Published online Jun 28, 2009. doi: 10.3748/wjg.15.2987
Revised: April 11, 2009
Accepted: April 18, 2009
Published online: June 28, 2009
AIM: To test whether oral L-81 treatment could improve the condition of mice with diabetes and to investigate how L-81 regulates microsomal triglyceride transfer protein (MTP) activity in the liver.
METHODS: Genetically diabetic (db/db) mice were fed on chow supplemented with or without L-81 for 4 wk. The body weight, plasma glucose level, plasma lipid profile, and adipocyte volume of the db/db mice were assessed after treatment. Toxicity of L-81 was also evaluated. To understand the molecular mechanism, HepG2 cells were treated with L-81 and the effects on apolipoprotein B (apoB) secretion and mRNA level of the MTP gene were assessed.
RESULTS: Treatment of db/db mice with L-81 significantly reduced and nearly normalized their body weight, hyperphagia and polydipsia. L-81 also markedly decreased the fasting plasma glucose level, improved glucose tolerance, and attenuated the elevated levels of plasma cholesterol and triglyceride. At the effective dosage, little toxicity was observed. Treatment of HepG2 cells with L-81 not only inhibited apoB secretion, but also significantly decreased the mRNA level of the MTP gene. Similar to the action of insulin, L-81 exerted its effect on the MTP promoter.
CONCLUSION: L-81 represents a promising candidate in the development of a selective insulin-mimetic molecule and an anti-diabetic agent.
-
Citation: Au WS, Lu LW, Tam S, Ko OKH, Chow BK, He ML, Ng SS, Yeung CM, Liu CC, Kung HF, Lin MC. Pluronic L-81 ameliorates diabetic symptoms in
db/db mice through transcriptional regulation of microsomal triglyceride transfer protein. World J Gastroenterol 2009; 15(24): 2987-2994 - URL: https://www.wjgnet.com/1007-9327/full/v15/i24/2987.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2987
Pluronic® surfactants or poloxamers are synthetic copolymers based on ethylene oxide and propylene oxide. They are synthesized by controlled addition of propylene oxide to the two hydroxyl groups of propylene glycol[1]. Pluronic surfactants are widely used in industries as defoaming and antifoaming agents in dishwashing, antifreeze, cutting and grinding fluids, water treatment, etc. They are also being investigated as drug delivery vectors[2] and cancer therapies[3]. Pluronic L-81 (L-81) contains 10% hydrophilic and 90% hydrophobic polyoxyethylene residues with a molecular weight of 2750.
L-81 has profound effects on the lipid metabolism of the intestine and the liver. Chronic feeding of rats with L-81 greatly reduced the lymphatic lipid transport from their intestine, without affecting the digestion and absorption of lipid into enterocytes. The absorbed lipid was however accumulated in the enterocytes, suggesting that L-81 interferes with lipoprotein assembly and/or exit of lipoproteins from the mucosal cells[4]. L-81 inhibits chylomicron formation but not very low density lipoprotein (VLDL) assembly in the intestine[5]. On the other hand, L-81 effectively inhibits VLDL and low-density lipoprotein (LDL) secretion in hepatocytes[6]. Assembly of VLDL in hepatocytes depends very much on the endoplasmic reticulum-residing protein microsomal triglyceride transfer protein (MTP). There is evidence that L-81 inhibits MTP activity in hepatocytes[7]. Consistent with these molecular effects, rodents fed with L-81 exhibited obvious weight loss, which is reversible upon withdrawal of the compound in the diet[48]. Because the inhibitory effect of L-81 on lipid transport is rapid and readily reversible, it is an attractive drug for controlling obesity.
Obesity, defined as a body mass index exceeding 30 kg/m2, is epidemic in many developed countries. For instance, more than 50% of the adults in the USA are either overweight or obese[9]. Obesity is a strong risk factor for the development of insulin resistance and type 2 diabetes[10]. The risk of developing type 2 diabetes increases in parallel with increasing severity of overweight and obesity[11]. On the other hand, weight reduction is associated with a decreased incidence of type 2 diabetes[12]. Obesity is also strongly associated with cardiovascular diseases and cancers[13]. Clearly, it would be of great clinical benefit if effective prevention and treatments for obesity and associated type 2 diabetes were established.
Although L-81 is a potent anti-obesity drug, its potential in alleviating obesity-induced insulin resistance and type 2 diabetes has not been fully explored. Here we aimed to test whether L-81 could ameliorate diabetic symptoms using a mouse model of type 2 diabetes, db/db mice. db/db mice have a mutant leptin receptor which results in high plasma triglyceride and cholesterol levels. db/db mice develop significant obesity, fasting hyperglycemia and hyperinsulinemia within 6 wk of age[14]. We also investigated the possibility that L-81 affects MTP activity through transcription regulation.
HepG2 cells were obtained from the American Type Culture Collection (ATCC) and maintained in basal medium (MEM supplemented with 1.5 g/L sodium bicarbonate, 2 mmol/L glutamate, 2 mmol/L sodium pyruvate) with 10% FBS. In a typical experiment, cells were seeded into 6-well (35 mm) culture plates, allowed to grow to 70% confluence, and then incubated with 3 mL of either the control medium (basal medium supplemented with 3% BSA) or experimental media (control medium plus test substances) at 37°C for the indicated time. At the end of the experiments, media were collected and analyzed for apolipoprotein B (apoB) and apoA-I by ELISA as described previously[15].
Total RNA was isolated from HepG2 cells by the guanidinium thiocyanate method and the relative levels of the MTP large subunit and β-actin mRNA were determined by the DNA excess solution hybridization assays as described previously[15].
The promoter-luciferase construct (MTP-250) which contains a 336-bp fragment encompassing position -250 to +86 of the human MTP promoter was generated by PCR as described in our earlier study[16] and cloned into promoterless pGL3-Basic vector (Promega, Madison, WI). For transfection, HepG2 cells were grown overnight (70% confluent) in 6-well plates and washed twice with serum-free medium. DNA-lipofectAMINE 2000 complexes, containing 1 &mgr;g MTP promoter-firefly luciferase construct, 0.1 &mgr;g pRL-SV40 renilla luciferase control vector, and 2 &mgr;g lipofectAMINE 2000 (Invitrogen) in 200 &mgr;L serum-free medium in each well, were allowed to form at room temperature for 30 min. The cells were then overlaid with the complex for 6 h at 37°C. After 16 h of recovery in complete culture medium, the cells were washed twice with serum-free medium, and experimental media with or without the indicated concentration of Pluronic L-81 were subsequently added. After 24 h, the cells were washed twice with ice-cool PBS and treated with passive lysis buffer (Pormega, Madison, WI). The lysates were assayed for both luciferase activities using the Dual-luciferase assay kit (Pormega, Madison, WI) according to the manufacturer’s instructions. Luciferase activities were determined by Lumat LB 9507 luminometer (Berthold).
Male and genetically diabetic BKS· Cg-m +/+ Leprdb (db/db) mice and their non-diabetic littermates C57BLKS/J (BKS) (5-6 wk of age; n = 3 mice per group) were obtained from Jackson Laboratories (Bar Harbor, ME). They were housed in environmentally controlled conditions with a 12-h light/dark cycle. Mice were fed a standard rodent chow diet (powdered) and water ad libitum in sterile cages. Animals were gathered together for 2 wk before the commencement of the experiment. Pluronic L-81 was kindly provided by BASF Corporation (Parsippany, NJ). To prepare food with the indicated amount of L-81 for treatment, L-81 was first dissolved in ethanol and sprayed on the powdered chow. The food was then air-dried to remove the carrier ethanol before it was used to feed the mice. Various parameters of the mice including body weight, food and water intake were monitored on a regular basis as indicated.
Animals (n = 3 mice per group) were fasted for 5 h before blood was sampled from the retro-orbital sinus. Plasma glucose, insulin, adiponectin, triglycerides, cholesterol, alkaline phosphatase (ALP), alanine aminotransferase (ALT) levels were measured using standard enzyme assay kits. For IPGTT, mice were first fasted for 5 h and then received an intraperitoneal administration of glucose (2 g/kg). Blood glucose levels were determined using the One-touch Ultra Blood Glucose Monitoring System (LifeScan Inc) from the tail blood samples at 0 (before glucose administration), 30, 60, 90, and 120 min after glucose administration. Tissues were collected and fixed in 10% phosphate-buffered formalin for histological analysis. Paraffin-embedded tissues were sectioned (5 &mgr;m thick) and stained with hematoxylin and eosin (HE) using standard procedures.
For each animal experiment, C57 lean mice and db/db mice (n = 3 mice per group) were either untreated (control) or treated with different concentrations of L-81 or 0.005% rosiglitazone (rosig). The experiment was performed 3 times to obtain data with statistical significance. Data shown were obtained from at least 3 independent experiments and presented as mean ± SD. One-way analysis of variance (ANOVA) was used to compare multiple experimental groups. Differences were considered to be statistically significant when P < 0.05.
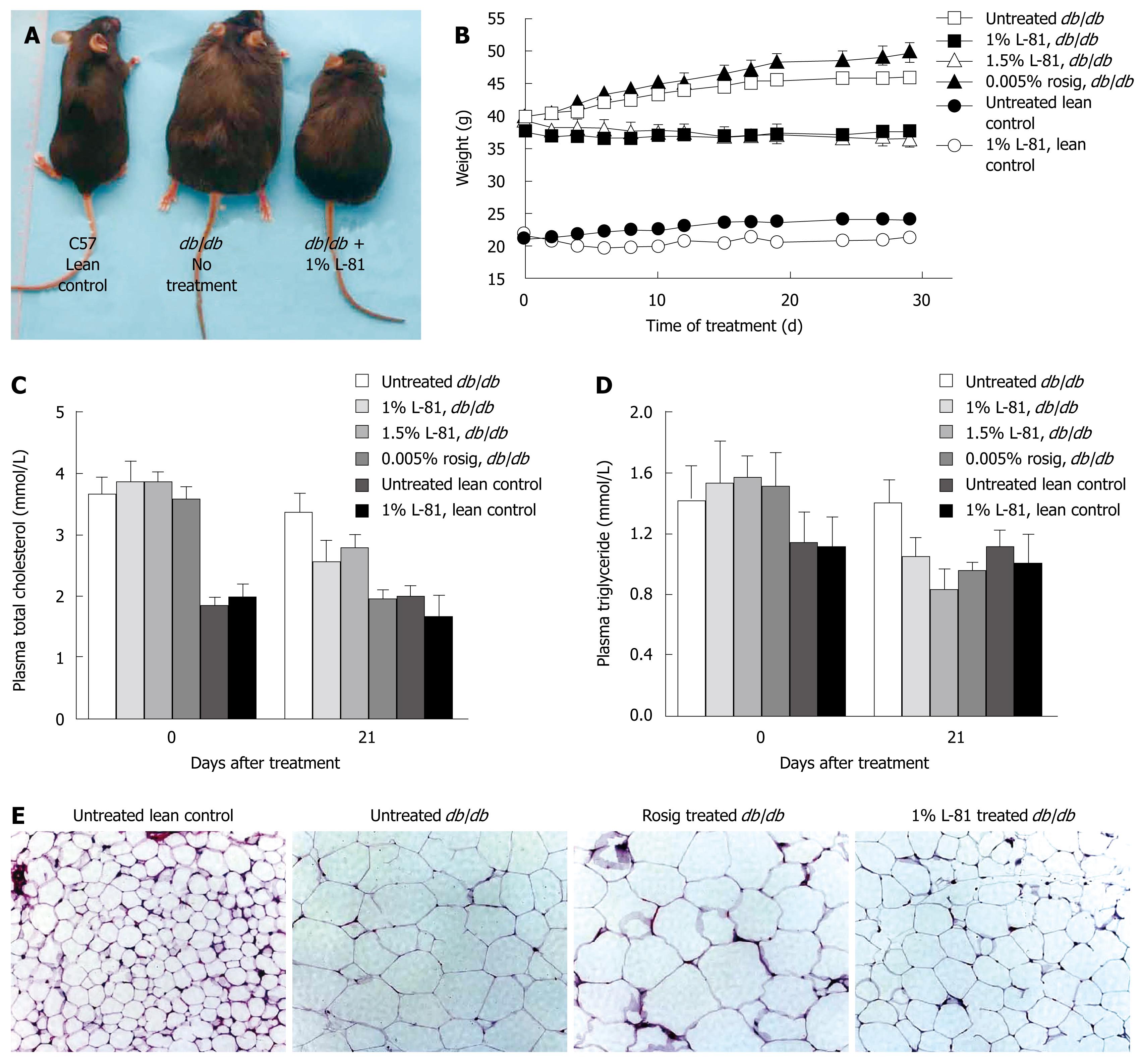
The nonionic surfactant L-81 has been shown to be an effective weight-reducing drug in rats[48]. We observed a similar effect in db/db mice. Thus, while db/db mice weighed 40 g on average at 5-6 wk of age, and their weight continued to increase during observation, mice treated with 1% or 1.5% L-81 for 30 d lost weight to a small extent (n = 3 mice per group) (Figure 1A and B). Their plasma total cholesterol and plasma triglycerol (TG) were also reduced by L-81 treatment (Figure 1C and D). Examination of their epididymal fat pads revealed that adipocytes in mice treated with L-81 were smaller than in the db/db controls, although they were still larger than those in C57 lean control mice (Figure 1E). These data showed that L-81 is, as already found in rats, a hypolipidemic agent in db/db mice.
While the weight control effect of L-81 is well documented, its implication in diabetes control is less well understood. So we asked, given the fact that L-81 is effective in control of obesity, is it able to ameliorate diabetes? To answer this we measured some diabetic parameters in L-81 treated db/db mice. In this study, we compared L-81 with rosig, a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist and an anti-diabetes drug[17].
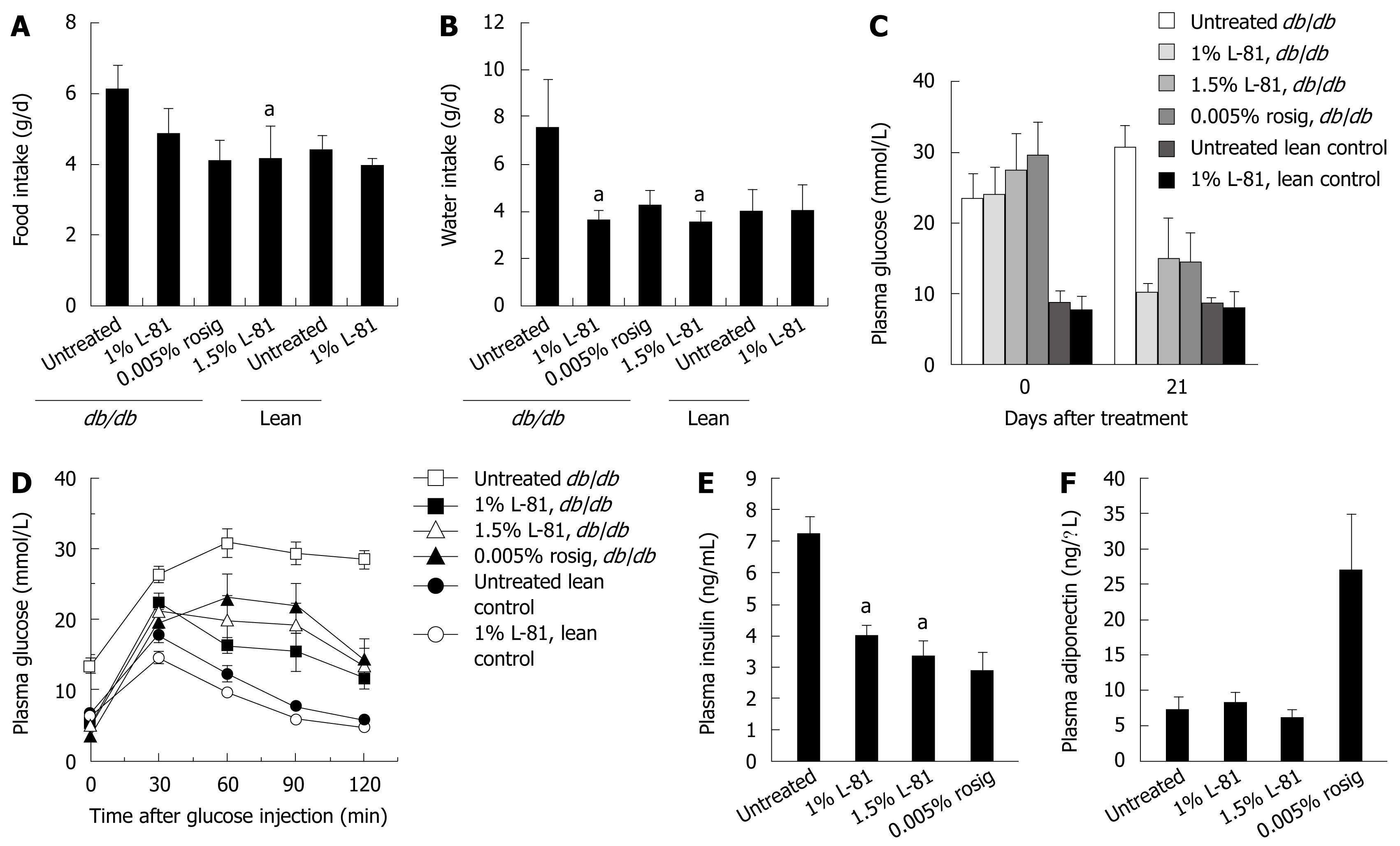
Hyperphagia and polydipsia are two common symptoms of diabetes. We first observed whether a 4-wk L-81 treatment could affect the feeding and drinking behaviors of the mice. Compared with lean controls, db/db mice ingested about 2 g more food and 4 g more water per day. Inclusion of 1% or 1.5% L-81, or 0.005% of rosig in their diet could reduce their food and water intake to the lean control level (Figure 2A and B).
Fasting plasma glucose levels of the mice were measured at the beginning and the end of a 21 d treatment period. As shown in Figure 2C, db/db mice exhibited elevated fasting blood glucose levels of about 25 mmol/L, as compared with 10 mmol/L in lean controls. Twenty one days of rosig or L-81 treatment reduced this parameter to near the lean control level (Figure 2C). db/db mice have a typical diabetic blood glucose profile after glucose injection (Figure 2D). Rosig or L-81-treated mice showed improved glucose tolerance, thus their blood glucose level started to drop slightly or stopped increasing at 30 min after injection (Figure 2D). However, the levels were never as low as in lean control mice.
db/db mice are insulin-resistant and therefore have an elevated plasma insulin level[14]. We found that db/db mice treated for 21 d with rosig or L-81 exhibited a lowered plasma insulin concentration (Figure 2E). The effects of rosig and L-81 were comparable. Rosig effectively increased the plasma level of adiponectin, an adipocytokine responsible for increasing tissue sensitivity to insulin. This, however, was not observed in L-81 treated mice (Figure 2F). Taken together, oral L-81 treatment can effectively improve diabetic symptoms in the db/db mouse model.
L-81 treated cells show decreased activity of MTP. However MTP purified from those cells exhibited normal TG transfer activity[7], suggesting that L-81 does not affect MTP activity post-translationally. We have shown previously that MTP activity is largely controlled at the transcription level[15]. We therefore wished to address the possibility that L-81 controls MTP activity by modulating the transcription of the MTP gene. To do this we transiently transfected a MTP promoter-luciferase reporter construct as previously described[16] into HepG2 and treated the cells with L-81.
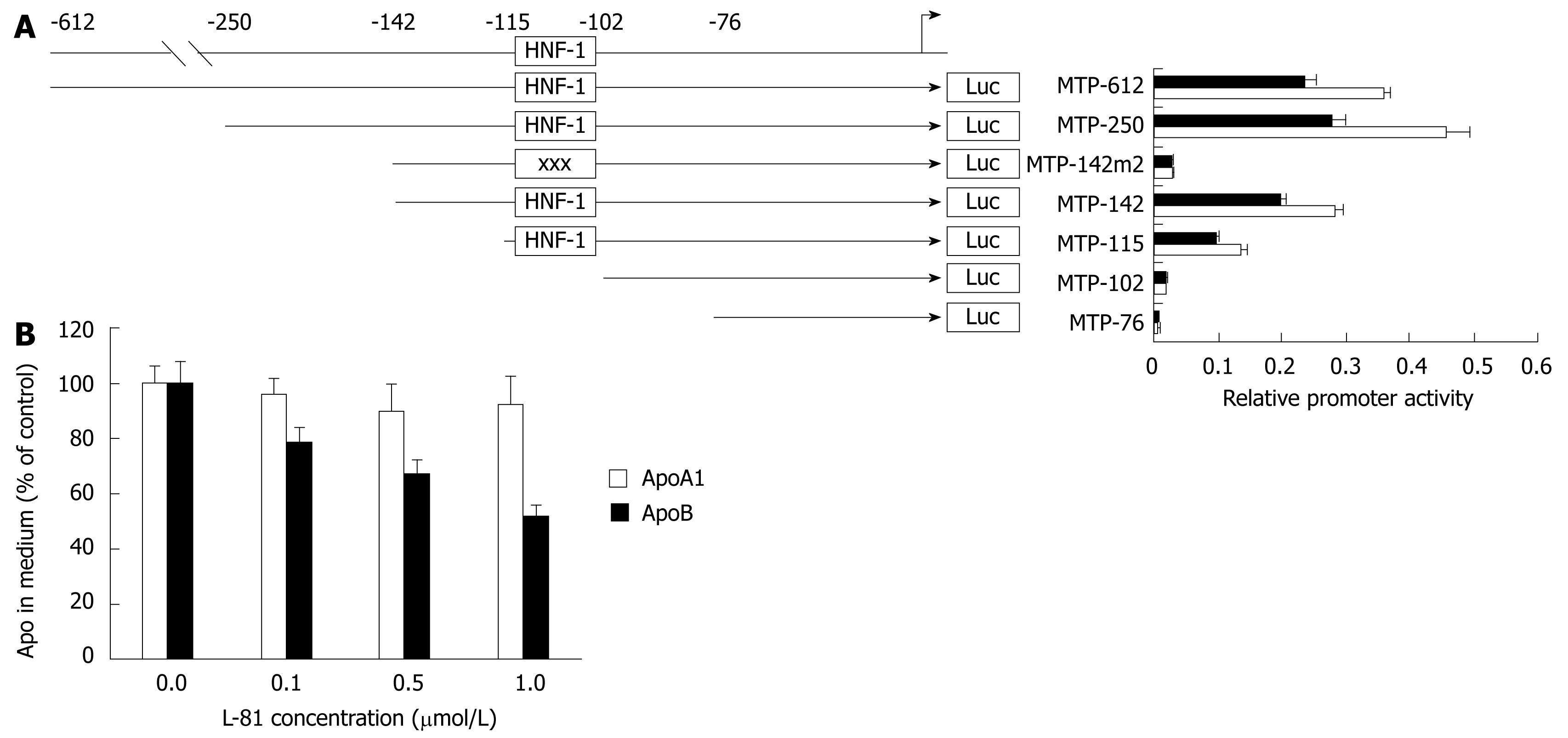
As shown in Figure 3A, the expression of luciferase driven by the full-length MTP promoter (-612 to +86) was reduced by about 25% in the presence of 1% L-81. This figure agrees with that observed previously[7]. Given that MTP plays an essential role in the assembly and secretion of apoB-containing lipoproteins, we tested the effect of L-81 on apoB secretion in HepG2 cells. As shown in Figure 3B, L-81 dose-dependently inhibited the apoB secretion in HepG2 cells, while no significant changes were observed in apoA-I secretion. By examining the cell morphology, lactate dehydrogenase activity (a marker of necrosis), and total cellular proteins in HepG2 cells, we also determined that in vitro L-81 treatment (0-3 &mgr;mol/L) had little or no cytotoxic effect on the cells (data not shown). Taken together, these results demonstrated that L-81 causes a reduction of apoB secretion in HepG2 cells at least partly via the inhibition of MTP expression.
To locate the sequence element responsible for mediating the inhibitory effect of L-81 on MTP promoter, we transfected HepG2 cells with various MTP promoter/luciferase reporter deletion constructs (see Figure 3A), and their relative promoter activities were determined from the cells treated with 1% L-81. It was found that the inhibitory effect of L-81 (and insulin) persists up to the deletion construct MTP-115 (Figure 3A), suggesting that L-81 (and insulin) responsive sequence resides between -102 and -115. A mutation of promoter sequence -102 to -108 [where a hepatocyte nuclear factor (HNF)-1 site is located] abolished the inhibitory effect, suggesting that this region is responsible for mediating the inhibition. Either deletion or mutation of the HNF-1 site caused an almost complete shutdown of the MTP promoter activity (Figure 3A), indicating that this site is involved not only in the regulation but also in the basal expression of the MTP gene.
Pharmacological inhibition of MTP transcription has been explored as a means of treating dyslipidemia. It is believed that by interfering with hepatic and intestinal VLDL packaging, MTP transcription inhibitors would reduce the plasma level of VLDL. Many MTP transcription inhibitors have been developed and put into clinical trials. However, from the clinical trial data, it is obvious that MTP transcription inhibitors very often cause hepatosteatosis (fatty liver)[18]. We were hence concerned whether L-81’s MTP transcription inhibitory effect would lead to hepatosteatosis and a decline of liver function.
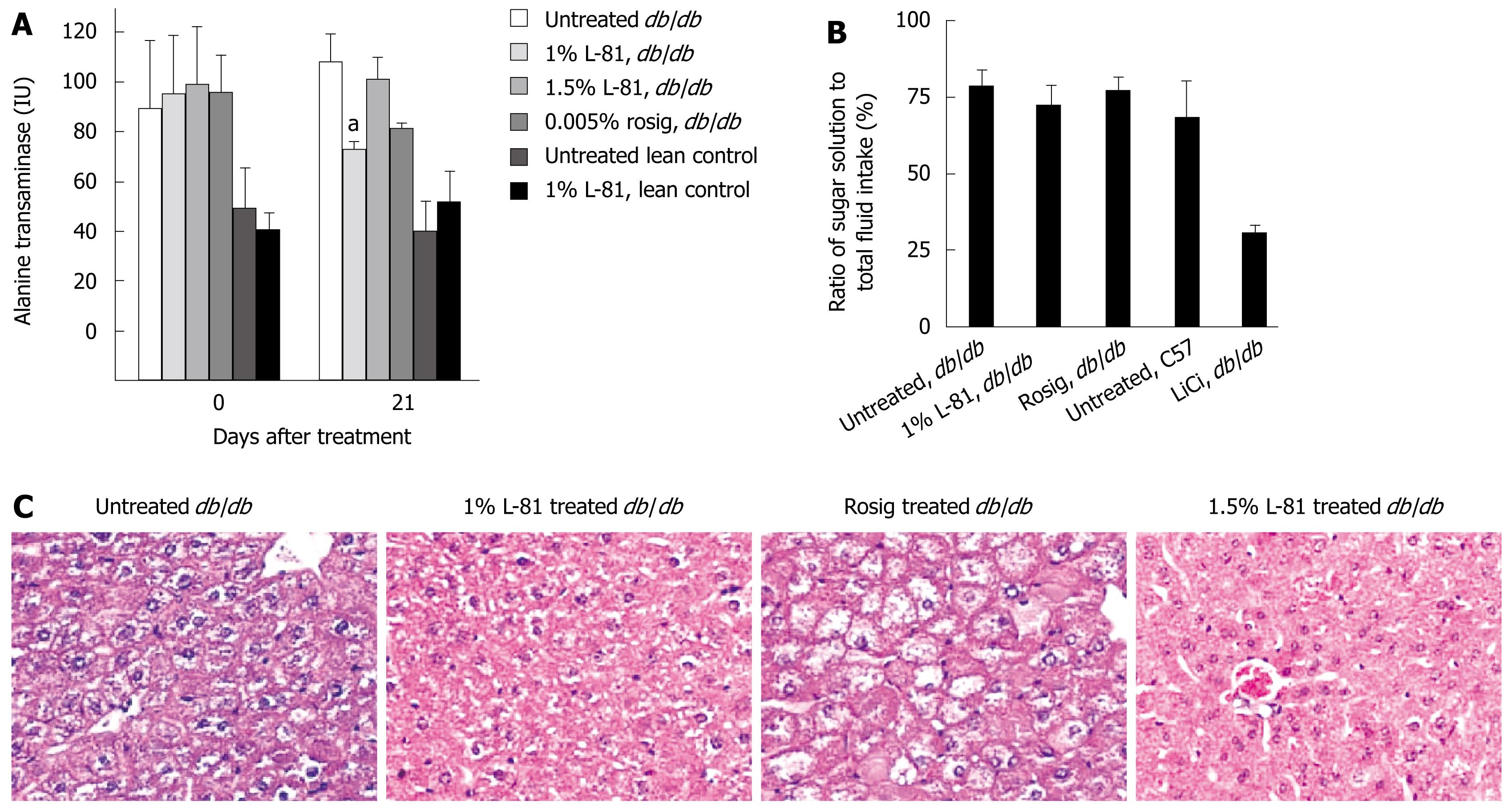
To check whether L-81 treatment caused any gross hepatic injury, we measured the plasma ALT level of db/db C57L mice after 21 d of L-81 treatment. In lean control mice, the presence of L-81 in the diet did not cause any significant difference in the plasma ALT level, indicating that L-81 did not induce liver injury (Figure 4A). Interestingly, we found that while db/db mice generally have a higher level of plasma ALT than C57L lean control mice, L-81 treatment did not result in any significant difference (Figure 4A). Histological examination of the livers of the mice revealed that L-81 treatment caused neither an enlargement of the liver nor any microscopic damage to the liver (Figure 4C).
Conditioned taste aversion (CTA) is a commonly used test to assess whether a particular substance or treatment would render animals ill. L-81 treated mice consumed 72% of their total fluid intake as saccharin in the 2-bottle test, a ratio comparable to that of the untreated db/db mice (Figure 4B). Treatment with rosig gave a similar response (Figure 4B). In contrast, administration with lithium chloride produced a robust CTA (Figure 4B). Taken together, we have not observed any toxicity associated with L-81 treatment.
The prevalence of obesity is now escalating globally and insulin resistance resulting from the increased fat mass has been identified as a key factor that could drive parallel increases in T2DM[19]. The prevalence of T2DM has reached a pandemic situation in which it is estimated to affect > 6% of the world population. Clinically, patients with T2DM display not only an elevated level of blood glucose, but also an elevated level of plasma lipids. These abnormal levels of circulating lipids are the primary cause of severe macrovascular diseases with poor prognosis, which is responsible for 80% of diabetic mortality and 75% of all hospitalizations for diabetic complications[20]. Indeed, diabetes is recognized as a coronary heart disease “risk equivalent”, and the risk of myocardial infarction in diabetic patients is equal to those of non-diabetic patients with known cardiac diseases[21]. Thus, new therapeutic strategies for the treatment of diabetes should not aim only to lower blood glucose level, but also to address diabetes-associated lipid disorders.
Our present study provides evidence for the first time that L-81 is not only a potent lipid-lowering compound but also an anti-diabetic agent in db/db mice. L-81 normalizes the levels of plasma lipids and reduces fat mass, and thereby attenuates obesity which is essentially linked to insulin resistance and a number of serious diabetes-associated macrovascular disorders such as coronary, cerebral and peripheral artery diseases[20]. The ability of L-81 to attenuate obesity may play an important role in mediating its antidiabetic effects in db/db mice. In fact, the relationship between obesity and insulin resistance/T2DM has been linked in a cause and effect association, as weight loss or gain correlates closely with increasing or decreasing insulin sensitivity, respectively[22–24].
Moreover, L-81 significantly improved fasting glycemic control and glucose tolerance in db/db mice. It also reduced hyperphagia and polydipsia, which are symptoms of diabetes mellitus. Remarkably, L-81 caused neither hepatotoxicity nor apparent sickness during the course of treatment. The fact that treatment with L-81 decreased food intake in the db/db mice is likely to be an element of the therapeutic action of L-81. Earlier studies showed that L-81 treatment did not affect food consumption in lean mice[825]. Our data on the lean mice are consistent with this previous observation. Compared with rosig, a PPAR-γ agonist[17] and a well marketed anti-diabetic drug, L-81 exhibited comparable potency in lipid and glycemic control without eliciting overt weight gain which is the side effect of the former[2627].
Consistent with previous studies, we observed that treatment with L-81 led to an accumulation of lipid stored in intestinal villi. Since the turnover rate of the intestinal epithelial lining is rapid, the excess lipids may be excreted together with obsolete cells. It is believed that the intestine is the principal site of action of L-81 when the drug is included in the diet[825]. Functionally, L-81 impairs the transepithelial lipid flux during fat absorption and arrests the trafficking of apoB-containing lipoproteins within the enterocytes, which leads to a cytosolic and endoplasmic reticulum lipid accumulation and thereby prevents the absorption of lipids[42829]. This action appears to be selective and does not affect other lipid metabolism such as fatty acid synthesis, cholesterol re-esterification, and more importantly the absorption of other nutrients[452930]. Taken together, our present results indicate that L-81 exhibits prominent antidiabetic activities in the diabetic db/db mice and suggest that L-81 may represent a new class of anti-diabetic agent conferring both efficacy and safety.
MTP exists in the lumen of the endoplasmic reticulum as a heterodimer with protein-disulfide isomerase and plays a pivotal role in the assembly and secretion of the apoB-containing lipoproteins[31]. Our in vitro studies showed that L-81 treatment induced a significant reduction in apoB secretion of HepG2 cells, at least partly via the inhibition of MTP gene expression. We also found that the expression of MTP transcripts in the liver of L-81-treated mice was significantly decreased, supporting our in vitro data which demonstrated that L-81 could reduce MTP expression. These results indicated that attenuation of the MTP gene expression by L-81 may partially contribute to the improvement of the lipid profiles in diabetic db/db mice. Moreover, we showed that L-81 inhibited MTP promoter activity, in a similar fashion as insulin, in L6 cells with an enforced expression of HNF-1α, suggesting that L-81 mimics insulin action and exerts an inhibitory effect on the MTP gene via the same HNF-1 element.
We have previously shown that the consensus HNF-1 element is functionally equivalent to the slightly modified HNF-1 element found on the MTP promoter in mediating the negative insulin response[32]. This finding implies that the HNF-1-mediated negative insulin response is not only limited to the MTP gene but also to other genes containing the HNF-1 binding motif. In addition to the MTP gene, the transcription of other HNF-1-containing genes may be elevated under diabetic conditions, which could potentially result in abnormal lipid and glucose metabolism and elevated plasma triglyceride, cholesterol and glucose levels. Thus, compounds like L-81 that can mimic the insulin effect and suppress the transcription of MTP and other HNF-1-containing genes should provide relief to these syndromes. Since HNF-1α contributes to the transcriptional regulation of many rate-limiting steps in gluconeogenesis and also binds to genes whose products are central to normal hepatic functions, including carbohydrate synthesis and storage, lipid metabolism, detoxification, and synthesis of serum proteins[33], the potential regulation of these genes by L-81 warrants further investigation in order to gain more insight into the mechanisms of L-81 action.
Obesity is common in many developed countries; more than 50% of the adults in the USA are either overweight or obese. The risk of developing type 2 diabetes and cardiovascular diseases increases in parallel with increasing severity of obesity. Therefore, it is crucial to establish effective prevention and treatments for obesity and its associated type 2 diabetes.
Pluronic L-81 (L-81) effectively inhibits absorption of dietary lipids from the intestine, and secretion of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) from the liver. In this study, the authors demonstrated that oral L-81 treatment can alleviate diabetes symptoms in a mouse model of diabetes (db/db mice) and suppress apolipoprotein B (apoB) secretion and microsomal triglyceride protein (MTP) gene transcription in HepG2 cells.
Although L-81 is a potent anti-obesity drug, its potential in alleviating obesity-induced insulin resistance and type 2 diabetes has not been fully explored. This is the first study to report that L-81 has anti-diabetic effects on db/db mice, and it mimics the action of insulin by reducing the transcription of the MTP gene.
By elucidating the molecular mechanism of the anti-diabetic actions of L-81, this study provides the basis for the development of L-81 as a selective insulin mimetic and anti-diabetic agent in the future.
Pluronic® surfactants are synthetic copolymers based on ethylene oxide and propylene oxide. They have been widely used in industries as defoaming and antifoaming agents in dishwashing, antifreeze, cutting and grinding fluids. L-81 contains 10% hydrophilic and 90% hydrophobic polyoxyethylene residues with a molecular weight of 2750.
The authors investigated the anti-diabetic actions of a nonionic surfactant, L-81, in a mouse model of diabetes. They found that L-81 markedly ameliorated the diabetes symptoms in db/db mice by reducing their body weight and plasma glucose, cholesterol and triglyceride levels. Similar to the action of insulin, L-81 was found to inhibit apoB secretion and MTP gene transcription in HepG2 cells. The results are important and lay down the foundation of developing L-81 as an anti-diabetic drug.
| 1. | Schmolka IR. A review of block polymer surfactants. J Am Oil Chem. 1977;54:110-116. |
| 2. | O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405-415. |
| 3. | Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54:759-779. |
| 4. | Tso P, Balint JA, Rodgers JB. Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am J Physiol. 1980;239:G348-G353. |
| 5. | Tso P, Drake DS, Black DD, Sabesin SM. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol. 1984;247:G599-G610. |
| 6. | Manowitz NR, Tso P, Drake DS, Frase S, Sabesin SM. Dietary supplementation with Pluronic L-81 modifies hepatic secretion of very low density lipoproteins in the rat. J Lipid Res. 1986;27:196-207. |
| 7. | Fatma S, Yakubov R, Anwar K, Hussain MM. Pluronic L81 enhances triacylglycerol accumulation in the cytosol and inhibits chylomicron secretion. J Lipid Res. 2006;47:2422-2432. |
| 8. | Brunelle CW, Bochenek WJ, Abraham R, Kim DN, Rodgers JB. Effect of hydrophobic detergent on lipid absorption in the rat and on lipid and sterol balance in the swine. Dig Dis Sci. 1979;24:718-725. |
| 9. | Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529. |
| 11. | Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581-1586. |
| 12. | Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481-486. |
| 13. | Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337-350. |
| 14. | Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22-31. |
| 15. | Lin MC, Gordon D, Wetterau JR. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J Lipid Res. 1995;36:1073-1081. |
| 16. | Au WS, Kung HF, Lin MC. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 2003;52:1073-1080. |
| 17. | Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201-2207. |
| 18. | Burnett JR, Watts GF. MTP inhibition as a treatment for dyslipidaemias: time to deliver or empty promises? Expert Opin Ther Targets. 2007;11:181-189. |
| 19. | Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137-145. |
| 20. | Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821-827. |
| 21. | Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. |
| 22. | Bak JF, Moller N, Schmitz O, Saaek A, Pedersen O. In vivo insulin action and muscle glycogen synthase activity in type 2 (non-insulin-dependent) diabetes mellitus: effects of diet treatment. Diabetologia. 1992;35:777-784. |
| 23. | Cancello R, Tounian A, Poitou Ch, Clement K. Adiposity signals, genetic and body weight regulation in humans. Diabetes Metab. 2004;30:215-227. |
| 24. | Freidenberg GR, Reichart D, Olefsky JM, Henry RR. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988;82:1398-1406. |
| 25. | Rodgers JB, Kyriakides EC, Kapuscinska B, Peng SK, Bochenek WJ. Hydrophobic surfactant treatment prevents atherosclerosis in the rabbit. J Clin Invest. 1983;71:1490-1494. |
| 26. | Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271:445-450. |
| 27. | Mukherjee R, Hoener PA, Jow L, Bilakovics J, Klausing K, Mais DE, Faulkner A, Croston GE, Paterniti JR Jr. A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol. 2000;14:1425-1433. |
| 28. | Pidlich J, Renner F, Ellinger A, Huttinger M, Pavelka M, Gangl A. Effect of pluronic L-81 on intestinal lipoprotein secretion in the rat. Dig Dis Sci. 1996;41:1445-1451. |
| 29. | Tso P, Balint JA, Bishop MB, Rodgers JB. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am J Physiol. 1981;241:G487-G497. |
| 30. | Nutting DF, Tso P. Hypolipidemic effect of intravenous pluronic L-81 in fasted rats treated with Triton WR-1339: possible inhibition of hepatic lipoprotein secretion. Horm Metab Res. 1989;21:113-115. |
| 31. | Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136-150. |
| 32. | Au WS, Lu L, Yeung CM, Liu CC, Wong OG, Lai L, Kung HF, Lin MC. Hepatocyte nuclear factor 1 binding element within the promoter of microsomal triglyceride transfer protein (MTTP) gene is crucial for MTTP basal expression and insulin responsiveness. J Mol Endocrinol. 2008;41:229-238. |