Published online Feb 14, 2008. doi: 10.3748/wjg.14.899
Revised: December 6, 2007
Published online: February 14, 2008
AIM: To investigate the effects of vitamins (A, C and E) on liver injury induced by ethanol administration during liver regeneration in rats.
METHODS: Male Wistar rats subjected to 70% partial hepatectomy were divided into five groups (groups 1-5). During the experiment, animals of Group 1 drank only water. The other four groups (2-5) drank 30 mL of ethanol/L of water. Group 3 additionally received vitamin A, those of group 4 vitamin C and those of group 5 received vitamin E. Subsequently serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin and bilirubin were measured colorimetrically. Lipid peroxidation (thiobarbituric-acid reactive substances, TBARS) both in plasma and liver was measured, as well as liver mass gain assessment and total DNA.
RESULTS: Compared with sham group, serum AST and ALT increased significantly under ethanol treatment (43% and 93%, respectively, with P < 0.05). Vitamin C and vitamin E treatment attenuated the ethanol-induced increases in ALT and AST activity. Ethanol treatment also decreased serum albumin concentration compared to sham group (3.1 ± 0.4 g/dL vs 4.5 ± 0.2 g/dL; P < 0.05). During liver regeneration vitamins C and E significantly ameliorated liver injury for ethanol administration in hepatic lipid peroxidation (4.92 nmol/mg and 4.25 nmol/mg vs 14.78 nmol/mg, respectively, with P < 0.05). In association with hepatic injury, ethanol administration caused a significant increase in both hepatic and plasma lipid peroxidation. Vitamins (C and E) treatment attenuated hepatic and plasma lipid peroxidation.
CONCLUSION: Vitamins C and E protect against liver injury and dysfunction, attenuate lipid peroxidation, and thus appear to be significantly more effective than vitamin A against ethanol-mediated toxic effects during liver regeneration.
- Citation: Ramírez-Farías C, Madrigal-Santillán E, Gutiérrez-Salinas J, Rodríguez-Sánchez N, Martínez-Cruz M, Valle-Jones I, Gramlich-Martínez I, Hernández-Ceruelos A, Morales-González JA. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World J Gastroenterol 2008; 14(6): 899-907
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/899.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.899
Alcohol liver disease is a world-wide medical complication of alcohol abuse[1]. Although important progress has been made in understanding its pathogenesis, the mechanisms involved in its development are as yet not fully understood. Oxidative stress is a key step in the pathogenesis of ethanol-associated liver injury. The deleterious effects of alcohol, at least partly involve oxidative injury that has been documented by measurement of oxidant radicals, alterations in oxidant/antioxidant balance and oxidant induced changes in cellular proteins and lipids[2].
After ethanol is absorbed, it is distributed to all tissue, and body fluids in direct proportion to blood levels[3]. Ethanol affects the liver more than any other organ[4], since its metabolism implies generation of free radicals that can damage cell structure and function, particularly when antioxidant mechanisms are not able to neutralize them[56]. Animal models show the mechanisms by which both acute alcohol administration and chronic alcohol consumption affect the liver. Chronic ethanol administration induces an increase in lipid peroxidation either by enhancing the production of oxygen reactive species, or by decreasing the level of endogenous antioxidants[7]. Short-term ethanol administration has been reported to decrease vitamins and increase superoxide generation in the liver[8]. The enhanced superoxide generation increases lipid peroxidation and induces liver dysfunction in rats that have been subjected to acute ethanol exposure[9].
One of the most prominent antioxidant defense systems are vitamins (A, C and E ) acting as co-factors for many enzymes, protecting cells against free radical-mediated damage[10], as free radical scavengers. Besides, large ethanol intakes have deleterious effects on nutritional status[78].
Recently, it has become clear that antioxidant nutrients, including vitamins, are important for neurological function[11–13]. High intake of vitamins E and C have been found to be associated with lower risk of Alzheimer's disease[14]. In addition, evidence for alcohol-induced oxidant injury comes from studies in which pretreatment with antioxidants such as vitamin E, vitamin C, and agents that enhance antioxidant capacity attenuate alcohol induced effects[15].
Nutrient intermediary metabolism is a vital function of the healthy adult liver. Fortunately, the liver appears to have a tremendous reserve capacity in this regard, since general nutritional status is well-preserved until severe hepatic parenchymal loss occurs. Indeed, several clinical and biochemical markers of nutritional status are often used to gauge the severity of liver dysfunction, with evidence of severe malnutrition implying end-stage liver disease. Hepatocyte proliferation occurs rarely in healthy adults, but it is necessary to replace liver cells lost by hepatic necrosis or resection. These facts have prompted speculation on whether decreased hepatic mass and metabolism are involved in the initiation and progression of hepatic regeneration following liver injury[16]. Restoration of normal hepatic structure and function is the goal of therapy for acute and chronic liver injury of varying etiologies. Consequently, strategies to optimize compensatory hepatic regenerative growth have tremendous therapeutic relevance. Better understanding of the mechanism regulating cellular proliferation, and organ regeneration will play a critical role in designing treatments to enhance liver regeneration[16]. Current knowledge suggests several approaches. Thus, novel agents that correct the fundamental cellular disturbances resulting from excessive ethanol consumption represent attractive therapeutic strategies.
In the present study, we investigated the effects of vitamins A, C and E in rats with liver injury induced by ethanol administration during liver regeneration. Specifically, we planed to evaluate: (a) whether the effect of the vitamin supplementation protects against ethanol-induced lipid peroxidation; (b) whether vitamin supplementation can attenuate ethanol-induced hepatic injury and dysfunction during liver regeneration.
Male Wistar rats (230-270 g, body weight) were obtained from Centro Médico Nacional “20 de Noviembre” ISSSTE-MEXICO. The rats were housed in animal quarters at the Escuela de Dietética y Nutrición ISSSTE-MEXICO. They were maintained at 22°C with a 12-h light/dark cycle and allowed to consume standard rat pellet chow (Purina de México SA) and water ad libitum before treatments. After 10 d of adaptation the procedure was begun. All procedures involving experimental animals were done according to our Federal Regulations for Animal Experimentation and Care (Ministry of Agriculture; SAGAR, Mexico).
Vitamins A, C and E and thiobarbituric acid were from Sigma (Sigma Chemical St. Louis MO). All other chemicals were from the best quality available.
Two-thirds partial hepatectomy (PH) was performed according to the technique of Higgins and Anderson[17]. The operations were carried out between 08:00 and 10:00 hours under light diethyl ether anesthesia and consisted of removal of the median and left lateral lobes of the liver. As controls, sham-operated rats were subjected to the same surgical procedure, without remission of the liver mass.
After surgery, rats were housed individually and fed with rat pellet chow. They were grouped (n = 30 for each experimental group) as follows: (1) control rats (sham operated and PH-only treated rats) who received water ad libitum; (2) PH rats who received a 30 mL/L ethanol solution in their drink container (PH-ethanol group) in a voluntary way, the intake being registered daily. Ethanol consumption was calculated in terms of g/kg per day. Ethanol consumption usually averaged 1.5 ± 0.5 g/kg per day and blood alcohol levels have been reported to vary between 75 mg/dL and 150 mg/dL[18–20]. On the other hand, PH-ethanol treated rats were divided into the following treatment groups: (a) PH-ethanol treated rats with intraperitoneal doses of vitamin A (PH-ethanol-Vitamin A group), 4000 UI per day[21]; (b) PH-ethanol treated rats with intragastric doses of vitamin C (PH-ethanol-Vitamin C group), 250 mg/kg per day[22]; and (c) PH-ethanol treated rats with intraperitoneal doses of vitamin E (PH-ethanol-Vitamin E group), 250 mg/kg per day[22]. All the treatments (ethanol solution and vitamins) were given daily during seven days; the animals' weights were recorded daily.
On the eighth day of treatment, animals were killed by decapitation previously anesthetized with sodium pentobarbital (40 mg/kg of b.w.). Blood samples were obtained and serum isolated. Serum was kept frozen at -70°C until use. The liver was isolated and weighed and it was placed quickly in an ice-cold PBS (phosphate saline buffer solution, pH 7.5) and washed until it was bloodless. The liver was placed in 9 volumes of ice-cold solution Tris-HCL buffer (50 mmol/L Tris-HCl, 180 mmol/L KCL, 10 mmol/L EDTA, and 0.02% butylated hydroxytoluene; pH 7.4) and homogenized using four to five strokes of a Teflon homogenizer spinning at 1000 r/min. Homogenate was divided into samples and frozen at -70°C until its subsequent use. Total protein concentration was determined by the Lowry method[23], using bovine serum albumin (BSA) as a standard.
Serum enzymes, alanine aminotransferase (ALT; EC 2.6.1.2) and aspartate aminotransferase (AST; EC 2.6.1.1) activities as indicators of the extent of liver injury were measured colorimetrically using a diagnostic kit (Sigma Chemical Co., St. Louis, MO) following the instructions provided by the manufacturers.
Serum albumin and total bilirubin concentrations, documenting the extent of liver dysfunction, were determined colorimetrically using a Sigma Diagnostic kit (Sigma Chemical Co., St. Louis, MO) following the instructions provided by the manufacturer.
To determine the thiobarbituric acid reactive substances (TBARS) in liver, homogenate was used according to method of Song[24]. Briefly, to 0.2 mL of the tissue homogenate, 0.2 mL of 8.1% sodium dodecyl sulfate, 1.5 mL of 20% acetic acid, 1.5 mL of 0.9% thiobarbituric acid, and 0.6 mL of distilled water were added and vortexed. The reaction mixture was placed in a water bath at 95°C for 1 h. After cooling on ice, 1.0 mL of distilled water and 5.0 mL of butanol/pyridine mixture (15:1, v/v), were added and vortexed. After centrifugation at 10 000 r/min for 10 min, the resulting upper phase was determined at 532 nm. The concentration of TBARS was calculated using 1, 1, 5, 3-tetraethoxypropane as a standard[24]. In the serum, the determination of TBARS concentration was made as described above with 200 &mgr;L serum.
Hepatic regeneration was determined by the calculation of liver weight restitution and total DNA concentration. The extent to which the liver mass had been reconstituted was also evaluated. Resected livers were weighed at the time of PH. This weight was divided by 0.7 to derive the initial (pre-PH) weight of each liver. Each post-PH liver remnant was also weighed at the time of death. For each rat, the weight of the remnant liver was normalized to the weight of that rat's entire liver at the time of PH and expressed as the percentage of the rat's initial liver weight according to the following formula: (wt of liver remnant/initial liver wt) × 100[25]. Data from all PH rats per treatment group were used to calculate the mean ± SE at the end of the experimental time. DNA concentrations were determined fluorometrically according to Labarca and Paigen[26].
All data are expressed as mean ± SE. Statistical analysis was performed using Student's t-test and ANOVA where appropriate. Differences between groups were considered to be statistically significant at P < 0.05.
The effect of the combination of ethanol and vitamins on the mortality and the body weight of the rats is shown in Table 1. It is observed that the ethanol group (PH-EtOH), presented a significant mortality (73.3%; P < 0.05) in comparison with the sham group and the group the PH-only group. On the contrary, the administration of the vitamins A, E and C in the ethanol group, decreased mortality, increasing significantly (P < 0.05) survival capacity as compared with the PH-EtOH group; 73.4% for the vitamin E group (PH-EtOH-VE), 66.7% for the vitamin C group (PH-EtOH-VC) and 46.7% for the vitamin A group (PH-EtOH-VA). As for the body weight, there were differences observed in the initial weight (after the hepatectomy) and the final weight (seven days after treatment) in the animals of all the experimental groups, which confirms that this parameter is not altered by the surgery or other used substances.
| Group | Mortality (%) | Initial body Weight’ (g) | Final body weight’ (g) | Differential between final and initial weight (g) |
| Sham | 0/30 (0) | 259 ± 10 | 274 ± 1 | 15 |
| PH | 0/30 (0) | 250 ± 11 | 267 ± 10 | 17 |
| PH-EtOH | 22/30 (73.3)a | 255 ± 10 | 276 ± 15 | 21 |
| PH-EtOH-VA | 16/30 (53.3)e | 255 ± 12 | 270 ± 12 | 15 |
| PH-EtOH-VC | 10/30 (33.3)e | 260 ± 12 | 275 ± 10 | 15 |
| PH-EtOH-VE | 8/30 (26.6)e | 242 ± 10 | 258 ± 11 | 16 |
Table 2 shows the results of the indicators (restitution of the liver mass, liver/body ratio and mgDNA/g liver ratio) associated with the process of hepatic regeneration. The PH-EtOH group had a significant decrease in the hepatic indicators compared to the PH-only group. Except for the PH-EtOH group, all the experimental groups treated with ethanol in combination with the vitamins showed a recovery in the hepatic regeneration process; but the group that obtained the major protection was the one treated with vitamin E (PH-EtOH-VE) and vitamin C (PH-EtOH-VC), obtaining values comparable to the PH-only group.
| Group | Restitution liver mass’ (%) | Resected liver mass’ (g) | Final liver weight’ (g) | Liver/body ratio’ (%) | mgDNA/g liver1 |
| Sham | None | None | 9.41 ± 0.3 | 3.41 ± 0.2 | 2.1 ± 0.2 |
| PH | 83.95 ± 3.0 | 6.9 ± 0.3 | 8.27 ± 0.3 | 3.09 ± 0.3a | 4.1 ± 0.4a |
| PH-EtOH | 54.89 ± 2.0c | 6.8 ± 0.4 | 5.33 ± 0.2c | 1.93 ± 0.1ac | 2.9 ± 0.1ac |
| PH-EtOH VA | 62.90 ± 3.9ce | 7.1 ± 0.3 | 6.38 ± 0.4c | 2.36 ± 0.3ace | 3.4 ± 0.2ace |
| PH-EtOH-VC | 79.00 ± 2.0c | 7.0 ± 0.5 | 7.90 ± 0.2e | 2.87 ± 0.2ae | 4.0 ± 0.3ae |
| PH-EtOH-VE | 81.00 ± 3.0c | 6.8 ± 0.2 | 7.86 ± 0.3e | 3.03 ± 0.3ae | 3.9 ± 0.4ae |
The difference with the PH-only group with the vitamin E group (PH-ETOH-VE) and the vitamin C group (PH-EtOH-VC) was 2.95% and 4.95% for the restitution of the liver mass, 0.06% and 0.22% for the liver/body ratio, and 0.2 and 0.1 of mgDNA/g of liver, respectively. On the other hand, the group treated with ethanol and vitamin A (PH-EtOH-VA) only had a recuperation of 62.9% for the restitution of the liver mass, being significantly different from the PH-only group (83.9%, P < 0.05), and 2.36% (vs 3.09%, P < 0.05) and 3.4% (vs 4.1%, P < 0.05) for the other parameters, liver/body ratio and mgDNA/g of liver, respectively.
The results of the liver metabolic integrity are presented in Table 3. There are similar concentrations of serum (bilirubin and albumin) both in animals with hepatectomy as well as the sham group animals. When ethanol was administered as in the (PH-EtOH) group, there was a significant decrease in serum albumin (31 g/L vs 47 g/L, P < 0.05) and an increase in bilirubin (17.2 mg/L vs 4.0 mg/L, P < 0.05) compared to the PH-only group. This serum alteration is normalized when ethanol and one of the vitamins is administered in combination, giving a better recuperation of both metabolites with vitamin C or E, since albumin increases its concentration to 42 g/L in the PH-EtOH-VC group and 46 g/L in the PH-EtOH-VE group, while in bilirubin their values are reduced to 4.5 mg/L and 2.9 mg/L respectively. The group treated with vitamin A did not reach values comparable to the PH-only group because its behavior was similar to the PH-EtOH group.
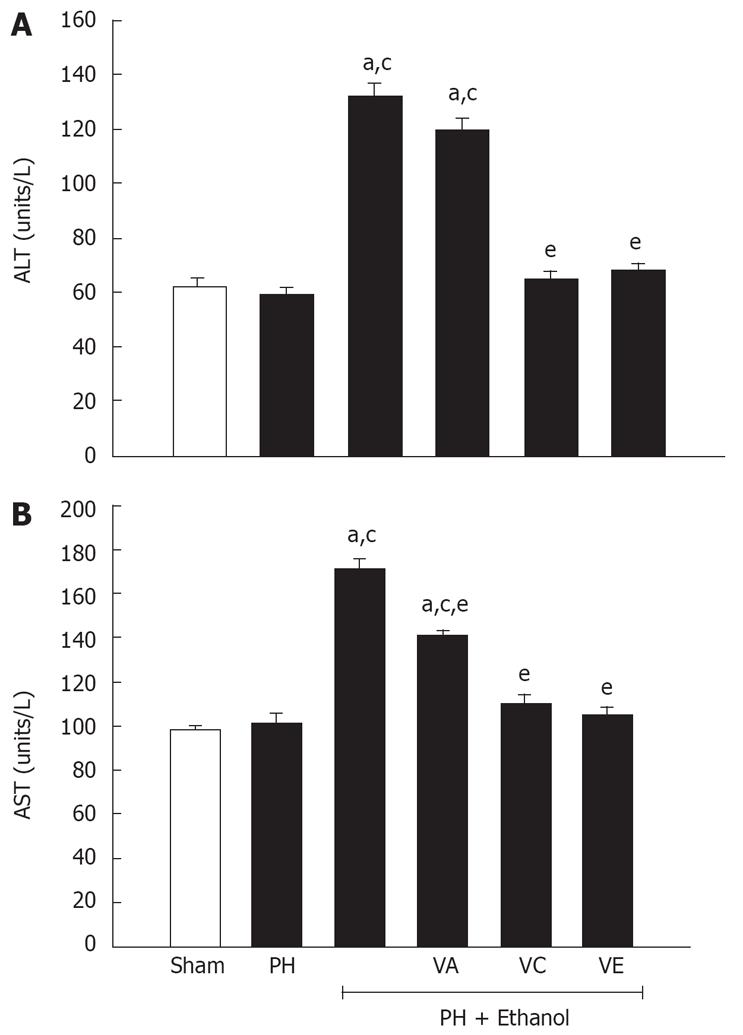
The protective effect of the vitamins was evaluated with the enzymatic activity of the alanine aminotransferase (ALT) and the aspartate aminotransferase (AST) because they reflect the liver function. As is shown in Figure 1, the ethanol increased significantly the serum concentration of both enzymes, observing an activity of 2-times for ALT (A) and 1.7-time for AST (B) compared to PH-only and sham groups. A similar effect is observed in the animals treated with ethanol plus the vitamin A, giving evidence of the low potential of this vitamin to promote hepatic regeneration. On the contrary, the partial hepatectomized rats treated with combinations of ethanol and vitamins C or E showed a significant decrease in the serum concentration of ALT and AST levels, reaching values comparable to the control group (sham group).
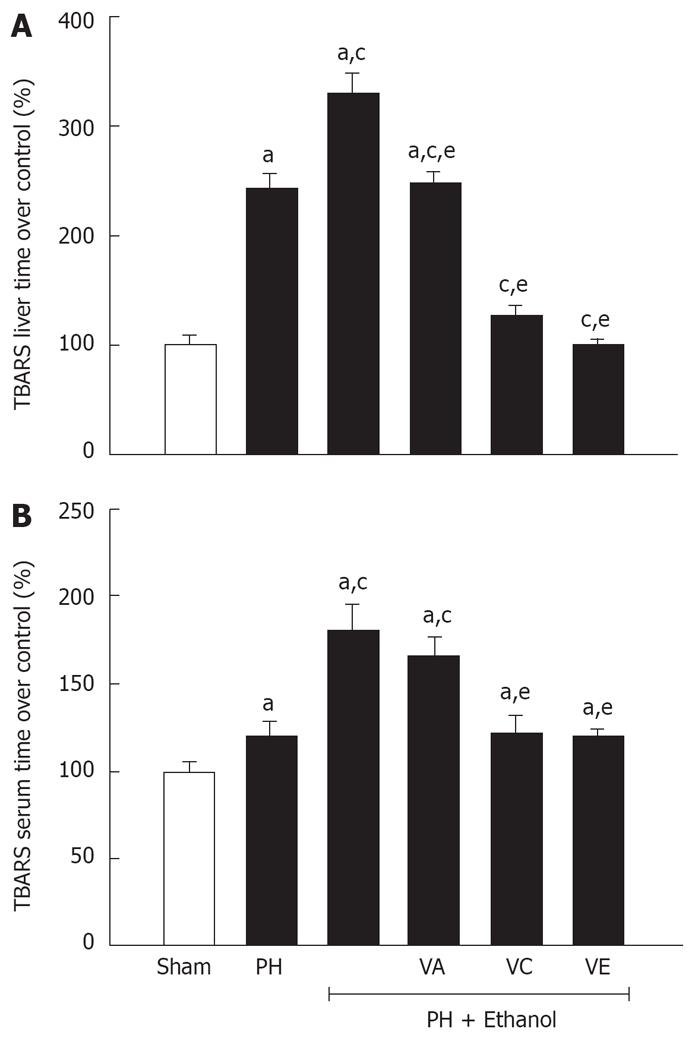
To evaluate the damage produced by the reactive oxygen species (ROS), the TBARS concentration was determined in liver and serum in the animals treated with ethanol and the diverse vitamins (Figure 2). An increase of almost double is observed in the hepatic concentrations of TBARS (panel A) in the rats with PH in comparison with the sham group. This increase becomes more evident, when the animals are treated for seven days with ethanol, with 14.78 nmol/mg, which is 3.3 times and 1.4 times greater than the sham group (4.48 nmol/mg) and the PH-only group (10.55 nmol/mg), respectively.
Contrary, the three groups with ethanol and a vitamin showed a decrease in the hepatic concentration of TBARS, although the most relevant protection was observed in the PH-EtOH-VC (4.92 nmol/mg) and PH-EtOH-VE group (4.25 nmol/mg), since these values were similar to the sham group (4.48 nmol/mg). It is important to mention, that the PH-EtOH-VA group (10.74 nmol/mg) only presented a reduction comparable to the PH-only group (10.55 nmol/mg).
In the case of the corresponding concentrations in serum (Figure 2, panel B), the rats of the PH-EtOH group presented a significant increase of 80% in relation to the sham group. On the contrary, when vitamin C or E was administrated to the rats intoxicated with ethanol, there were no differences observed in the serum concentrations of TBARS in comparison with the rats of the PH-only group. Contrary, when vitamin A was administered, the serum TBARS group was similar to the PH-EtOH group, observing an increment of 20% and 22% with respect to the PH-EtOH-VC and the PH-EtOH-VE groups respectively.
Epidemiologic studies agree about the existing correlation between the ingestion of alcohol and the high incidence of hepatic cancer, but the exact mechanism of the hepatocarcinogenesis is still a ground research because ethanol by itself is not a carcinogenic compound[27–303132]. Besides several mechanisms involved in the development of a hepatocarcinogenic process which have been identified, the formation of acetaldehyde through the oxidation of ethanol by the action of the cytochrome P-4502E1 (CYP2E1) continues to be the most accepted one because of the production of reactive oxygen species (ROS) during the biotransformation, which consequently can alter cellular regeneration[33].
It has been observed that the chronic consumption of alcohol increases the activity of CYP2E1. This enzyme has been related to the lipoperoxidation process and the excessive generation of hydroxyl ethyl radicals, resulting in a decrease in endogenic antioxidants' levels[34]. In other words, the oxidative stress produced by ethanol is critical in post alcoholic hepatogenesis due to the production of free radicals which damage the cellular structure and function, especially if natural antioxidant mechanisms cannot neutralize them[2833].
These observations support the idea that pre-treatment with substances that reduce the activity of CYP2E1 or that inactivate the ROS, could reduce the hepatotoxic effects of ethanol. Following this hypothesis, the objective of this study was to evaluate the antioxidant potential of three vitamins A, C, and E against the hepatic damage produced by the sub chronic administration of ethanol during the cellular regeneration after a partial hepatectomy (PH).
Initially, it was observed that ethanol (1.5 g/kg) increased mortality, which supports the toxicity of ethanol when it is given in a subchronic way; confirming its capacity to produce different alterations in the organism, like nuclear condensation, cellular edema with abundant accumulation of fat, and an abnormal mitochondrial activity[35].
On the other hand, when ethanol was given in combination with the different vitamins, the survival percentage increased (Table 1), supporting the evidence of the importance of these compounds in the adequate development of an organism and backs up the studies carried out by different researchers, showing the capacity of vitamins to interact with free radicals decreasing the toxicity to the organism[1015]. Normally, the excessive and constant ingestion of ethanol produces a weight decrease in the individuals because alcohol modifies several metabolic pathways and at the same time, displaces an adequate diet for the necessity of alcohol consumption, but the opposite was observed in this experiment (Table 1), which may be explained by inadequate doses of ethanol or the insufficient experimentation time to be able to produce the dependency to this substance[1536].
In relation to the ethanol effect over the liver mass, the liver/body ratio and the mgDNA/g liver (Table 2), the data of the present work gives evidence of the utility of these indicators in the evaluation of the cellular regeneration showing that the given doses of ethanol (1.5 g/kg) decreases these parameters significantly. This agrees with the study of Yoshida[37], where an immunoblotting assay was used to evaluate the same dose of ethanol over cellular regeneration that altered PH and determined that alcohol attenuated the hepatic activity of the thymidylate synthase and the thymidine kinase at 24 h altering surgery, and affecting the quantity of DNA as well. The Northern blot analysis confirmed that the protein levels of the enzymes are proportional to the quantity of mRNA, so ethanol is capable of inhibiting the synthesis of DNA by reducing the mRNA levels of these enzymes during hepatic regeneration[37].
The previous study and this one agrees with the suggestions of other authors to consider the PH as the good in vivo model most adequate to study cellular proliferation because it is a relatively simple technique and with the advantage that after surgery, only a short time is required to evaluate hepatic regeneration. Besides, with the quantification of liver mass, liver/body ratio and mgDNA/g liver (Table 2) hepatic regeneration is evaluated indirectly; it has been observed that it depends on the integrity of the mitochondrial respiration mechanism and the cellular capacity of ATP formation, the proper formation of proteins and therefore the synthesis of mRNA and DNA[38–40]. Unlike most other terminally differentiated cells, the hepatocytes in adult liver retain the capacity to proliferate. Indeed, following 70% partial hepatectomy, most hepatocytes proliferative at least once. Hepatocellular proliferation continues until the prehepatectomy liver mass is restored. This compensatory hyperplasia being within minutes of resection is typically completed within 2 wk in rats and in less than one month in humans[17].
Since the 80's the equilibrium between the generation of free radicals and the endogenous antioxidant mechanisms have been recognized. It has been established that this balance can be altered during oxidative stress induced by different xenobiotics and it can also be reestablished when antioxidant compounds like ascorbic acid (vitamin C), alpha-tocopherol (vitamin E), or β-carotene (vitamin A) are administrated[4142]. The results of this study (Figure 2) support this observation, since the 3 vitamins showed a protective effect against the generation of hydroxyethyl radicals generated by the ethanol; although the most significant efficiency was with vitamin C and E, where the hepatic regeneration reached 94% and 96% respectively (Table 2). This result and previous studies support the basis of the potential protector effect of these vitamins in their capacity to scavenge free radicals, however, the difference in percentage of hepatic regeneration suggests that vitamin E might be more efficient than vitamin C. Thus, it is important to mention that there are differences between the vitamins in their physicochemical properties. For example, vitamin C is a hydrophilic compound and a strong reductor agent which reacts with O2 and even faster with the OH groups[43]. Therefore, the treatment with vitamin C could reduce the formation of hydroyxethyl radicals by the inhibition of oxygen radical intermediates. Besides, it has been observed in vitro, vitamin C is capable of reducing the 1-hydroxyethyl/PBN adducts and at the same time inhibits the adducts previously formed[44].
On the other hand, vitamin E also reacts with the oxygen radicals, but it has been observed that its efficiency depends on the radical species that is attacking. This has been shown through in vivo studies where it has been observed that vitamin E has more capacity to scavenge peroxyl radicals than to inhibit hydroxyl radicals[45].
Navasumrit[46] determined that a pre-treatment with vitamin E (100 mg/kg; intraperitoneal for five days) inhibits the 1-hydroxyethilic radicals generated by the ethanol, and it is more effective than pre-treatment with vitamin C (400 mg/kg; intraperitoneal for four days); attributing the result to the fact that 1-hydroxyethilic radicals are primarily generated in lipid compartments of the cell. This also supports the basis that the CYP enzymes (located in membranes of the smooth endoplasmic reticulum) are the most active site for the generation of oxygen reactive radicals, and suggest the correlation between the induction of CYP2E1 and the generation of the 1-hydroxyethilic radicals. This evidence with the fluorescent analysis (laser scanner confocal fluorescence image analysis system) of hepatocytes[47] suggests that the 1-hydroxyethilic radicals are primarily located in the hepatocyte plasma membrane and therefore the lipophilic property of vitamin E gives it a major facility to inhibit the generation of those radicals in comparison with vitamin C, which is hydrophilic (Figure 2).
On the other hand, the reduced effect of vitamin A in relation to hepatic regeneration fits with the report of Portari[42], who evaluated the action of β-carotene in the chronic administration of ethanol in rats. They conclude that because it is a pro-oxidant compound, its mechanism of action could be diminished when the serum and hepatic concentrations are low. As a consequence of this, the reduced effect observed here could be attributed to the intention of the organism to recuperate the serum concentration of this vitamin which was reduced by the extreme consumption of ethanol. To support this possibility, it is important to mention that some studies have indicated that ethanol interferes in several metabolic pathways of vitamin A, that β-carotene is a precursor of this vitamin, that retinol is the alcoholic form of vitamin A. Thus, both retinol and ethanol share the same oxidant activity, and are biotransformed by dehydrogenases, so metabolic interactions that reduce the normal concentration of vitamin A can occur[48].
As it has already been mentioned, the chronic consumption of ethanol damages hepatic cells. The serum concentrations of albumin and bilirubin and the enzymatic activities of AST and ALT are the most sensitive tests used for the diagnosis of hepatic diseases[49]. In this study, it was observed that the rats treated with ethanol presented a decrease in the concentration of albumin, as well as an increase in the serum concentrations of bilirubin, ALT, and AST (Table 3 and Figure 1). The decrease in albumin supports the previous report, where the same dose of ethanol was applied to evaluate the hepatic regeneration and it was analyzed whether toxicity was modified when ethanol was administered intraperitoneally or intragastrically. Both studies prove the hepatotoxic potential of ethanol and show evidence that low serum albumin is directly related with a decrease in the normal ATP generated in the liver; the organ which is only affected by the ethanol and not by the partial hepatectomy where the level of this protein is comparable to the sham group[40]. On the other hand, in the groups combined with ethanol and vitamins C and E, the concentrations of serum albumin and bilirubin were almost normalized (Table 3), which confirms the protective effect of this vitamin over the free radicals generated by the ethanol, as well as its efficiency in the cellular regeneration of the liver (Table 2 and Figure 2).
The enzymatic elevation can probably be attributed to the damaged structural integrity of the hepatic cells, because these enzymes are located in the cytoplasm and are released into circulation after cellular damage. If injury involves other organelles, such as mitochondria, then the soluble enzymes such as AST compartmentalized will also be similarly released indicating that alcohol consumption causes both plasma membrane and organelle membrane damage. On the administration of vitamins to alcoholic rats we observed decreased levels of serum AST and ALT, suggesting the protection capacity of these vitamins to preserve normal structural liver (Figure 1).
On the other hand, increased serum patterns of LDH, ALT and AST activities after 70% PH in rats have been reported previously[50]. The increase of these serum enzyme activities during the onset of liver proliferation could be related to cell necrosis or enhanced cell membrane permeability. Indeed, it has been concluded that increased serum activities of some enzymes after PH could be due to early release either from damaged cells or by cells with altered permeability, probably involving enhanced synthesis and release of enzymes[40].
It is known that ethanol inhibits liver regeneration, resulting in the increase in free radicals produced by the metabolism of ethanol, causing cellular damage as well as the alteration of liver functions. Our results propose that vitamin C and E which are free radical scavengers block the inhibitory effect of regeneration caused by the ethanol by decreasing the damage in the cellular membrane (MDA). This decrease in damage caused by the free radicals produced by the metabolism of ethanol also decreased the indicators of liver damage such as TGO and TGP, as well as the ones for physiological state (albumin and bilirubin) causing an increase in the indicators of liver regeneration (restitution of the liver mass, liver/body ratio and mgDNA/g liver ratio), and consequently reducing mortality.
In conclusion, in this study, the groups treated with ethanol plus the vitamins C or E had better response in terms of liver regeneration, showing less oxidative stress, and had better liver function than the PH-EtOH and the PH-Ethanol-vitamin A groups.
Evidence demonstrates that oxidative stress plays an important etiologic role in the development of alcoholic liver disease. Vitamins are one of the most promising antioxidant defense systems. Recently, it has become clear that antioxidant nutrients, including vitamins, are important for the liver. In addition, evidence for alcohol-induced oxidant injury comes from studies in which pretreatment with antioxidants such as vitamin E, vitamin C, and agents that enhance antioxidant capacity attenuate alcohol induced effects.
The aim of this study is to analyze the effects of vitamins A, C and E in rats with liver injury induced by ethanol administration during liver regeneration, as liver protector agents.
This study clearly shows that vitamin C and E, which are free radical scavengers, block the inhibitory effect of regeneration caused by the ethanol by decreasing the damage in the cellular membrane (MDA); causing an increase in the liver regeneration indicators (restitution of the liver mass, liver/body ratio and mgDNA/g liver ratio), and consequently reducing mortality.
The treatment of vitamin C and E can be used as a hepatoprotective agent from the damage caused by ethanol. It could be used as a protector of the damaged produced by ethanol, opening a window to new therapeutic strategies, of novel agents which correct fundamental cellular disturbances resulting from excessive alcohol consumption.
Partial hepatectomy: the operations consisted of removal of the median and left lateral lobes of the liver. Liver regeneration after partial hepatectomy in the rat has been widely employed as an experimental model to study mammalian cell proliferation.
This is an interesting study, aiming to identify the effects of vitamins A, C and E in rats, as hepatoprotective agents of the damage produced by ethanol during liver regeneration. The authors concluded that vitamin C and E are excellent therapeutic agents against the liver damage produced by ethanol.
| 1. | Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408-413. |
| 2. | Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994;29:513-522. |
| 3. | Kinoshita H, Ijiri I, Ameno S, Fuke C, Fujisawa Y, Ameno K. Inhibitory mechanism of intestinal ethanol absorption induced by high acetaldehyde concentrations: effect of intestinal blood flow and substance specificity. Alcohol Clin Exp Res. 1996;20:510-513. |
| 4. | Fallon H. Methods of Clinical Surveillance: Effects on Liver and Other Organs. In: Vouk VB, Butler GC, Upton AC, Parke DV, Asher SC, editors. Methods for Assessing the Effects of Mixtures of Chemicals. SCOPE 1987; 317-333. |
| 5. | Luczaj W, Skrzydlewska E. Antioxidant properties of black tea in alcohol intoxication. Food Chem Toxicol. 2004;42:2045-2051. |
| 6. | Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27:91-96. |
| 7. | Sivaram AG, Suresh MV, Indira M. Combined effect of ascorbic acid and selenium supplementation on alcohol-induced oxidative stress in guinea pigs. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:397-401. |
| 8. | Masini A, Ceccarelli D, Gallesi D, Giovannini F, Trenti T. Lipid hydroperoxide induced mitochondrial dysfunction following acute ethanol intoxication in rats. The critical role for mitochondrial reduced glutathione. Biochem Pharmacol. 1994;47:217-224. |
| 9. | Videla LA, Valenzuela A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implications. Life Sci. 1982;31:2395-24074. |
| 10. | Duthie GG, Bellizzi MC. Effects of antioxidants on vascular health. Br Med Bull. 1999;55:568-577. |
| 11. | Siushansian R, Wilson JX. Ascorbate transport and intracellular concentration in cerebral astrocytes. J Neurochem. 1995;65:41-49. |
| 12. | Grundman M, Grundman M, Delaney P. Antioxidant strategies for Alzheimer's disease. Proc Nutr Soc. 2002;61:191-202. |
| 13. | Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209-216. |
| 14. | Berman K, Brodaty H. Tocopherol (vitamin E) in Alzheimer'’s disease and other neurodegenerative disorders. CNS Drugs. 2004;18:807-825. |
| 15. | McDonough KH. Antioxidant nutrients and alcohol. Toxicology. 2003;189:89-97. |
| 16. | Diehl AM. Nutrition, hormones, metabolism, and liver regeneration. Semin Liver Dis. 1991;11:315-320. |
| 17. | Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arc Pathol. 1931;12:186-202. |
| 18. | DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967;91:331-336. |
| 19. | Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457-462. |
| 20. | Morales-Gonzalez JA, Gutierrez-Salinas J, Hernandez-Munoz R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol Clin Exp Res. 1998;22:1557-1563. |
| 21. | Pinaire J, Smith JR, Cho WK, Crabb DW. Effects of vitamin A deficiency on rat liver alcohol dehydrogenase expression and alcohol elimination rate in rats. Alcohol Clin Exp Res. 2000;24:1759-1764. |
| 22. | Ozdil S, Yanardag R, Koyuturk M, Bolkent S, Arbak S. Protective effects of ascorbic acid, DL-alpha-tocopherol acetate, and sodium selenate on ethanol-induced gastric mucosal injury of rats. Biol Trace Elem Res. 2004;99:173-189. |
| 23. | Lowry OH, Rosebrough , N . J., Farr, A. L. and Randall, R. J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265-275. |
| 24. | Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice small star, filled. J Nutr Biochem. 2003;14:591-597. |
| 25. | Orrego H, Crossley IR, Saldivia V, Medline A, Varghese G, Israel Y. Long-term ethanol administration and short-and long-term liver regeneration after partial hepatectomy. J Lab Clin Med. 1981;97:221-230. |
| 26. | Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344-352. |
| 27. | Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18:97-111. |
| 28. | Gutierrez-Salinas J. Daño en el hígado por radicales libres derivados de oxígeno. Alcohol, Alcoholismo y Cirrosis. Hidalgo, Mexico: Universidad Autónoma del Estado de Hidalgo 2007; 97-109. |
| 29. | Seitz HK, Pôschl G, Stickel F. Alcohol and colorectal cancer. Exogenous factors in colonic carcinogenesis. London: Kluwer, Dordrecht, Boston 2003; 128-141. |
| 30. | Doll R, Forman D, La Vecchia C. Alcoholic beverages and cancers of the digestive tract and larynx. Health issues related to alcohol consumption. 2nd ed. Cornwall: MPG Books 1999; 351-393. |
| 31. | Scheppach W, Bingham S, Boutron-Ruault MC, Gerhardsson de Verdier M, Moreno V, Nagengast FM, Reifen R, Riboli E, Seitz HK, Wahrendorf J. WHO consensus statement on the role of nutrition in colorectal cancer. Eur J Cancer Prev. 1999;8:57-62. |
| 32. | Stickel F, Schuppan D, Hahn EG, Seitz HK. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut. 2002;51:132-139. |
| 33. | Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155-165. |
| 34. | Seitz HK, Matsuzaki S, Yokoyama A, Homann N, Vakevainen S, Wang XD. Alcohol and cancer. Alcohol Clin Exp Res. 2001;25:137S-143S. |
| 35. | Gutierrez-Salinas J, Aranda-Fraustro A, Paredes-Diaz R, Hernandez-Munoz R. Sucrose administration to partially hepatectomized rats: a possible model to study ethanol-induced inhibition of liver regeneration. Int J Biochem Cell Biol. 1996;28:1007-1016. |
| 36. | Seitz HK, Suter PM. Ethanol toxicity and nutritional status. 2nd ed. Nutritional toxicology. London, New York: Cotsones, Taylor and Francis 2002; 122-154. |
| 37. | Yoshida Y, Komatsu M, Ozeki A, Nango R, Tsukamoto I. Ethanol represses thymidylate synthase and thymidine kinase at mRNA level in regenerating rat liver after partial hepatectomy. Biochim Biophys Acta. 1997;1336:180-186. |
| 38. | Orrego H, Israel Y, Crossley IR, Mahmoud AA, Peters PA, Varghese G, Wanless IR. Effect of chronic alcohol intake on hepatic fibrosis and granulomas in murine schistosomiasis mansoni. Hepatology. 1981;1:416-418. |
| 39. | Nakata R, Tsukamoto I, Nanme M, Makino S, Miyoshi M, Kojo S. Alpha-adrenergic regulation of the activity of thymidylate synthetase and thymidine kinase during liver regeneration after partial hepatectomy. Eur J Pharmacol. 1985;114:355-360. |
| 40. | Morales-Gonzalez JA, Gutierrez-Salinas J, Yanez L, Villagomez-Rico C, Badillo-Romero J, Hernandez-Munoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci. 1999;44:1963-1974. |
| 41. | Crabb DW, Pinairs J, Hasanadka R, Fang M, Leo MA, Lieber CS, Tsukamoto H, Motomura K, Miyahara T, Ohata M. Alcohol and retinoids. Alcohol Clin Exp Res. 2001;25:207S-217S. |
| 42. | Portari GV, Jordao Junior AA, Meirelles MS, Marchini JS, Vannucchi H. Effect of beta-carotene supplementation on rats submitted to chronic ethanol ingestion. Drug Chem Toxicol. 2003;26:191-198. |
| 43. | Halliwell B, Gutteridge JMC. Protection against oxidants in biological systems: the superoxide theory of oxygen toxicity. Free radicals in biology and medicine. 2nd ed. Oxford: Claredon Press 1989; 86-186. |
| 44. | Stoyanovsky DA, Wu D, Cederbaum AI. Interaction of 1-hydroxyethyl radical with glutathione, ascorbic acid and alpha-tocopherol. Free Radic Biol Med. 1998;24:132-138. |
| 45. | Niki E. Alpha-Tocopherol. Handbook of antioxidants. New York: Marcel Dekker Inc 1996; 3-25. |
| 46. | Navasumrit P, Ward TH, Dodd NJ, O’Connor PJ. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis. 2000;21:93-99. |
| 47. | Clot P, Parola M, Bellomo G, Dianzani U, Carini R, Tabone M, Arico S, Ingelman-Sundberg M, Albano E. Plasma membrane hydroxyethyl radical adducts cause antibody-dependent cytotoxicity in rat hepatocytes exposed to alcohol. Gastroenterology. 1997;113:265-276. |
| 48. | Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071-1085. |
| 49. | Kumar Rajagopal S, Manickam P, Periyasamy V, Namasivayam N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem. 2003;14:452-458. |
| 50. | Sekas G, Cook RT. The evaluation of liver function after partial hepatectomy in the rat: serum changes. Br J Exp Pathol. 1979;60:447-452. |