INTRODUCTION
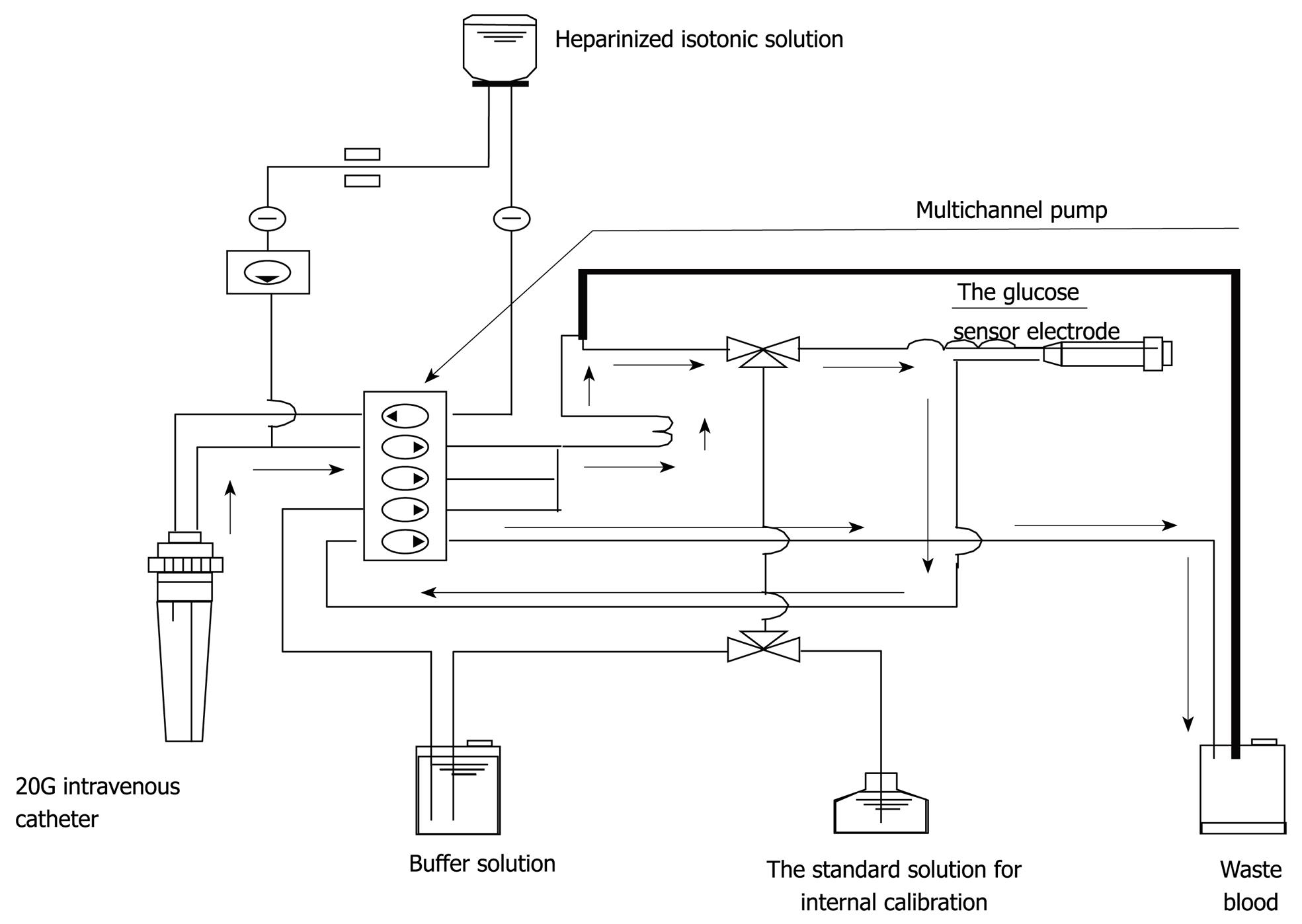
Figure 1 The whole circuit of the STG-22TM.
Arrows indicate the direction of blood sampling.
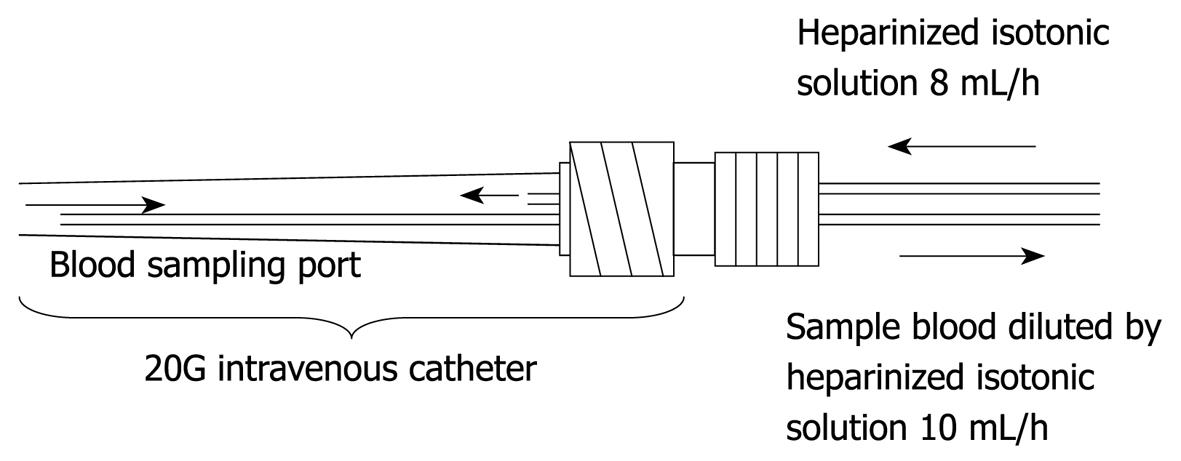
Figure 2 This figure shows the dual lumen catheter technique.
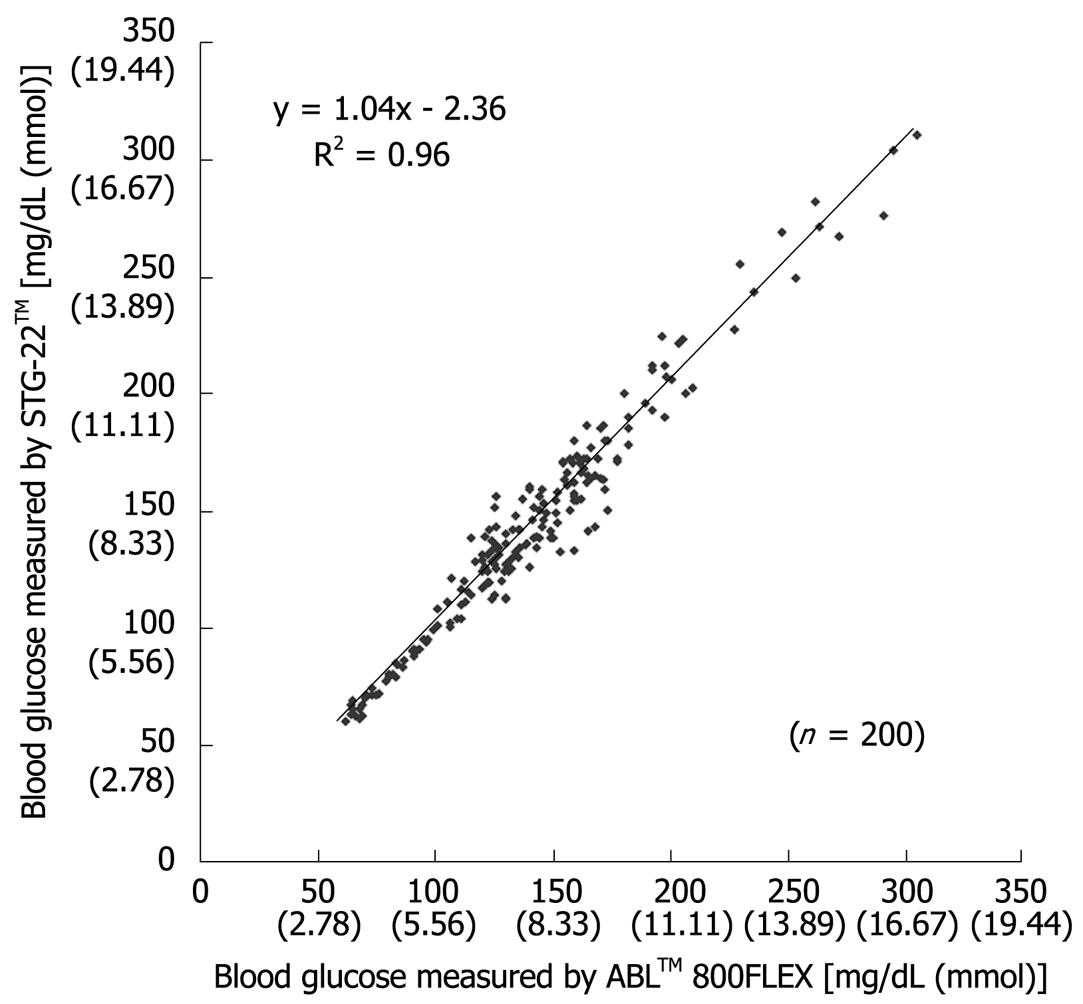
Figure 3 Scatter plot of individual blood glucose levels measured by both STG-22TM and ABLTM 800FLEX during surgery[20].
Figure 4 Scatter plot of individual blood glucose levels measured by both STG-22TM and ABLTM 800FLEX in post-surgical patients[21].
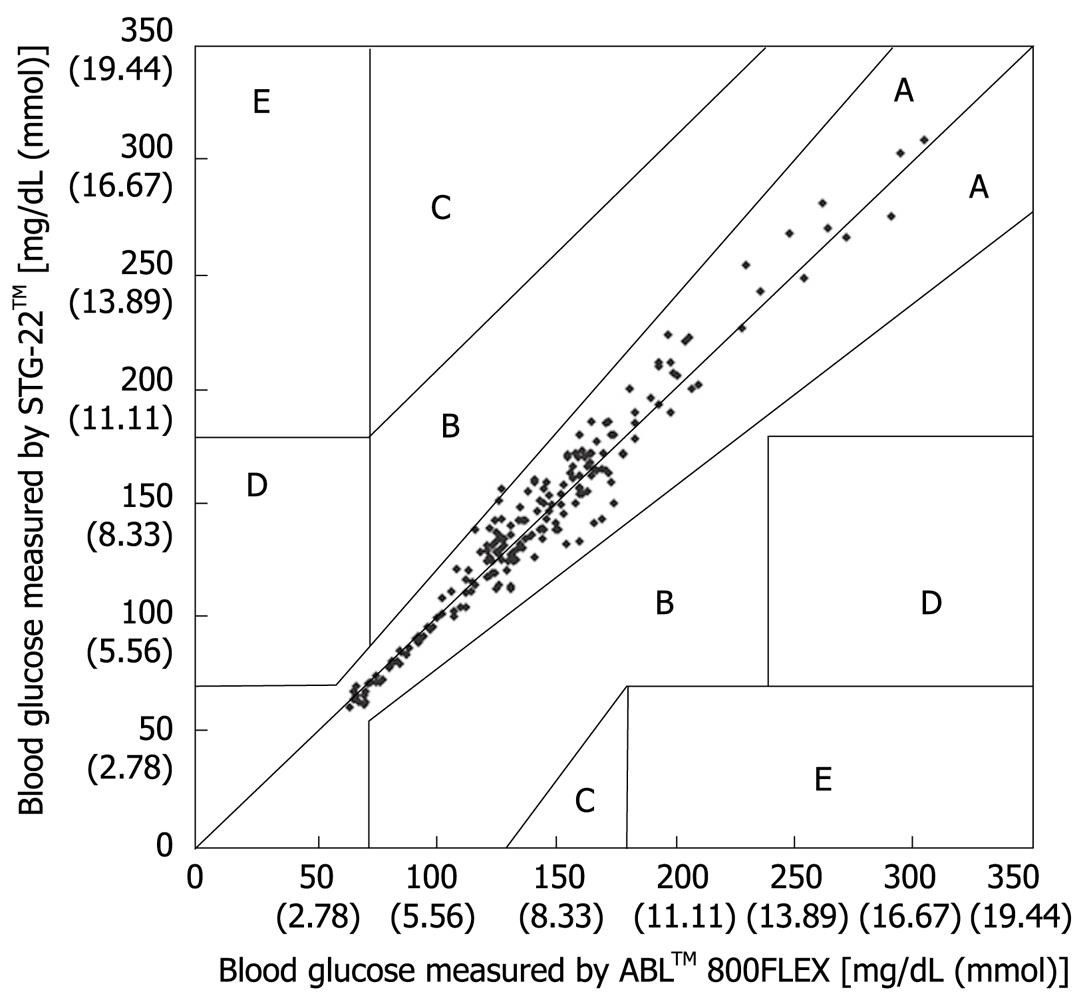
Figure 5 Error grid analysis for evaluation of blood glucose measured by STG-22TM compared with those measured by ABLTM 800FLEX.
Zone A, B: Accurate or acceptable; Zone C: Unnecessary corrections that could lead to a poor outcome; Zone D: Dangerous failure to detect and treat; Zone E: ‘Erroneous treatment’[21].
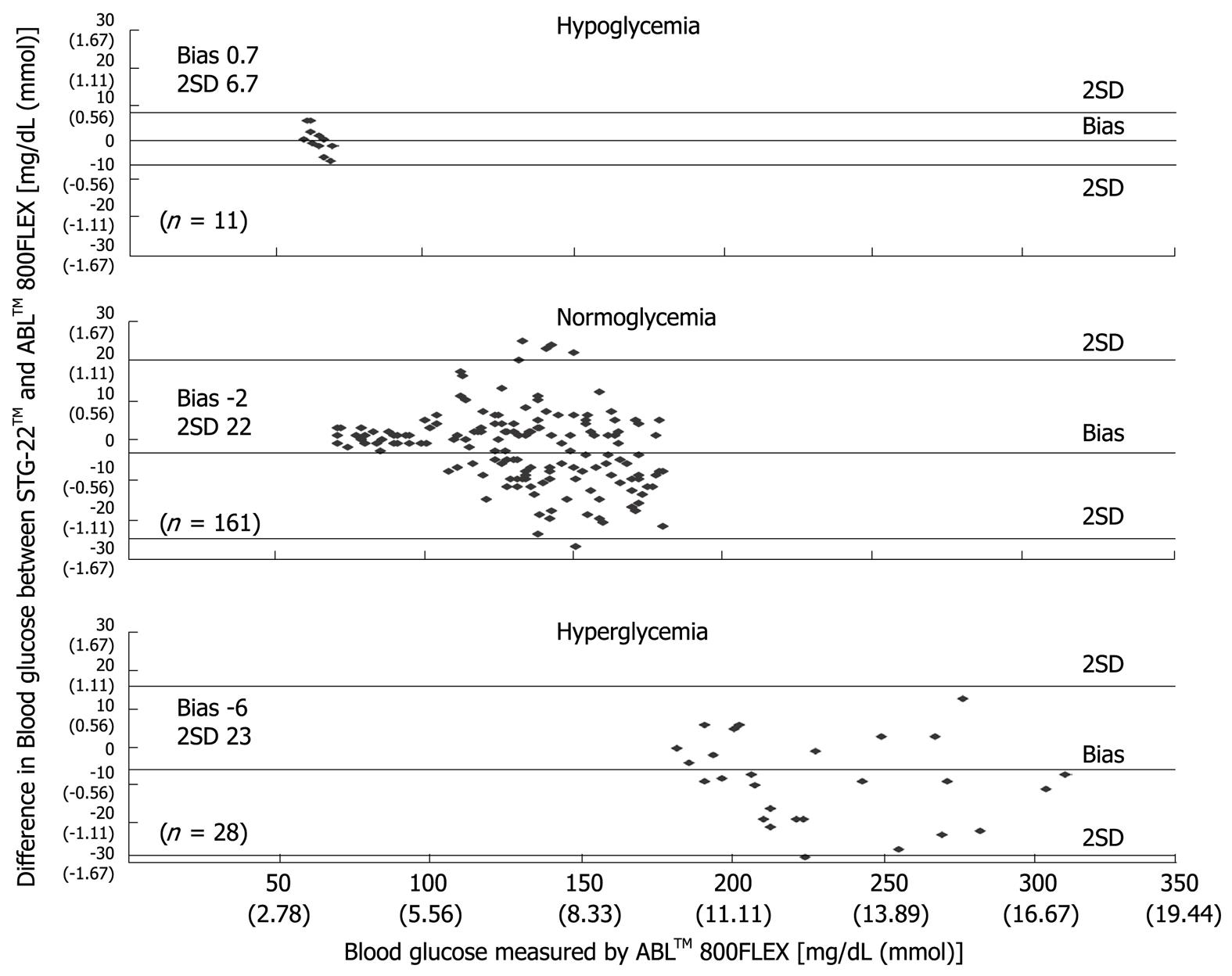
Figure 6 Bland-Altman plot of blood glucose measurements from STG-22TM and ABLTM 800FLEX[21].
Management of the respiratory and cardiovascular systems is the most important task of an anesthesiologist. Traditionally, the purpose of intraoperative glycemic control is to prevent three major problems: hypoglycemia, ketoacidosis, and hyperglycemia-associated osmotic diuresis (dehydration). So far, particular attention has been given only to the case of abnormal hyperglycemia. On the other hand, postoperative glycemic control appears to influence a patient’s prognosis, and as a result, this has drawn attention to intraoperative glycemic control[1,2]. In this report, the recent concept of glycemic control, problems of glycemic control procedures and effects of intraoperative glycemic control on a patient’s prognosis are reviewed based on recent publications.
NEW CONCEPT OF GLYCEMIC CONTROL
The concept of glycemic control in the care of critically ill patients largely changed after van den Berghe et al[3] published a report in 2001. They comparatively investigated two groups of patients admitted to a surgical intensive care unit. In the first group, insulin control was instituted when the blood glucose level exceeded 100 mg/dL in order to maintain the blood glucose level in the target range from 80 to 110 mg/dL (strict glycemic control group). In the second group, insulin control was instituted if the blood glucose level exceeded 215 mg/dL in order to maintain the blood glucose level in the target range from 180 to 200 mg/dL (conventional glycemic control group). The results of insulin control were remarkable. The mortality rate of patients during the stay in the intensive care unit was 4.6% for the strict glycemic control group vs 8.0% for the conventional treatment group. The mortality rate among patients who remained in the intensive care unit for five or more days was 10.6% for the strict glycemic control group vs 20.2% for the conventional treatment group. The in-hospital mortality rate was reduced by 3.7% by strict glycemic control. However, the test protocol has been questioned since there is a large discrepancy between the actual mortality rate of 26.3% in the examined group and the mortality rate predicted by APACHE II score (median score 7). To address this criticism, a study was conducted in a medical intensive care unit and results were reported in 2006[4]. This study, where long-term prognosis following a similar glycemic control was monitored for one year or longer, showed the benefits of strict glycemic control in patients who remained in the intensive care unit for three or more days, but not in those who stayed for a shorter period. The mortality rate of the strict glycemic control group was 41.5% compared with the 50.9% of the conventional treatment group. However, the underlying detailed mechanism was not clarified and various basic and clinical studies to clarify this mechanism are presently ongoing.
DOES INTRAOPERATIVE GLYCEMIC CONTROL INFLUENCE PATIENT PROGNOSIS?
To date, there have been no randomized controlled trials to assess the effects of intraoperative glycemic control on patient prognosis. However, it is likely that intraoperative hyperglycemia decreases immunocompetence and increases the incidence of wound infection[5-10]. Various types of anesthetic agents also alter blood glucose level[11]. Furthermore, it has been reported that the incidence of wound infection is markedly high among hyperglycemic patients whose blood glucose level was reported to be 149 mg/dL or above intraoperatively[12,13]. Moreover, every 18 mg/dL increase in blood glucose level from 110 mg/dL is reported to increase the risk of wound infection by 17%[14]. Based on these results, intraoperative glycemic control that maintains blood glucose at a level not higher than 150 mg/dL is considered necessary. On the other hand, intraoperative glycemic control reportedly increases the risks of hypoglycemia due to unstable actions of insulin owing to hypothermia and impaired tissue perfusion[15,16]. Therefore, the establishment of concrete glycemic control procedures is eagerly anticipated, and the development of a continuous intraoperative blood glucose monitoring system is considered very important.
CURRENT STATUS OF GLYCEMIC CONTROL PROCEDURES
As mentioned above, concrete glycemic control procedures for achieving strict glycemic control have not yet been established. The detailed procedures described by van den Berghe et al[3] in 2001 were not very clear. According to another line of thought, full-time staff can be designated to carry out frequent blood glucose measurements in order to achieve glycemic control. While this validates this line of thought, it can not be executed in general practice. To perform accurate and safe glycemic control, the following elements are considered important: (1) frequency of blood glucose measurements, (2) accuracy of blood glucose measurement results, (3) accuracy of the insulin dosing algorithm, and (4) blood collection volume.
FREQUENCY OF BLOOD GLUCOSE MEASUREMENT
As for the frequency of blood glucose measurement, continuous monitoring is preferable to intermittent measurements. Two types of system capable of continuous blood glucose monitoring are currently used. One measures glucose concentration in the intracellular substance obtained by directly inserting an electrode subcutaneously, and the other collects venous blood continuously for blood glucose monitoring[17,18]. The former system is based on the near proportional relation between glucose concentration in the intracellular substance and blood glucose level. However, the measurement stability of this type of system is in question because rapid changes in blood glucose level are not readily reflected in the intracellular substance. On the other hand, an artificial pancreas (STG-22TM) was developed by Nikkiso (Tokyo, Japan) and this device is considered the only system in the world representing the latter type of system, which collects venous blood continuously and performs blood glucose monitoring. Furthermore, this system carries out direct measurements of blood glucose and can therefore show rapid changes in blood glucose level (Figures 1 and 2). Although STG-22TM is expected to serve as a standard blood glucose monitoring system, it cannot be used for all patients because of its high cost. Generally, intermittent blood glucose measuring systems are highly versatile and can be used in any facility. However, many difficulties are encountered in their use for strict glycemic control, mostly attributed to increased workloads from frequent measurements and large measurement errors[19]. It is considered that the achievement of strict glycemic control is currently supported by the efforts and dedication of medical staff as well as through proper education[18].
PROBLEMS ENCOUNTERED REGARDING THE VOLUME OF BLOOD COLLECTED
To achieve successful strict glycemic control, blood glucose measurement must be performed at least every hour. Since around 0.5 mL of blood is required for a single blood glucose measurement, a total of 12 mL of blood in point-of-care blood glucose testing must be collected daily. When blood glucose control is unstable, more frequent measurements are needed and approximately 20 mL of blood must be collected daily solely for blood glucose measurement. This becomes a considerable burden for patients with anemia and for pediatric patients, and thus the development of blood glucose measurement technologies requiring no blood collection is greatly anticipated.
ACCURACY OF BLOOD GLUCOSE MEASUREMENT RESULTS
Measurement errors become an issue during strict glycemic control for maintaining blood glucose at a level between 80 and 110 mg/dL. According to the standards established by the International Organization for Standardization, the errors defined for glucose concentrations of 4.1 mmol (74 mg/dL) or above must not be greater than 20%, and those for glucose concentrations of 4.1 mmol (74 mg/dL) or below must not be greater than ± 0.8 mmol (14 mg/dL), and these values are not guaranteed by the accuracy of portable blood glucose measurement systems. STG-22TM, which is currently being used for a clinical study by our group, is a highly accurate and reliable system. Its measurement errors are below 21% during intraoperative measurement[20] (Figure 3) and not more than 15% during postoperative measurements[21] (Figures 4, 5 and 6). These findings suggest that STG-22TM can sufficiently perform closed loop control of blood glucose based on measurement results.
ACCURACY OF INSULIN DOSING ALGORITHMS
Sliding scale dosing of insulin, described previously as a special feature, is not described here. A program that calculates insulin dosage based on the blood glucose level, rate of its change and target blood glucose level is expected to be used widely in clinical applications, since such a program has been reported to decrease the incidence of hypoglycemia by approximately 0.3%[22]. On the other hand, STG-22TM is the only system equipped with a closed-loop algorithm that enables continuous measurement of blood glucose levels and provides updated results. This system utilizes Shichiri’s algorithm, which calculates insulin dosage on the basis of the fact that glucose-stimulated insulin secretion is based on the proportional-derivative action[23]. The results of our previous studies have proven that use of STG-22TM achieves good postoperative glycemic control among patients who received surgical treatments[24,25]. Here, we aim to further evaluate the applicability of this system to intraoperative glycemic control.
NEW BLOOD GLUCOSE MEASUREMENT TECHNOLOGY
A continuous noninvasive blood glucose monitoring system is an ideal instrument for strict glycemic control. Pasic et al[26] previously developed a blood glucose measurement system by combining microdialysis and an optic fiber, and they succeeded in performing continuous in vitro measurement for 3 d. Maruo et al[27] have also developed a near-infrared based blood glucose monitoring system, and its application to patients in the intensive care unit showed a good outcome. However, these systems present disadvantages in terms of convenience and accuracy, making strict glycemic control difficult. Further improvements to these systems are needed.
NEW INSULIN DOSING TECHNOLOGIES
Recently, an insulin inhalant has been approved in the US and Europe. In terms of drug assimilation, the lung possesses a large surface area for drug absorption. Moreover, the rate of solute absorption is fast in the alveolar epithelium, and mucosal clearance in the alveoli is slower than that in the bronchi. Therefore, the lung is a very suitable route for administering high-molecular-weight insulin. Because of the reasons mentioned above, the lung can therefore possibly be used as a new route for administering drugs when peripheral circulation is impaired. In support of this, Barnett et al[28] have reported that long-term inhaled insulin therapy has no adverse effects on pulmonary function and thus its wide application is expected in the future.
CONCLUSION
A highly accurate continuous blood glucose monitoring system and closed-loop control of blood glucose are essential for anesthesiologists to achieve strict intraoperative glycemic control. STG-22TM is the only system that performs both functions; however, it requires 50 mL of blood per day for continuous blood glucose monitoring, and the collection of such a volume is invasive and remains laborious. With technological progress, the development of blood glucose monitoring systems with improved convenience, accuracy and minimal invasiveness are anticipated in the future.
Peer reviewer: Christa Buechler, PhD, Regensburg University Medical Center, Internal Medicine I, Franz Josef Strauss Allee 11, 93042 Regensburg, Germany
S- Editor Tian L L- Editor O’Neill M E- Editor Yin DH