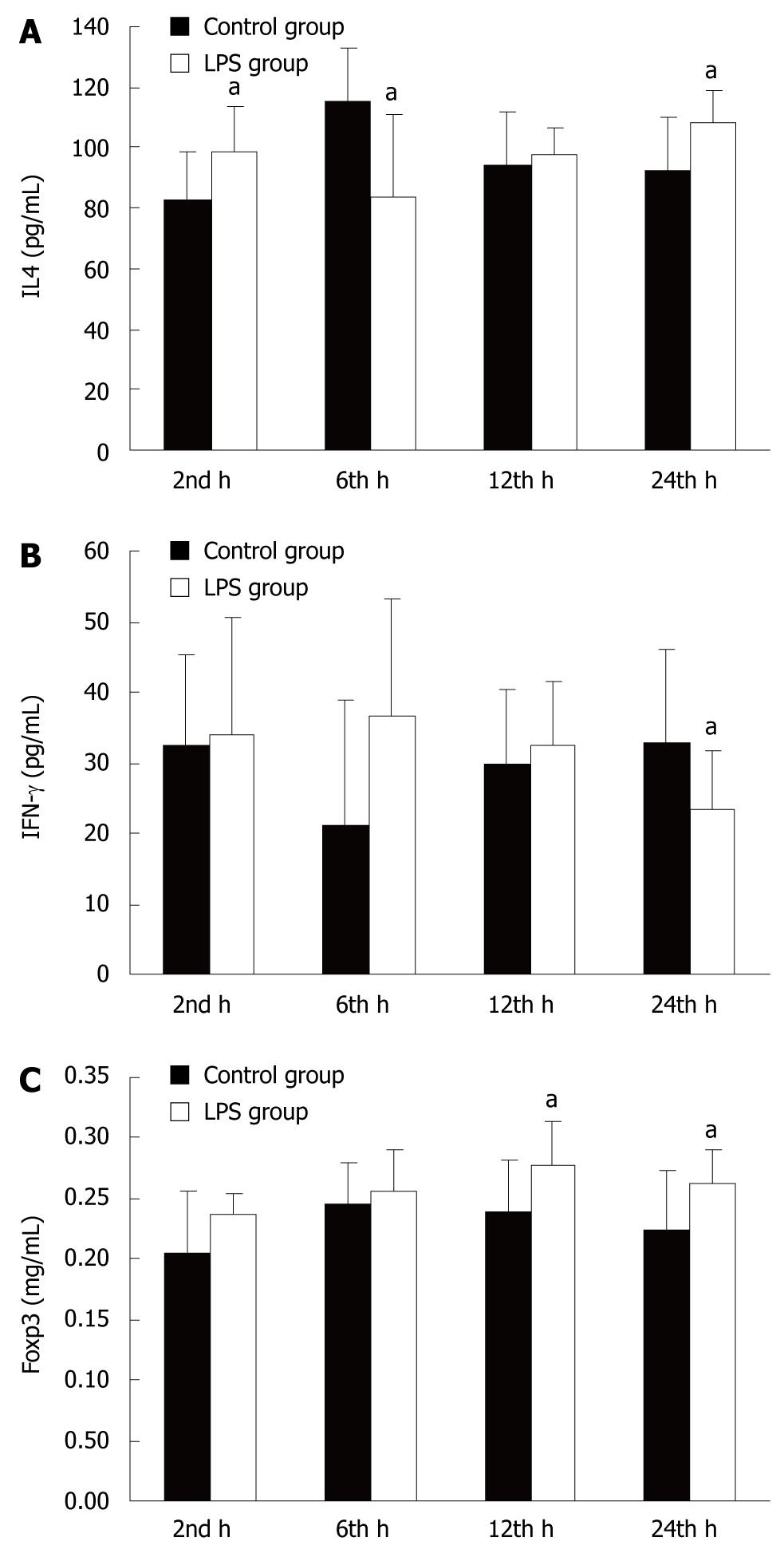
As shown in Figure 4, the level of IL-4 was significantly increased in the small intestinal mucosa after 2 h before significantly decreasing after 6 and 12 h in LPS-treated animals compared with controls. The level of IFN-γ was increased slightly after 2, 6 and 12 h before decreasing markedly by 24 h. The level of Foxp3 was significantly increased after 12 and 24 h.
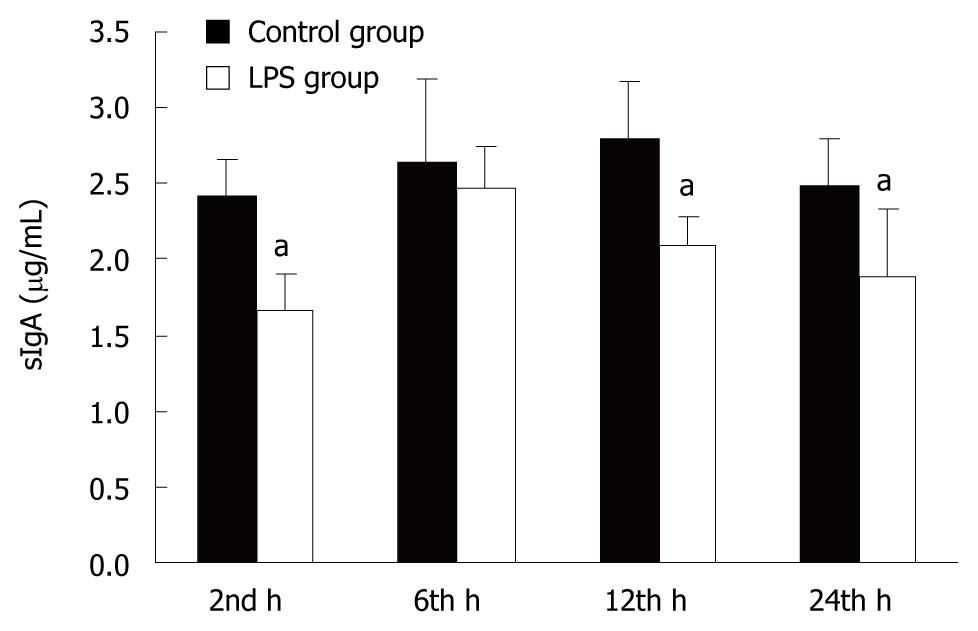
As shown in Figure 5, LPS induced a significant decrease in sIgA levels in rat intestinal mucus after 2, 12 and 24 h compared with controls.
This study has demonstrated that LPS-induced endotoxemia results in small intestinal mucosa injury. The number of M-cells, DCs, CD8+ T cells and IgA+ B cells were decreased and the number of Tr cells and apoptotic lymphocytes were increased significantly. The number of CD4+ T cells was increased in the early stages before slightly decreasing by the end of the study period. Similarly, the level of IL-4 was significantly increased at early time points and then reversed by the 24 h time point, while IFN-γ levels were also increased slightly at early time points before decreasing markedly by the end of the study. The level of Foxp3 was increased whereas the level of sIgA decreased.
Many researchers have demonstrated that the digestive tract is the largest immune organ in the body[18]. The immune response of the digestive tract mucosa primarily relies on humoral immunity. The induction site of mucosal immune responses is at the Peyer’s patches and the effector site is at the mucosa[19]. The mucosal immune response involves a number of processes: the M-cells of the Peyer’s patches collect granular antigens and pass them to DCs and macrophages which can then activate the T cells. The activated T cells will further activate B cells and the latter will produce antigen-specific sIgA after homing to the effector site.
During endotoxemia, oxidative stress causes direct damage to cells and tissues and is involved in inflammatory cytokine production. The response of the immune system to LPS is an inflammatory reaction in the early phase and anti-inflammatory reaction in the later period[20].
LPS can activate phagocytes, stimulating them to release large amounts of cytokines and inflammatory factors which can further induce microcirculatory disturbances and intestinal epithelium injury, resulting in intestinal barrier damage[21]. In this study, it was observed that the small intestinal mucosa was injured in rats with endotoxemia, suggesting that the intestinal barrier was damaged and that the most severe damage occurred 12 h after LPS injection. This study also demonstrated that the immune function of the small intestinal mucosa changed most significantly after 12 h, indicating that the immune barrier dysfunction was a part of intestinal mucosal barrier injury.
To investigate the changes to local immune induction during endotoxemia we observed the numbers of M-cells and DCs in the intestinal mucosa. M-cells are a special kind of intestinal epithelium mucosa cell that can play a role by delivering antigen to antigen presenting cells (APCs). It is believed that the M-cell is the first step in intestinal immunity[22]. It has been observed that the number of M-cells decreased and the intestinal barrier was damaged during chronic intestinal inflammation and bacterial invasion[23]. In this study, we found the same change in M-cells during acute LPS stimulation, suggesting that endotoxemia could impair the first step of intestinal immunity. DCs are a kind of APC and a previous study by Hotchkiss et al[24] demonstrated DC depletion in patients with sepsis. In this study, a decrease in DC number was observed in the intestinal mucosa, suggesting the same change in rats with endotoxemia. The decreases in M-cell and DC numbers in the small intestinal mucosa in rats with endotoxemia implied that the induction of local immune responses was impaired.
T lymphocytes, especially the Tr cells, play a major regulatory role in mucosal immunity. T cells can be classified into CD4+ T cells and CD8+ T cells according to the different protein markers on their cell surface. TH cells and Tr cells belong to the CD4+ T cell family. TH cells can be further divided into TH1 and TH2, where TH1 secretes inflammatory factors such as IFN-γ to mediate the protective immune response while TH2 secretes anti-inflammatory factors such as IL-4 to mediate the non-specific immune response[25]. A previous study demonstrated a TH1/TH2 drift[26], suggesting that the immune response progressed from being active to a suppressed state during sepsis, while a further study demonstrated that it was Tr cells that mediated this drift[27]. In addition, other studies have demonstrated that Tr cells could selectively kill and directly suppress B cells[28,29]. It was reported that circulating Tr numbers were markedly elevated and induced immunoparalysis in sepsis[30]. The development and function of Tr cells can be investigated by the expression level of Foxp3[31]. CD8+ T cells include cytotoxic T (Tc) cells and suppressor T (Ts) cells, which are the effector cells of cell immunity and suppressors of TH cells, respectively. In accordance with Bruder et al’s study[16], we inferred Tr cell numbers from a single marker of NPR-1 positive cells in our study. We only observed the intraepithelial NPR-1 positive cells to exclude interference since NRP-1 can be expressed by other cells such as DCs. Our study showed that CD4+ T cells increased in the early stages of endotoxemia before sepsis and then slightly decreased in the later phase, while CD8+ T cells decreased and Tr cells increased in the small intestinal mucosa in rats with endotoxemia. It should also be noted that in this study the level of IL-4 increased at the beginning and then decreased by the end of the study whereas the level of IFN-γ demonstrated the opposite change and the level of Foxp3 increased. Though a lot of activated immunological cells can secrete IL-4 and IFN-γ, the effects of IL-4 and IFN-γ remained unchanged. Similarly, Foxp3 is not exclusively expressed by Tr cells but also by activated effector T cells. The combination of an increase in NRP-1 cells and intestinal Foxp3 levels could suggest that Tr cells are increased. These results suggest a trend of immune changes whereby cellular immunity progressed from an active to a suppressed state during endotoxemia before sepsis. It can be concluded from this study that the rise in the number and function of Tr cells is one of the major reasons for immunosuppression in the small intestinal mucosa of rats with endotoxemia before sepsis.
IgA+ B cells are identified by positive IgA staining, because only B cells produce IgA or have surface IgA. A previous study showing decreases in the level of sIgA, the number of IgA+ B cells and the number of Gram-negative bacteria enclosed by sIgA in the intestinal tract during stress suggested that humoral immune function was inhibited dramatically[32]. Our study demonstrated that the level of sIgA and the number of IgA+ B cells diminished during endotoxemia, suggesting that LPS-induced endotoxemia inhibited humoral immune function of the digestive tract in accordance with the previous report. One explanation for this could be due to the rise in the number and function of Tr cells, as demonstrated in our study, which could lead to the suppression of B cell function.
Mongini et al[33] reported that relocation of endotoxin after injury can increase the number of apoptotic lymphocytes. Our research demonstrated a similar change, with an increase in the number of apoptotic lymphocytes in the intestinal mucosa of rats with endotoxemia. This could contribute to the immunosuppression of the small intestine in rats with endotoxemia.
In conclusion, our study showed that mucosal immune barrier dysfunction was a part of intestinal mucosal barrier injury. Cellular immunity was active in the early phase of endotoxemia and suppressed in later periods, humoral immunity was abnormal and lymphocyte apoptosis was increased. Our study suggests that the increased number and function of Tr cells and the increase in lymphocyte apoptosis are the reasons for intestinal mucosal immunodeficiency. Based on these findings, an earlier protective or immunoregulative treatment aimed at gastrointestinal immune function may be of benefit to severely infected patients. We suggest future studies could be designed to test this hypothesis.
Immune dysfunction is one of the most frequent complications within the gastrointestinal mucosal barrier during sepsis. Mucosal barrier dysfunction plays an important role in the pathophysiology of sepsis by promoting bacterial stasis, bacterial overgrowth, and bacterial translocation, which can lead to the development of secondary infections and multiple organ failure. The changes of gastrointestinal immune function may appear at the beginning or even before sepsis.
Intestinal mucosal barriers consist of a mechanical barrier, a chemical barrier, an immunological barrier, and a biological barrier. The immunological barrier is considered as the first line of defense of the intestinal mucosa from bacterial invasion and plays an important role in the overall defense. This study observed the changes in the gastrointestinal immunological barrier during endotoxemia.
Previous studies have discussed the changes to immunity during sepsis, but what happens to immunity, specifically the gut immunity, during endotoxemia before sepsis is not clear. In this study, changes to the number and function of intestinal mucosal immune cells in rats with endotoxemia were observed to investigate whether dysfunction of the immunological barrier occurred during endotoxemia and to elucidate the potential mechanism of this dysfunction.
By observing the changes of gastrointestinal immune function, the study shows that the gastrointestinal immune dysfunction occurs during endotoxemia before sepsis. Thus, a protective or an immunoregulative treatment of gastrointestinal immune function should be used earlier in severely infected patients.
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria, act as endotoxins and elicit strong immune responses in animals. Endotoxemia is the immune responses to LPS in animals. Cytokines: non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, which act as intercellular mediators. Sepsis is defined as infection plus systemic manifestations of infection.
This is a descriptive study of the effect of intravenous LPS on intestinal immune cells and cytokine levels in the rat. Straightforward and generally well written paper.
Peer reviewer: Dr. Adrian G Cummins, Department of Gastroenterology and Hepatology, (DX 465384), 28 Woodville Road, Woodville South, 5011, South Australia, Australia
S- Editor Wang JL L- Editor Logan S E- Editor Ma WH
| 1. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. |
| 2. | Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, Fang Q, Xu Q, Wang D, Jin Y. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538-2546. |
| 3. | Brun-Buisson C. [Epidemiology of severe sepsis]. Presse Med. 2006;35:513-520. |
| 4. | Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584-1589. |
| 5. | Garrison RN, Spain DA, Wilson MA, Keelen PA, Harris PD. Microvascular changes explain the "two-hit" theory of multiple organ failure. Ann Surg. 1998;227:851-860. |
| 6. | Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196-208. |
| 7. | MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228. |
| 8. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. |
| 9. | Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329-343. |
| 10. | Ellen JM, Lammel CJ, Shafer MA, Teitle E, Schachter J, Stephens RS. Cervical secretory immunoglobulin A in adolescent girls. J Adolesc Health. 1999;25:150-154. |
| 11. | Tyler BM, Cole MF. Effect of IgA1 protease on the ability of secretory IgA1 antibodies to inhibit the adherence of Streptococcus mutans. Microbiol Immunol. 1998;42:503-508. |
| 12. | Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol. 1998;6:13-18. |
| 13. | Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem Pharmacol. 2007;73:793-804. |
| 14. | Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA. Immunocytochemical characterization of the follicle-associated epithelium of Peyer’s patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol. 1996;71:363-370. |
| 15. | Chen-Woan M, Delaney CP, Fournier V, Wakizaka Y, Murase N, Fung J, Starzl TE, Demetris AJ. A new protocol for the propagation of dendritic cells from rat bone marrow using recombinant GM-CSF, and their quantification using the mAb OX-62. J Immunol Methods. 1995;178:157-171. |
| 16. | Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623-630. |
| 17. | Su GL, Walgenbach KJ, Heeckt PH, Wang Q, Halfter W, Whiteside TL, Bauer AJ. Increased expression of interferon-gamma in a rat model of chronic intestinal allograft rejection. Transplantation. 1996;62:242-248. |
| 18. | Takahashi I, Kiyono H. Gut as the largest immunologic tissue. JPEN J Parenter Enteral Nutr. 1999;23:S7-S12. |
| 19. | Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853-879. |
| 20. | Jones-Carson J, Fantuzzi G, Siegmund B, Dinarello C, Tracey KJ, Wang H, Fang FC, Vazquez-Torres A. Suppressor alphabeta T lymphocytes control innate resistance to endotoxic shock. J Infect Dis. 2005;192:1039-1046. |
| 21. | Aranow JS, Fink MP. Determinants of intestinal barrier failure in critical illness. Br J Anaesth. 1996;77:71-81. |
| 22. | Meynell HM, Thomas NW, James PS, Holland J, Taussig MJ, Nicoletti C. Up-regulation of microsphere transport across the follicle-associated epithelium of Peyer’s patch by exposure to Streptococcus pneumoniae R36a. FASEB J. 1999;13:611-619. |
| 23. | Kucharzik T, Lugering N, Rautenberg K, Lugering A, Schmidt MA, Stoll R, Domschke W. Role of M cells in intestinal barrier function. Ann N Y Acad Sci. 2000;915:171-183. |
| 24. | Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493-2500. |
| 25. | Hauer AC, Breese EJ, Walker-Smith JA, MacDonald TT. The frequency of cells secreting interferon-gamma and interleukin-4, -5, and -10 in the blood and duodenal mucosa of children with cow’s milk hypersensitivity. Pediatr Res. 1997;42:629-638. |
| 26. | Liu JH, Zhang YQ, Huang B, Fang Q, Huo X, Hu H. [The effects of endotoxin on the Th1/Th2 cells and immune modulation of Astragalus membranaceus]. Zhonghua Erke Zazhi. 2003;41:613-614. |
| 27. | Purcell EM, Dolan SM, Kriynovich S, Mannick JA, Lederer JA. Burn injury induces an early activation response by lymph node CD4+ T cells. Shock. 2006;25:135-140. |
| 28. | Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925-3932. |
| 29. | Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180-4183. |
| 30. | Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068-2071. |
| 31. | Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331-337. |
| 32. | Van Leeuwen PA, Boermeester MA, Houdijk AP, Meyer S, Cuesta MA, Wesdorp RI, Rodrick ML, Wilmore DW. Pretreatment with enteral cholestyramine prevents suppression of the cellular immune system after partial hepatectomy. Ann Surg. 1995;221:282-290. |
| 33. | Mongini C, Ruybal P, Garcia Rivello H, Mocetti E, Escalada A, Christiansen S, Argibay P. Apoptosis in gut-associated lymphoid tissue: a response to injury or a physiologic mechanism? Transplant Proc. 1998;30:2673-2676. |