Published online Jul 7, 2009. doi: 10.3748/wjg.15.3142
Revised: May 30, 2009
Accepted: June 6, 2009
Published online: July 7, 2009
AIM: To investigate the presence of high-risk human papilloma virus (HPV) in esophageal squamous cell carcinomas (ESCCs) in a non-selected Mexican population.
METHODS: Cases with a pathological diagnosis of squamous cell carcinoma of the esophagus were obtained from Department of Pathology files, at the National Cancer Institute in Mexico City during the period between 2000 and 2008. Slides from each case were reviewed and cases with sufficient neoplastic tissue were selected for molecular analysis. DNA was extracted from paraffin-embedded tissue samples for polymerase chain reaction analysis to detect HPV DNA sequences. Demographic and clinical data of each patient were retrieved from corresponding clinical records.
RESULTS: HPV was detected in 15 (25%) of ESCCs. HPV-16 was the most frequently observed genotype, followed by HPV-18; HPV-59 was also detected in one case. Unfortunately, HPV genotype could not be established in three cases due to lack of material for direct sequencing, although universal primers detected the presence of HPV generic sequences. No low-risk HPV genotypes were found nor was HPV-16/18 co-infection. HPV presence in ESCC was not significantly associated with gender, age, alcohol consumption, smoking, anatomic location, or histologic grade. All patients belonged to low and very low socioeconomic strata, and were diagnosed at advanced disease stage. Male patients were most commonly affected and the male:female ratio in HPV-positive ESCC increased two-fold in comparison with HPV-negative cases (6.5:1 vs 3.1:1).
CONCLUSION: High prevalence of high-risk HPV in ESCC in Mexico does not support the hypothesis that HPV-associated ESCC is more common in areas with higher ESCC incidence rates.
- Citation: Herrera-Goepfert R, Lizano M, Akiba S, Carrillo-García A, Becker-D’Acosta M. Human papilloma virus and esophageal carcinoma in a Latin-American region. World J Gastroenterol 2009; 15(25): 3142-3147
- URL: https://www.wjgnet.com/1007-9327/full/v15/i25/3142.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3142
With regard to incidence and prevalence, esophageal cancer exhibits striking geographical variations due to unknown factors between countries, as well as between different regions of the same country. According to the World Health Organization, incidence-rate spectra are located between Western Africa -at the low-risk end, and China at the high-risk end, including the so-called “Asian esophageal cancer belt”[1].
Among Latin American countries, Mexico and Peru have the lowest mortality rates for esophageal carcinoma, in both males and females, whereas Brazil, Argentina and Chile, have the highest mortality rates[2]. Mexico has a low esophageal cancer risk, with mortality rates in male and female inhabitants ca 2 and ca 1 per 100 000, respectively[3].
Esophageal carcinoma has been related to tobacco, alcohol, fungal toxins, nutritional deficiencies, and hot food and beverages[4]. In addition, human papilloma virus (HPV), a major cause of carcinoma of the cervix uteri throughout the world, is suspected of being related to the development of this carcinoma. Meta-analyses by Syrjänen[5] (2002) showed that HPV DNA was detected in 15.2% of esophageal squamous cell carcinomas (ESCCs). To date, total or partial sequencing identified > 200 genotypes of HPV, which are categorized into the following four cervical oncogenicity-based risk groups: high, probably high, low and undetermined risks[6]. High-risk HPV is considered to cause neoplastic transformation of normal epithelial cells, through expression of early transforming viral proteins E6 and 7, resulting in cell cycle-machinery deregulation and expression of several transforming oncogenes[7].
High-risk HPV-associated ESCC is hypothesized to be consistently more common in countries with higher ESCC risk[8]. In a recent study by Castillo et al[9], HPV DNA was detected in 34% and 19% of esophageal carcinomas in Colombia and Chile, respectively; HPV-16 was the most frequent genotype in both countries. It is noteworthy that the HPV detection rate in ESCCs was found to be two-fold higher in Colombia than in Chile, whereas the esophageal cancer mortality rate showed an inverse relationship.
The aim of the present study was to investigate the presence of high-risk HPV DNA jn ESCCs, among a non-selected Mexican population.
Cases with a pathological diagnosis of squamous cell carcinoma of the esophagus were obtained from Department of Pathology files, at the National Cancer Institute in Mexico City during the period between 2000 and 2008. Slides from each case were reviewed and cases with sufficient neoplastic tissue were selected for molecular analysis. Because the majority of the cases of esophageal cancer seen at our Institution correspond to advanced clinical stages, elective surgery is not performed. Subsequently, DNA was extracted from paraffin-embedded tumor samples, obtained during a panendoscopic procedure; adjacent normal esophageal mucosa was not sampled. Demographic and clinical data of each patient were retrieved from corresponding clinical records.
Twenty micrometer sections of formalin-fixed and paraffin-embedded tumors were de-waxed by incubation with N-octane and washings with 100% ethanol. This process was repeated twice, and the pellet was dried. Deparaffinized samples were digested with 1 mL of lysis buffer (Tris-HCl 10 mmol/L pH 8.0, EDTA 0.1 mol/L pH 8-0, SDS 0.5%, Proteinase K 200 &mgr;g/mL, RNase A 20 &mgr;g/mL) at 55°C for 3 h. DNA was extracted with phenol/chloroform precipitations as described by Sambrook et al[10]. To test DNA suitability for polymerase chain reaction (PCR) amplification the DNA obtained was amplified for: β-globin gene (PCO4/GH2O) under conditions described by Resnick et al[11]. Samples were later submitted to HPV amplification with three sets of the following universal primers recognizing distinct size fragments of L1 gene: L1C1/L1C2, MY09/MY11, and GP5/GP6[12–14]. HPV type-specific amplification was also performed with primers designed to amplify the E6 gene of HPV type 16 and 18 as described by Lizano et al[15].
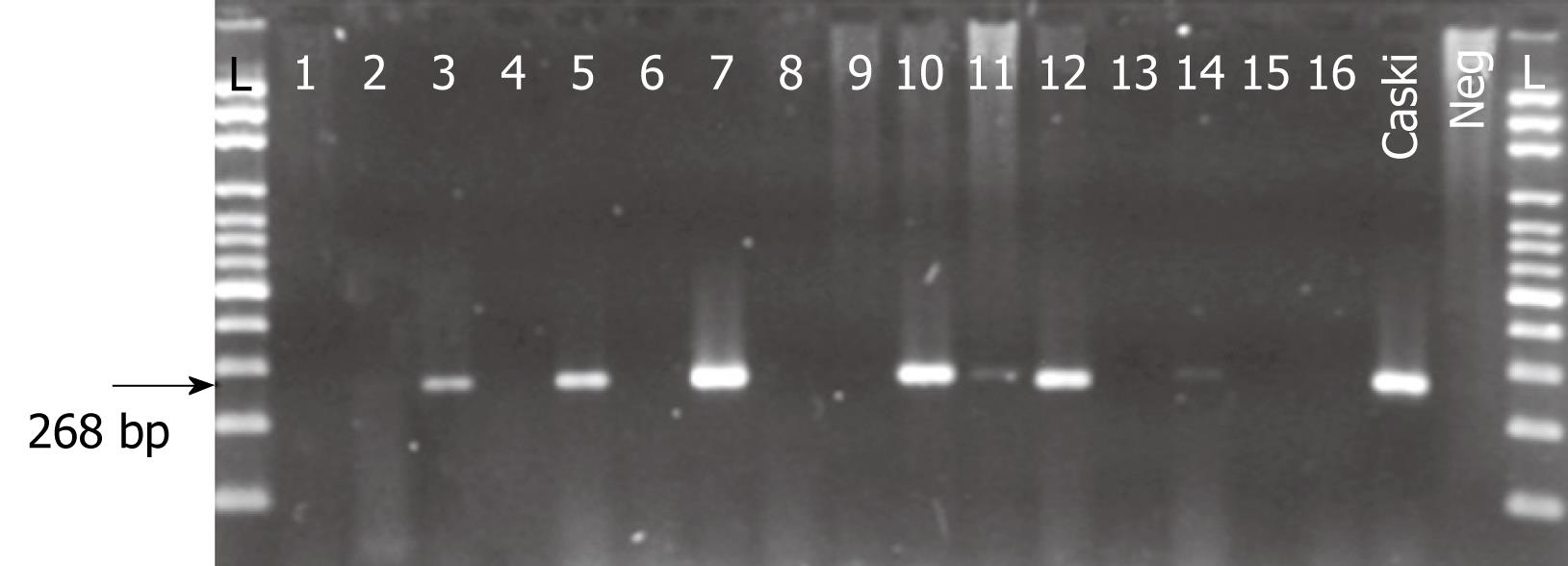
HPV PCR products were electrophoresed in a 1.2% agarose gel and visualized by ethidium bromide staining. HPV typing was carried out by direct sequencing of PCR products by means of the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The resulting sequences were analyzed in BLAST data bank for comparison with known HPV sequences. HPV 16 and 18 specific amplification was conducted for every DNA sample. DNA extracted from CaSki and Hela HPV containing cell lines were used as positive controls.
Fisher’s exact test was applied for categorical variables. A probability value P < 0.05 was considered statistically significant.
In 60 cases of ESCC, DNA quality was adequate for PCR analysis to detect HPV DNA sequences, as demonstrated by β-globin gene amplification. There were 47 male and 13 female patients, with a mean age of 62.7 years (range, 27-85 years) and 61.2 years (range, 47-80 years), respectively. Thirty-six subjects consumed alcohol (ranging from occasional to 60 years drinkers) but frequency and alcoholic-beverage type could not be precisely assessed. In 14 subjects, previous history of alcohol consumption was unknown and ten patients denied alcohol consumption. Smoking habit was recorded in 28 patients (from occasional to up to 60 years of smoking; frequency was not known), whereas in 16 subjects smoking habit was unknown; 16 patients did not smoke. Clinical history of esophageal achalasia was not recorded in any case, and there were previous symptoms of gastro-esophageal reflux disease (GERD) in two patients. Finally, oesophageal surgery was performed in one patient 26 years previously due to direct trauma. All patients belonged to low and very-low socioeconomic strata, the majority of these were agricultural workers.
All patients were diagnosed at advanced disease stage. Among these, 42 patients (70%) were treated with chemo- and/or radiotherapy and three subjects underwent esophagectomy immediately after neoadjuvant therapy. Finally, 18 patients (30%) refused any treatment. During follow-up, seven patients (11.7%) died of oesophageal carcinoma, whereas one of these died of causes not related to the neoplastic disease. The majority of patients were alive but with disease at 1 year or less of follow-up, but did not return for subsequent medical care. Regardless of the presence of HPV, all patients with ESCCs belonged to low and very-low socioeconomic strata, the majority of these were agricultural workers (data not shown). HPV presence in ESCCs was also unrelated to anatomic location, histologic grade or patient condition (alive or dead) at hospital discharge.
HPV universal primers and HPV-16 and -18 specific primers detected the presence of HPV DNA in 15 (25%) of 60 ESCCs. Cases of HPV DNA consisted of 13 males and two females with a mean age of 63.4 years (range, 37-76 years) and 62.5 years (range, 47-78 years), respectively. There were no statistical differences or associations (P > 0.05) between HPV status and gender, age, previous history of alcohol consumption and smoking, anatomic location, histologic grade or patient condition (alive or dead) at hospital discharge (Table 1).
| Total (%) | HPV- (%) | HPV+ (%) | P value | |
| Total | 60 (100) | 45 (75) | 15 (25) | |
| Gender | 0.485 | |||
| Female | 13 (100) | 11 (85) | 2 (15) | |
| Male | 47 (100) | 34 (72) | 13 (28) | |
| Alcohol consumption | 0.377 (0.420) | |||
| No | 10 (100) | 9 (90) | 1 (10) | |
| Yes | 36 (100) | 27 (75) | 9 (25) | |
| Unknown | 14 (100) | 9 (64) | 5 (36) | |
| Smoking | 0.274 (0.450) | |||
| No | 16 (100) | 14 (88) | 2 (12) | |
| Yes | 28 (100) | 21 (75) | 7 (25) | |
| Unknown | 16 (100) | 10 (63) | 6 (37) | |
| Esophageal location | 0.330 | |||
| Upper third | 14 (100) | 9 (64) | 5 (36) | |
| Upper/middle third | 4 (100) | 2 (50) | 2 (50) | |
| Middle third | 28 (100) | 23 (82) | 5 (28) | |
| Middle/lower third | 10 (100) | 7 (70) | 3 (30) | |
| Lower third | 4 (100) | 4 (100) | 0 (0) | |
| Histologic grade | 0.222 | |||
| Basaloid/poorly differentiated | 1 (100) | 1 (100) | 0 (0) | |
| Poorly differentiated | 20 (100) | 17 (85) | 3 (15) | |
| Moderately-differentiated | 38 (100) | 27 (71) | 11 (29) | |
| Well-differentiated | 1 (100) | 0 (0) | 1 (100) |
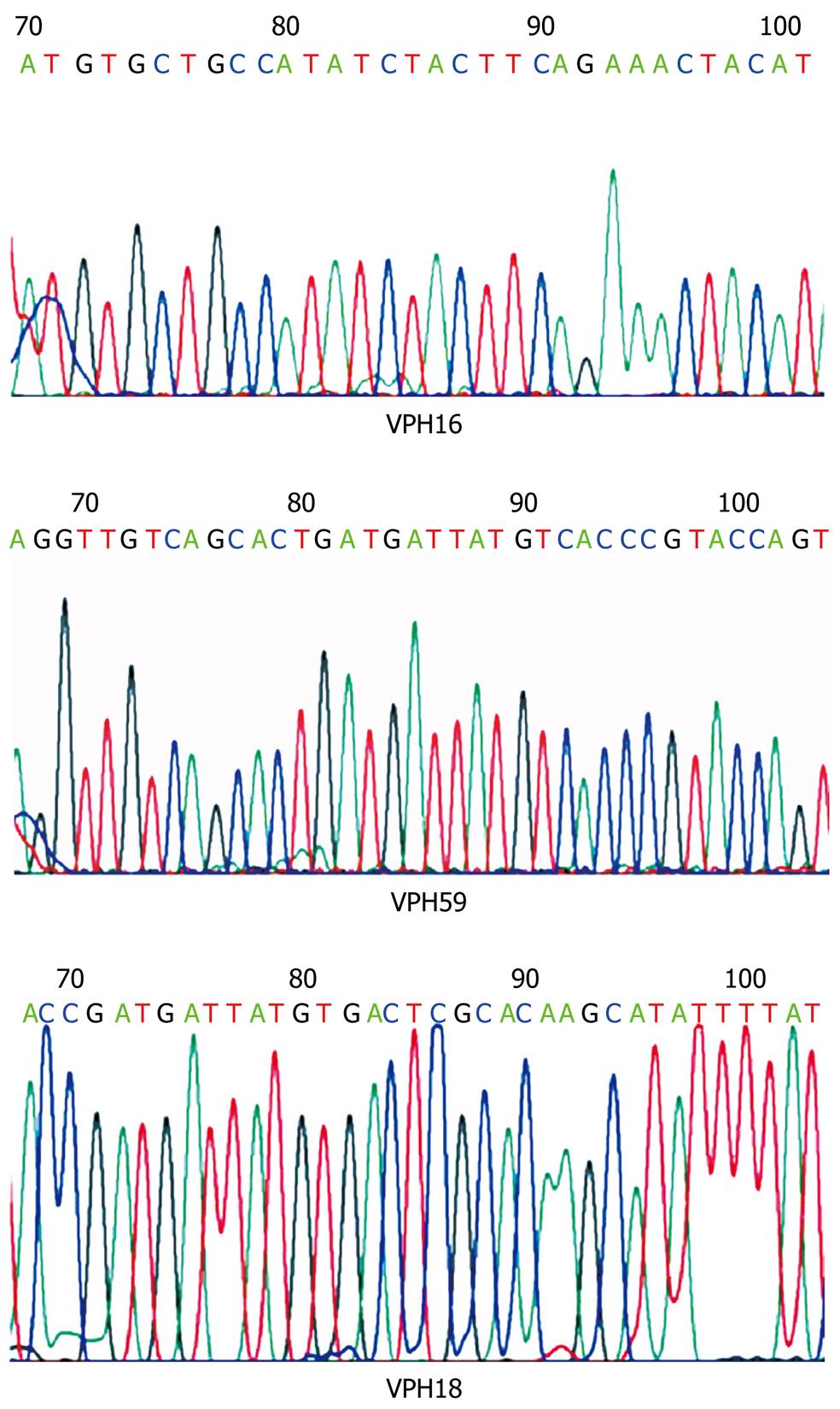
Direct PCR-product sequencing detected 12 cases of high-risk HPV. As summarized in Table 2, HPV-16 was the most frequently detected genotype, followed by HPV-18; interestingly HPV-59 was also detected in one case (Figures 1 and 2). In three of 15 HPV-positive cases (20%), HPV genotype could not be specified although universal primers detected the presence of HPV generic sequences. Low-risk HPV DNA sequences were not detected and we found no HPV-16/18 co-infections among the cases under study.
| HPV genotype | n (%) |
| HPV-16 | 6 (40) |
| HPV-18 | 5 (33) |
| HPV-59 | 1 (7) |
| Unknown | 3 (20) |
| Total | 15 (100) |
PCR analysis in this study demonstrated the presence of high-risk HPV in 25% of ESCCs from a small set of Mexican patients; HPV-16 was the most frequent viral genotype followed by HPV-18. Interestingly, HPV-59 was also detected in one case; to our knowledge, this HPV genotype has not been previously reported in other ESCC studies. No low-risk HPV genotype was detected. In addition, HPV-16/18 co-infection was not found.
High-risk HPV-genotypes distribution in Mexican ESCCs observed in the present study is in agreement with that reported in the studies of cervical carcinomas in Mexico, in which HPV-16 is the most frequent genotype, followed by HPV-18, then by other high-risk HPV genotypes[16]. A similar high-risk HPV distribution was observed in a South American study of ESCC in Colombia and Chile[9], as well as a small Mexican series of lung carcinoma[17]. HPV-16 is not only the most prevalent high-risk genotype in cervical carcinomas[18] and in young females with normal Papanicolaou smears[19], but also in HPV-associated ESCC, worldwide[5].
HPV prevalence in Mexican women, as analyzed in cervical smears, has been reported to range 16.7%-23%, depending on the age group[20], and detected in 92% of cervical cancers[21]. A study of HPV prevalence in men showed a 62% HPV global positivity in samples from external genitalia of Mexican men, with 13% oncogenic types[22].
Since the Syrjänen[5] studies in the early 1980s, several studies have been conducted in different countries and in different geographical regions of the same country, to identify HPV DNA in ESCC. Utilizing molecular methods, the majority of these studies have shown the presence of high-risk HPV in a variable proportion of cases[23–27]; others, however, have failed to demonstrate HPV traces in EC, even from highly prevalent regions[2829]. Differences in such figures could be attributed, first, to the sensitivity and specificity of molecular methods employed to detect HPV DNA[5]; it is widely accepted that PCR is the most sensitive method for detecting HPV DNA, and can detect as few as 20 copies or less[30].
According to the concept of “Condemned Mucosa Syndrome” proposed by Pillai and Nair[31], HPV promotes neoplastic transformation in a previously damaged mucosa with the aid of other carcinogenic agents. With regard to alcohol consumption and/or smoking, there were no substantial differences between HPV-positive and HPV-negative ESCCs, in this study. The present study also examined the possible association between the presence of HPV DNA and esophageal disorders involving membrane damage, such as achalasia and GERD. However, patients in the present study had no clinical history of esophageal achalasia. In two cases of ESCC in which there were previous symptoms of GERD and in another case with direct trauma-related esophageal surgery 26 years previously, HPV DNA was not detected.
The majority of our Mexican patients suffering from ESCC, were native to rural and semi-urban areas of Mexico, thus indicating low yearly income and a low level of education. Indeed, low socioeconomic status is characterized by, among others, nutritional deficiencies, poor hygiene habits and lack of health and public services, including drinking water supply, conditions that taken together could also predispose to esophageal carcinogenesis. Low socioeconomic status has also been associated with an increased risk of cervical intraepithelial neoplasia and cervical invasive carcinoma, among HPV-positive Mexican women[32].
Interestingly, the male:female ratio in HPV-positive ESCC increased two-fold in comparison with HPV-negative cases (6.5:1 vs 3.1:1). This ratio is higher than that reported from Colombia and Chile (0.75:1 vs 1.1:1)[9]. However, the gender-ratio between HPV-positive and -negative cases was not statistically significant. This finding does not allow us to draw any conclusion, due in part, to the small number of cases under study. No such comparison has been explored in other ESCC series, but its meaning warrants further research.
The etiological role of HPV in mucosal malignancy development has been supported on the basis of high-risk HPV localization and expression of viral oncoproteins in neoplastic cell nuclei, the characteristic squamous and basaloid morphology of malignant tumors, the occurrence of HPV-associated malignant neoplasms in anatomic sites where HPV is known to cause benign papillomas and where HPV direct exposure is suspected, and the elevation of serum antibodies against E6/E7 in patients harboring HPV-associated invasive cancers, among others[33]. The etiological significance of HPV detected in ESCCs is yet to be established. It is noteworthy that the present study was unable to find the association of basaloid morphology with HPV presence in ESCC. Although HPV-positive ESCC frequency was high, the mere presence of viral DNA does not necessarily imply its etiological involvement. It is necessary to conduct further studies to examine viral DNA integration into host carcinoma cells, localization in malignant cells, and monoclonality of HPV-positive cells.
The HPV transmission route detected in ESCCs is also of interest. HPV infection of the esophageal mucosa is highly suspected to occur in a direct fashion. In a recent case-control study[34], HPV oral infection was strongly associated with a sub-group of oropharyngeal squamous cell carcinomas, in which high-risk sexual behaviors (i.e. oral, vaginal) were recorded, regardless of alcohol and tobacco use. Notwithstanding the association between oral HPV infection and sexual behavior, a Finnish HPV Family Study[35] has shown that persistent high-risk HPV infection in a mother is a major risk factor for oral and genital infections by this virus in her offspring; this susceptibility appears to be modulated by the immune system. Thus, it could be argued that previous high-risk HPV oral infection, may predispose asymptomatic carriers for further ESCC development.
In conclusion, one fourth of ESCCs diagnosed in a Mexican population were found to harbor high-risk HPV DNA. Elevated high-risk HPV prevalence of ESCC in Mexico, where ESCC incidence is relatively low, does not support the hypothesis that HPV-associated ESCC is more common in areas with higher ESCC incidence rates. Further studies are warranted to evaluate the etiological significance of HPV detected in Mexican ESCCs.
Esophageal carcinoma is a dismal disease which exhibits striking geographical variations in incidence and prevalence between countries and between different regions of the same country, due to unknown factors. Western Africa has the lowest incidence rates and China the highest. Among Latin America countries, Mexico and Peru have low mortality rates for esophageal carcinoma, whereas Brazil, Argentina and Chile have the highest. In Mexico, mortality rates in male and female inhabitants are ca 2 and ca 1 per 100 000, respectively.
Esophageal carcinoma is multifactorial in origin; tobacco, alcohol, fungal toxins, nutritional deficiencies, hot food and beverages, as well as infectious agents, are related to esophageal carcinogenesis. Among infectious agents, high risk human papilloma virus (HPV), a major cause of carcinoma of the cervix uteri throughout the world, is strongly implicated in the etiology of esophageal carcinoma. High-risk HPV DNA sequences have been detected in approximately 15.2% of esophageal squamous cell carcinomas (ESCCs) worldwide, HPV-16 and -18 being the most frequent genotypes.
In this study, the authors found the presence of high-risk HPV DNA in 25% of ESCCs. HPV-16 and -18 were the most frequent genotypes, but they also demonstrated the presence of HPV-59 in one of the cases, a genotype not previously reported in ESCCs anywhere. On the other hand, the results do not support the hypothesis that HPV-associated ESCC is more frequent in areas with higher ESCC incidence rates, because ESCC is not a common cancer in Mexico. Interestingly, the male:female ratio in HPV-positive ESCC increased two-fold in comparison with HPV-negative cases (6.5:1 vs 3.1:1).
By knowing the prevalence of high-risk HPV-associated ESCC, this study among others, may contribute to the design of future strategies for the prevention of HPV-related malignancies, through the development of effective vaccines.
HPVs are DNA viruses that infect basal skin and mucosal cells. HPVs are categorized according to their cervical oncogenicity-based risk, with high, probably high, low and undetermined risks.
This study examined HPV infection in ESCCs in Mexican patients. This is an interesting study and the manuscript is written well.
| 1. | Stewart BW, Kleihues P. World cancer report. IARC Press: Lyon 2003; 223-227. |
| 2. | Bosetti C, Malvezzi M, Chatenoud L, Negri E, Levi F, La Vecchia C. Trends in cancer mortality in the Americas, 1970-2000. Ann Oncol. 2005;16:489-511. |
| 3. | Malvezzi M, Bosetti C, Chatenoud L, Rodríguez T, Levi F, Negri E, La Vecchia C. Trends in cancer mortality in Mexico, 1970-1999. Ann Oncol. 2004;15:1712-1718. |
| 4. | Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737-1746. |
| 5. | Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-728. |
| 6. | Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527. |
| 7. | Riley RR, Duensing S, Brake T, Münger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862-4871. |
| 8. | Lam AK. Molecular biology of esophageal squamous cell carcinoma. Crit Rev Oncol Hematol. 2000;33:71-90. |
| 9. | Castillo A, Aguayo F, Koriyama C, Torres M, Carrascal E, Corvalan A, Roblero JP, Naquira C, Palma M, Backhouse C. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World J Gastroenterol. 2006;12:6188-6192. |
| 10. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press: New York 1989; 9.16. |
| 11. | Resnick RM, Cornelissen MT, Wright DK, Eichinger GH, Fox HS, ter Schegget J, Manos MM. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82:1477-1484. |
| 12. | Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990;71:173-181. |
| 13. | van den Brule AJ, Meijer CJ, Bakels V, Kenemans P, Walboomers JM. Rapid detection of human papillomavirus in cervical scrapes by combined general primer-mediated and type-specific polymerase chain reaction. J Clin Microbiol. 1990;28:2739-2743. |
| 14. | Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res. 1991;82:524-531. |
| 15. | Lizano M, De la Cruz-Hernández E, Carrillo-García A, García-Carrancá A, Ponce de Leon-Rosales S, Dueñas-González A, Hernández-Hernández DM, Mohar A. Distribution of HPV16 and 18 intratypic variants in normal cytology, intraepithelial lesions, and cervical cancer in a Mexican population. Gynecol Oncol. 2006;102:230-235. |
| 16. | Berumen-Campos J. [Human papilloma virus and cervical cancer]. Gac Med Mex. 2006;142 Suppl 2:51-59. |
| 17. | Castillo A, Aguayo F, Koriyama C, Shuyama K, Akiba S, Herrera-Goepfert R, Carrascal E, Klinge G, Sánchez J, Eizuru Y. Human papillomavirus in lung carcinomas among three Latin American countries. Oncol Rep. 2006;15:883-888. |
| 18. | Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621-632. |
| 19. | Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991-998. |
| 20. | Lazcano-Ponce E, Herrero R, Muñoz N, Cruz A, Shah KV, Alonso P, Hernández P, Salmerón J, Hernández M. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412-420. |
| 21. | Piña-Sánchez P, Hernández-Hernández DM, López-Romero R, Vázquez-Ortíz G, Pérez-Plasencia C, Lizano-Soberón M, González-Sánchez JL, Cruz-Talonia F, Salcedo M. Human papillomavirus-specific viral types are common in Mexican women affected by cervical lesions. Int J Gynecol Cancer. 2006;16:1041-1047. |
| 22. | Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, Papenfuss MR, Abrahamsen M, Jolles E, Nielson CM. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036-2043. |
| 23. | Yao PF, Li GC, Li J, Xia HS, Yang XL, Huang HY, Fu YG, Wang RQ, Wang XY, Sha JW. Evidence of human papilloma virus infection and its epidemiology in esophageal squamous cell carcinoma. World J Gastroenterol. 2006;12:1352-1355. |
| 24. | Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929-934. |
| 25. | Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200-1203. |
| 26. | Matsha T, Erasmus R, Kafuko AB, Mugwanya D, Stepien A, Parker MI. Human papillomavirus associated with oesophageal cancer. J Clin Pathol. 2002;55:587-590. |
| 27. | Shuyama K, Castillo A, Aguayo F, Sun Q, Khan N, Koriyama C, Akiba S. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. Br J Cancer. 2007;96:1554-1559. |
| 28. | Gao GF, Roth MJ, Wei WQ, Abnet CC, Chen F, Lu N, Zhao FH, Li XQ, Wang GQ, Taylor PR. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. Int J Cancer. 2006;119:1354-1359. |
| 29. | Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, Abnet CC, Zhao P, Taylor PR, Mark SD. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119:579-584. |
| 30. | Shibata DK, Arnheim N, Martin WJ. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988;167:225-230. |
| 31. | Pillai MR, Nair MK. Development of a condemned mucosa syndrome and pathogenesis of human papillomavirus-associated upper aerodigestive tract and uterine cervical tumors. Exp Mol Pathol. 2000;69:233-241. |
| 32. | Flores YN, Bishai DM, Shah KV, Lazcano-Ponce E, Lörincz A, Hernández M, Ferris D, Salmerón J. Risk factors for cervical cancer among HPV positive women in Mexico. Salud Publica Mex. 2008;50:49-58. |
| 33. | Gillison ML, Shah KV. Chapter 9: Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003;50:57-65. |
| 34. | D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956. |
| 35. | Rintala MA, Grénman SE, Puranen MH, Isolauri E, Ekblad U, Kero PO, Syrjänen SM. Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin Microbiol. 2005;43:376-381. |