INTRODUCTION
For a potent and long-lasting tumor therapy it is desirable to eradicate the primary tumor and also to induce an anti-tumor immunity to prevent the spread and recurrence of tumor cells. Recent advances in tumor immunology have identified various tumor-associated antigens, and this has facilitated the development of vaccine strategies for cancer. However, the use of tumor-associated antigens as a vaccine component is limited to cancer patients with a known tumor antigen[1]. To circumvent this limitation, some cancer vaccination strategies use killed tumor cells or lysates delivered in combination with adjuvants or cytokines, which probably include both known and unknown antigens[23]. Furthermore, gene transfer of cytokines, MHC molecules, costimulatory molecules or tumor antigens to tumor cells has been used to enhance the visibility of tumor cells to immune effector cells[45].
In 1994, Polly Matzinger presented a new theory called the danger model, suggesting a specific immune response develops as a result of danger detection rather than discrimination between self and non-self antigens[67]. According to the danger model, the immune surveillance system fails to detect tumor antigens because transformed cells do not send any danger signals[7]. Danger signals are thought to act by stimulating dendritic cells to mature so that they can present foreign antigens and stimulate T cells[8–11]. During infections, microbial components provide signals that alert the immune system to danger and promote the generation of immunity[812]. Dying mammalian cells have also been found to release danger signals[13–16]. In the absence of such signals there is often no immune response or tolerance may develop.
Linardakis et al demonstrated in a syngeneic murine B16 melanoma model that the expression of fusogenic membrane protein G from vesicular stomatitis virus (VSV-G) can enhance the efficacy of a weak allogeneic vaccine[17]. Fusogenic membrane glycoproteins were introduced as a new class of therapeutic genes for cancer gene therapy by Bateman et al, who demonstrated that expression of these proteins alone resulted in a significantly greater tumor growth control than suicide prodrug systems[18]. The fusion of viral envelopes with cellular membranes is an essential step mediating the entry of enveloped viruses into host cells. This process is mediated by specific viral proteins such as the fusion (F) protein of paramyxoviruses[19–21]. A member of this virus family, human respiratory syncytial virus (RSV), encodes three envelope glycoproteins, namely the major attachment glycoprotein (G)[22], the small hydrophobic (SH) protein, which blocks TNF-α mediated apoptosis[23], and the fusion glycoprotein (F), which mediates virus-cell and cell-cell fusion[24], creating the characteristic syncytia for which the virus is named.
Previously, we demonstrated in a syngeneic bilateral subcutaneous MC38 and Colon26 colon cancer model in immunocompetent mice that the injection of one tumor with a replication-defective adenovirus encoding RSV-F resulted not only in tumor growth reduction of the treated tumor, but also of the second, untreated contralateral tumor[25]. We observed qualitatively similar effects with fusogenic membrane proteins of measles virus (MV-H/F)[26]. The effects were associated with a tumor-specific cytotoxic T cell (CTL) response and a pronounced infiltration of tumors with natural killer cells and macrophages.
In an attempt to promote the development of improved tumor vaccination strategies that rely on intratumoral expression of viral fusogenic membrane proteins, in this study we elucidated factors that might influence the induction of a systemic anti-tumor response. Using the same bilateral subcutaneous tumor models we demonstrated that treatment of one cutaneous tumor with a replication-defective adenovirus encoding RSV-F alone (Ad.RSV-F) or in combination with RSV-G (Ad.RSV-F/G) resulted in improved survival and the induction of a systemic anti-tumor immune response. Although in vitro transduction of tumor cells with Ad.RSV-F/G resulted in significantly larger syncytia compared with transduction of tumor cells with Ad.RSV-F, the in vivo treatment outcome was not significantly influenced by the size of cell-cell fusion. Treatment of animals with an adenovirus encoding a soluble, non-fusogenic form of RSV-F (Ad.RSV-Fsol) or RSV-G (Ad.RSV-G) had no anti-neoplastic effect, indicating that the anti-neoplastic effects are not primarily mediated by intrinsic immunological properties of RSV envelope glycoproteins. In both tumor models we observed the induction of a systemic anti-tumor response only when RSV glycoprotein expression in the tumor cells caused syncytium formation and apoptosis, independent of the size of syncytia.
MATERIALS AND METHODS
Cells and cell culture
The murine colon adenocarcinoma cell line MC38 was obtained from Steven A. Rosenberg, NCI, NIH, Bethesda, MD. The murine adenocarcinoma cell line Colon26 was purchased from CLS (Heidelberg, Germany). The human embryonic kidney cell line 293 was obtained from Microbix Biosystems, Inc. (Toronto, Canada). The T-REx-293 cells, which stably express a tetracycline-dependent repressor, were purchased from Invitrogen (San Diego, CA, USA). Cell lines were propagated in Dulbecco’s modified Eagle medium with high glucose (Invitrogen, Karlsruhe, Germany), supplemented with 10% heat-inactivated fetal bovine serum and 50 &mgr;g/mL gentamicin. All cell lines were routinely tested for Mycoplasma and found to be free of contamination.
Viruses
The replication-defective Ad5-based vector Ad.GFP, which encodes enhanced green fluorescent protein (GFP) driven by the CMV-IE promoter, has been described previously[27]. As a vector control we used Ad.Null, a replication-defective adenovirus generated with the Ad-Easy-1 system[28] that does not encode a transgene.
The adenovirus vector Ad.RSV-F, which carries a codon-optimized cDNA for native RSV-F[29], has been described previously[25]. The vector Ad.RSV-Fsol encodes a non-fusogenic soluble form of RSV-F, which lacks the transmembrane domain and cytoplasmic tail (Δ524-574). The vector Ad.RSV-G encodes the RSV (ATCC VR26) major attachment protein G, which was codon optimized for expression in human cells (GeneArt, Regensburg, Germany). In all adenovirus vectors, the RSV envelope glycoproteins were under the transcriptional control of the TetO2 promoter[30]. The vector Ad.RSV-F/G encodes the RSV-F and RSV-G proteins under the transcriptional control of a bi-directional TetO2 promoter. All RSV glycoprotein encoding adenovirus vectors were generated using the Ad-Easy-1 system[28], and are Ad5-based and E1-, E3-deleted. Infectious vectors were rescued using T-REx-293 cells in the absence of tetracycline.
All vectors were purified using the Vivapure AdenoPACK 100 kit (Vivascience, Hannover, Germany). Vector particle concentration was determined by spectrophotometry as described previously[31] and expressed as viral particles (VP)/mL. The particle-to-PFU ratios of all vector preparations were about 30:1.
Analysis of cell-cell fusion
About 95%-100% confluent MC38 or Colon26 cell monolayers were transduced with equal amounts of Ad.GFP to enhance syncytium visibility and Ad.Null, Ad.RSV-F, Ad.RSV-F/G, Ad.RSV-G, or Ad.RSV-Fsol, at a multiplicity of infection (MOI) of 1000 VP/cell. Forty-eight hours after vector transduction, cells were analyzed by inverse fluorescence microscopy. Digital images were captured using a high-resolution still camera (Olympus DP50, Tokyo, Japan) attached to a fluorescence microscope (Olympus BX51, Tokyo).
Analysis of apoptosis
Fourteen hours after treatment, the annexin V binding and caspase-3/7 activity of MC38 and Colon26 cells was analyzed using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA) or Caspase-Glo™ 3/7 assay (Promega, USA), respectively, as described previously[32]. Changes in mitochondrial membrane potential (ΔΨm) were analyzed by flow cytometry using the BD MitoScreen Kit (BD Biosciences), as described previously[32].
In vivo studies
The Animal Care and Use Committee of the Ruhr-University Bochum approved all described studies. Six- to eight-week-old female C57BL/6 and BALB/c mice were obtained from Janvier (Le Genest-St-Isle, France). To generate the syngeneic bilateral subcutaneous syngeneic tumor model, C57BL/6 or BALB/c mice received subcutaneous injections of 1 × 105 MC38 or Colon26 cells, respectively, in 100 &mgr;L into the right hind flank and 1 × 104 cells in 100 &mgr;L into the left hind flank. Animals were randomly assigned to treatment groups (n = 5 for each tumor model) when the tumor on the right hind flank reached a volume of about 200 mm³ and the tumor on the left side was palpable. On day 0 and 2, animals received 6 × 109 VP of Ad.Null, Ad.RSV-F, Ad.RSV-F/G, Ad.RSV-G, or Ad.RSV-Fsol in 100 &mgr;L of PBS into the tumor on the right flank. Tumor growth was monitored at least once a week, minimum and maximum perpendicular tumor axes were measured using vernier calipers, and tumor volume was calculated using the simplified formula of a rotational ellipse (l×w²× 0.5). The skin thickness of 0.4 mm was subtracted from the measurements. To generate effector cells, mice were sacrificed and spleens were harvested and weighed 28 d after virus inoculation. When animals seemed to be in distress or the tumor weight exceeded 10% of the body weight, animals were euthanized by CO2 asphyxia.
CTL assay
We analyzed the CTL response to tumor cells, using the lactate dehydrogenase (LDH)-based CytoTox 96 (Promega) assay according to the manufacturer’s instructions. In brief, target cells (MC38 or Colon26) were plated at a density of 5 × 103 cells per well in round-bottomed 96-well plates. Target cells were then mixed with effector cells at the indicated ratios and co-incubated for 4 h. LDH release was determined measuring absorbance at 490 nm with a plate reader, and the specific lysis was calculated from triplicate samples as follows:
Specific lysis(%)=[(Experimental A490– Effector spontaneous A490 - Target spontaneous A490)/ (Target maximum A490 - Target spontaneous A490)] × 100.
Statistical analysis
The statistical software package SPSS 15 (SPSS Inc., Chicago, USA) was used for data analysis. For comparative analysis of survival rates across treatment groups, Kaplan-Meier analysis with the log-rank test was used. Tumor volumes and spleen weights were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey's honestly significantly different (HSD) test. The sizes of syncytia were determined with the image analysis software ImageJ 1.38 (http://rsb.info.nih.gov/ij). The calculated areas of 30 syncytia in each treatment group were compared using the Mann-Whitney Rank Sum Test.
RESULTS
Intratumoral expression of RSV-F alone or in combination with RSV-G induces an antineoplastic effect on the treated tumor as well as on the untreated contralateral tumor
In the subcutaneous syngeneic bilateral MC38 and Colon26 colon cancer model we analyzed in more detail factors that are necessary to induce a systemic anti-tumor response by intratumoral expression of RSV envelope glycoproteins encoded by replication-defective adenovirus vectors (Figure 1). To analyze whether enhanced syncytium formation improves the tumor vaccination effect, animals were treated with Ad.RSV-F or an adenovirus encoding RSF-F as well as RSV-G (Ad.RSV-F/G). Furthermore, we elucidated whether syncytium formation is necessary to induce a systemic anti-tumor response or whether this effect is mediated by intrinsic immunological properties of RSV envelope glycoproteins. Animals were treated with an adenovirus encoding a soluble, non-fusogenic RSV-F without the transmembrane domain and cytoplasmic tail (Ad.RSV-Fsol). Treatment of animals with Ad.RSV-G served as a control. In addition we analyzed whether the tumor cells needed to undergo apoptosis to induce a systemic anti-tumor response.
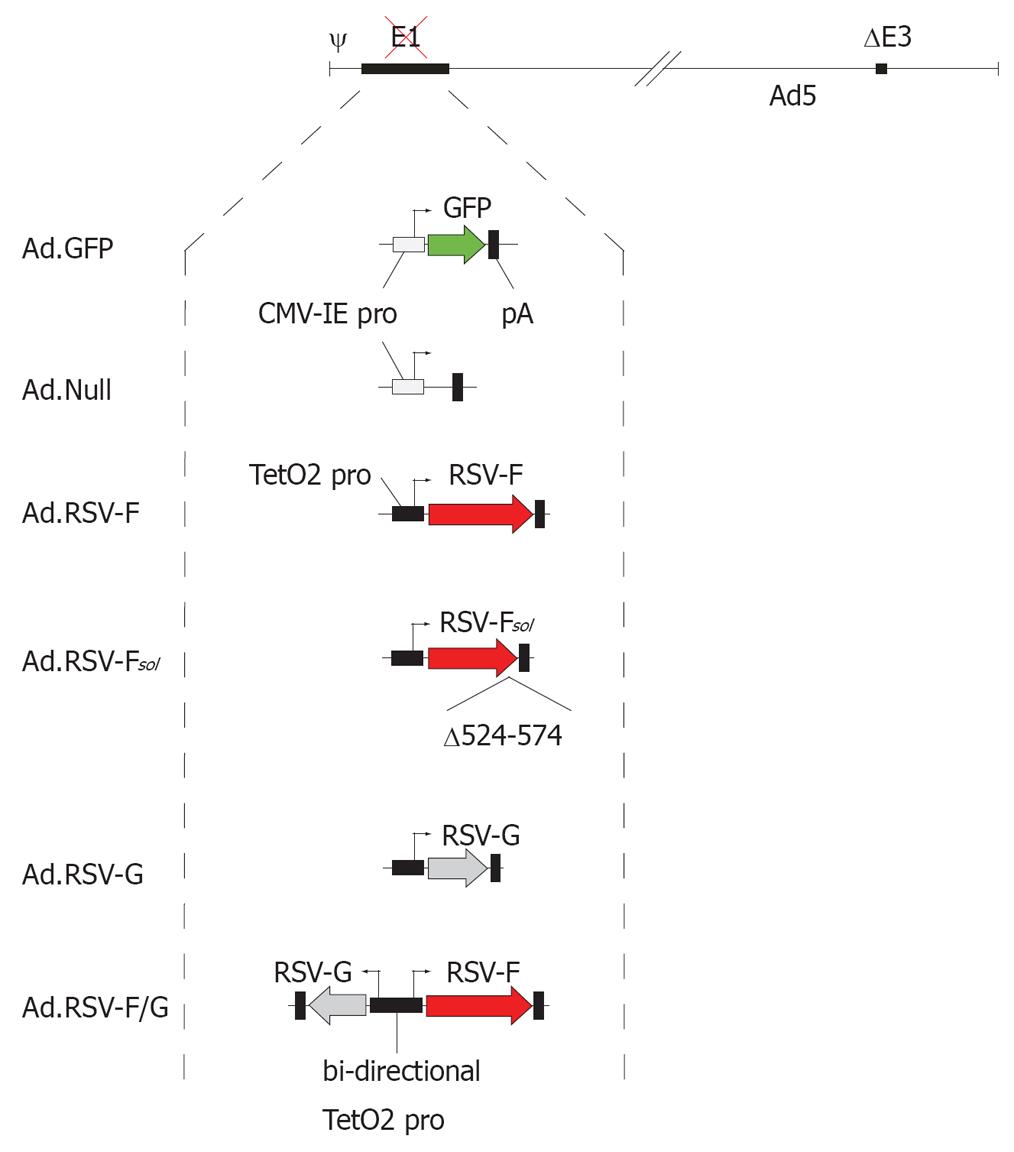
Figure 1 Adenovirus vector design.
All adenovirus vectors used in this study are E1- and E3-deleted and Ad5-based. The vector Ad.GFP encodes green fluorescent protein and the vector Ad.Null served as an adenovirus vector control. The RSV glycoprotein encoding vectors carried the transgene under the transcriptional control of the doxycycline-repressible TetO2 promoter. The vector RSV-Fsol encodes a soluble form of the RSV fusion protein F without the transmembrane domain and cytoplasmic tail (Δ524-574).
As shown in Figure 2, injection of Ad.RSV-F or Ad.RSV-F/G into the right tumor resulted in about 80% and 87% reduction, respectively, in the size of vector-treated tumors at day 28 (P < 0.001). Importantly, treatment of the right tumor with Ad.RSV-F or Ad.RSV-F/G also resulted in about 60% reduction of the left, untreated tumor compared with Ad.Null-treated animals (P < 0.001). By contrast, treatment with Ad.Null, Ad.RSV-G or Ad.RSV-Fsol had no significant anti-neoplastic effect on either vector-treated or contralateral tumors (P > 0.05).
Figure 2 Intratumoral expression of RSV-F or RSV-F/G induces an anti-neoplastic effect.
In a bilateral subcutaneous syngeneic MC38 or Colon26 colon cancer model, the indicated adenovirus vectors were inoculated on d 0 and d 2 into the tumor on the right flank. No viral vectors were inoculated into the tumor on the left flank. A: The volume of the tumor on the right flank was measured at d 28 and presented as box-and-whisker plots, showing minimum, 25th percentile, median, 75th percentile, and maximum tumor volume; B: The volume of the tumor on the left flank, which did not receive direct viral vector injections, was measured at d 28 and the volume reduction relative to Ad.Null-treated control animals is presented as bar graphs (mean ± SD).
To assess whether these results are unique to MC38 cells and C57BL/6 mice (H-2b), we repeated the syngeneic bilateral tumor model under identical conditions with Colon26 cells in BALB/c mice (H-2d), which have contrasting susceptibilities to certain intracellular pathogens[3334]. As shown in Figure 2, the results were qualitatively similar to that obtained with MC38 cells.
Intratumoral expression of RSV-F alone or in combination with RSV-G results in enhanced survival
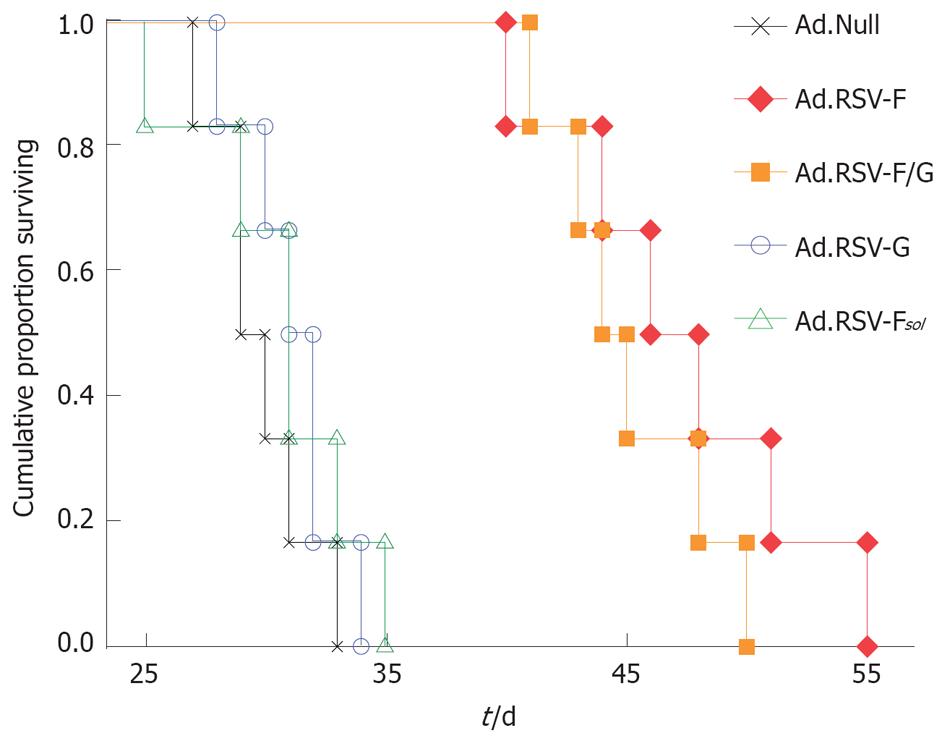
Next, we analyzed in the syngeneic bilateral MC38 subcutaneous colon cancer model whether intratumoral expression of RSV envelope glycoproteins also results in improved survival. Kaplan-Meier survival analysis revealed that animals treated with Ad.Null had a median survival of 29 d (Figure 3). Animals that received intratumoral injections of Ad.RSV-G or Ad.RSV-Fsol had a median survival of 31 d (P > 0.05). Treatment of mice with Ad.RSV-F or Ad.RSV-F/G resulted in a significantly improved outcome with median survivals of 46 d and 44 d, respectively (P < 0.001).
Figure 3 Kaplan-Meier survival analysis.
In the bilateral subcutaneous syngeneic MC38 colon cancer model described in Figure 2, one tumor was treated with the indicated adenoviral vectors, and survival time was monitored up to d 55.
Expression of RSV-F alone or in combination with RSV-G results in syncytium formation in MC38 and Colon26 cells
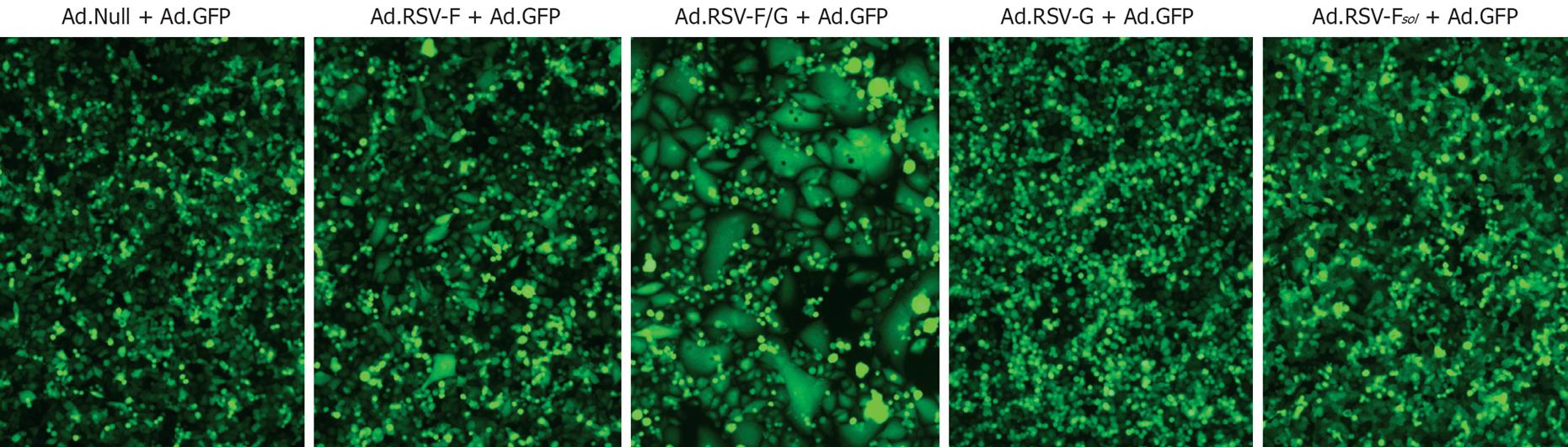
Next, we determined whether the size of syncytium formation correlates with the treatment outcome. As shown in Figure 4, no cell-cell fusion was detectable in MC38 cells transduced with Ad.Null, Ad.RSV-G or Ad.RSV-Fsol. The median syncytia area of monolayers transduced under identical conditions with Ad.RSV-F or Ad.RSV-F/G was about 3 times and 25 times larger than the median area of single MC38 cells transduced with Ad.Null. Transduction of cell monolayers with Ad.RSV-F/G resulted in significantly larger syncytia than Ad.RSV-F (P = 0.001). Similar data were obtained with Colon26 cells (data not shown).
Figure 4 Fluorescence micrographs.
MC38 cells were transduced in vitro with indicated RSV glycoprotein encoding adenoviruses and an adenovirus encoding GFP. Transduction with Ad.GFP enhanced the visibility of syncytia. Representative pictures 48 h after transduction are shown (× 200). Similar data were obtained with the Colon26 cells (data not shown).
Expression of RSV-F alone or in combination with RSV-G results in apoptosis
To examine whether the cells need to undergo apoptosis after expression of RSV envelope glycoproteins to induce a systemic anti-tumor response, we analyzed early and late events of programmed cell death. We first analyzed the binding of annexin V-FITC to externalized phosphatidylserine, which reflects reversible membrane damage, as a marker for the early stages of apoptosis in combination with vital staining[35]. As shown in Figure 5A, there was no significant annexin V binding to MC38 or Colon26 cells transduced with Ad.Null. Fourteen hours after transduction with Ad.RSV-G or Ad.RSV-Fsol, the median percentage of annexin V-positive cells was about 6%. Transduction of the tumor cell lines with Ad.RSV-F or Ad.RSV-F/G resulted in about 32% annexin V-positive cells.
Figure 5 Analysis of apoptosis.
A: MC38 or Colon26 cells were transduced in vitro with indicated adenovirus vectors and early apoptotic events were analyzed by flow cytometric measurements of phosphatidylserine translocation to the outer membrane by annexin V binding; B: Because apoptosis is essentially executed by proteases of the caspase family, we analyzed caspase-3/7 activity. Relative caspase activities are given as means ± SD of three independent experiments; C: To measure mitochondrial alterations we determined the mitochondrial membrane potential ΔΨm. Ad.Null served as a control.
Induction of apoptosis is essentially executed by the activation of caspases in response to extrinsic or intrinsic stimuli. Activation of initiator caspases results in the downstream activation of effector caspases that cleave key cellular proteins leading to controlled cell death[36]. To assess the involvement of caspases, we measured the proteolytic activity of the effector caspases-3/7 using a luminogenic substrate assay. The caspase-3/7 activity of untreated MC38 or Colon26 cells was normalized to 100%. As shown in Figure 5B, 36 h after transduction of MC38 or Colon26 cells with Ad.RSV-G or Ad.RSV-Fsol, the median caspase-3/7 activity was about 107%. Treatment with Ad.RSV-F or Ad.RSV-F/G resulted in about 142% caspase activity.
In addition, we analyzed the mitochondrial membrane potential ΔΨm, an important parameter of mitochondrial alterations, 36 h after transduction of the cells with the adenoviral vectors, by staining with the cationic dye JC-1, which selectively enters into mitochondria and reversibly changes color from green to red as the membrane potential increases. In healthy cells with high mitochondrial ΔΨm, JC-1 spontaneously forms complexes with intense red fluorescence. In apoptotic or unhealthy cells with low ΔΨm, JC-1 remains in the monomeric form, which shows green fluorescence. As shown in Figure 5C, transduction of MC38 or Colon26 cells with Ad.RSV-F or Ad.RSV-F/G resulted in a clearly stronger loss of ΔΨm compared with cells transduced with Ad.Null, Ad.RSV-G or Ad.RSV-Fsol.
Treatment of animals with Ad.RSV-F or Ad.RSV-F/G but not with Ad.RSV-G or Ad.RSV-Fsol results in significantly increased spleen weight
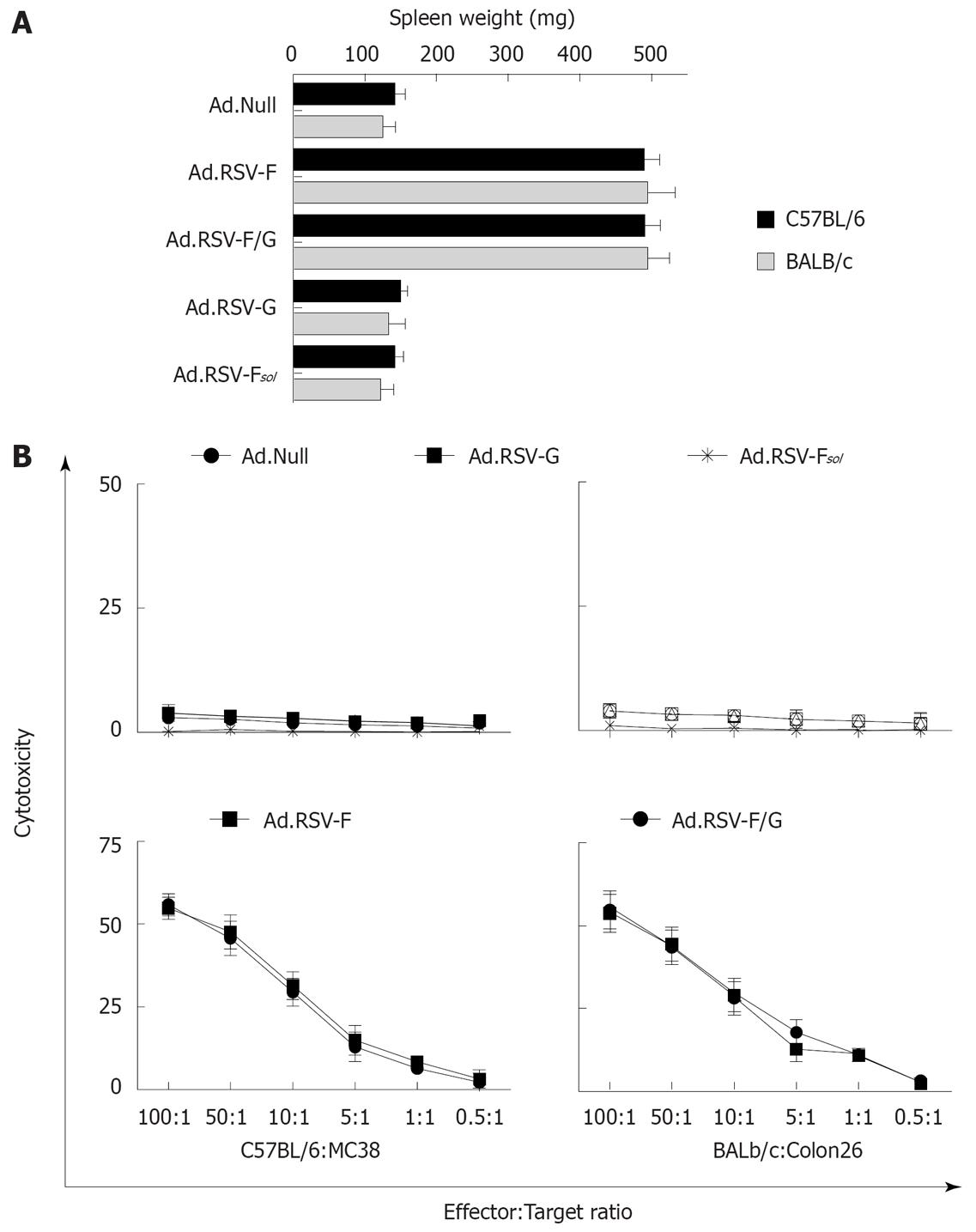
Splenomegaly is often associated with a cellular immune response. To elucidate whether the anti-neoplastic effect on the untreated contralateral tumor is immune-mediated we determined the spleen weights of the animals on d 28 (Figure 6A). We observed about a 245% increase in median spleen weights in the MC38 and Colon26 cancer model animals treated with Ad.RSV-F or Ad.RSV-F/G, compared to Ad.Null-treated animals. Treatment of animals with Ad.Null, Ad.RSV-G or Ad.RSV-Fsol had no significant effect on spleen weight.
Figure 6 Effects of indicated treatments on the spleen weight and cytotoxic T cell induction.
A: In the bilateral subcutaneous syngeneic MC38 and Colon26 tumor model described in Figure 2, animals were euthanized at day 28 and spleen weight was determined (mean ± SD); B: In addition, we determined the cytotoxic activity of spleen-derived T cells against target MC38 or Colon26 tumor cells in these animals using an LDH release assay. Data of all animals were expressed as the average percentage of specific LDH release from three independent experiments (mean ± SD).
Intratumoral expression of RSV-F or RSV-F/G but not of RSV-G or RSV-Fsol induces a tumor cell-specific CTL response
As the splenomegaly suggests the induction of a cellular immune response, we next determined whether there is a tumor-specific CTL response mediating the observed anti-neoplastic response against the untreated tumor. As shown in Figure 6B, in an LDH release assay we observed no cytotoxicity of splenocytes derived from Ad.Null-treated mice with or without tumors against the MC38 or Colon26 target cells. Splenocytes from animals treated with Ad.RSV-F or Ad.RSV-F/G showed a cytotoxicity of about 55% at an effector to target ratio of 100:1. By contrast, we observed only a slight lysis of target cells by splenocytes from animals treated with Ad.RSV-G or Ad.RSV-Fsol.
DISCUSSION
We demonstrated previously in syngeneic murine tumor models that intratumoral expression of viral fusogenic membrane proteins can induce a systemic anti-tumor response associated with a tumor-specific CTL response[2637]. In this study we analyzed whether in situ tumor vaccination by intratumoral expression of RSV envelope glycoproteins is influenced by the efficiency of cell-cell fusion, the mode of tumor cell death, or whether the effect is mediated by intrinsic immunological properties of the RSV-F protein.
The key findings of our current study were as follows. First, despite the limited intratumoral spread and transduction efficiency of the replication-defective adenovirus vectors[38], intratumoral injection of Ad.RSV-F or Ad.RSV-F/G resulted in significantly improved tumor growth reduction of both vector-inoculated tumors and contralateral untreated tumors. The improved anti-neoplastic treatment efficacy also resulted in a significantly improved survival. The effect was not mediated by an adenovirus vector viremia, as we did not observe this effect in nude mice (data not shown) and we did not detect adenovirus vector beyond d 1 in the serum of vector-treated C57BL/6 mice[39]. This indicates that the systemic anti-tumor response depends on an intact immune system. This is supported by our demonstration of the induction of a tumor-specific CTL response and a massively increased spleen weight. This qualitatively confirms our data obtained in a bilateral subcutaneous colon cancer model using HSV-1 or adenovirus vectors encoding the fusogenic membrane proteins F and H of measles virus or RSV-F, showing that the expression of viral fusogenic membrane proteins can serve as a tumor vaccination platform[252637]. Furthermore the data are in concert with previous studies demonstrating that expression of VSV-G encoded by a plasmid vector can enhance the immunogenicity of tumor cells[174041].
Second, we demonstrated that transduction of tumor cells with Ad.RSV-F/G resulted in clearly larger syncytia than transduction with Ad.RSV-F. This result is in concert with a previous observation[42]. However, as we used codon-optimized cDNA for the expression of the RSV glycoproteins[29], we observed cell-cell fusion also after transduction of the tumor cells with Ad.RSV-F alone. In other paramyxoviruses, expression of the fusion protein alone is not sufficient to mediate cell-cell fusion[43]. Transduction of the murine colon cell lines with Ad.RSV-G or Ad.RSV-Fsol did not cause detectable cell-cell fusion.
Third, there was no significant difference in the local and distant anti-tumor effect in animals that were treated with Ad.RSV-F or Ad.RSV-F/G, although the syncytia of cells transduced with Ad.RSV-F/G were clearly larger in vitro. This indicates that the treatment outcome was not significantly influenced by the size of cell-cell fusion.
Fourth, our data indicate that only RSV envelope glycoproteins which cause syncytium formation (RSV-F or RSV-F/G) are able to induce apoptosis, in contrast to RSV-G or RSV-Fsol. Importantly, in our experimental setting, only membrane proteins which are able to induce apoptosis are associated with a local and distant anti-tumor effect. Because the expression of RSV-Fsol did not induce a distant anti-tumor response, this effect is most likely not mediated by intrinsic immunological properties of RSV-F. This supports previous data indicating the mechanisms by which tumor cells are killed may be critical for the induction of a specific anti-tumor immunity[4445]. However, because we did not include a fusion-disabled RSV-F, we cannot rule out the possibility that the transmembrane domain of RSV-F per se is responsible for the observed effects.
In an earlier study we elucidated by Western blot analysis some of the molecular pathways leading to cell death by expression of viral fusogenic membrane proteins. We demonstrated that induction of apoptosis was independent of functional p53 and was mediated via a mitochondrial death pathway triggered by modulation of Bcl-2 family proteins[32]. In addition, we demonstrated increased protein levels of the heat shock proteins (HSP) 60, 70 and 90α[46].
According to the danger model, growing tumors do not provide a danger signal to dendritic cells, and thus, do not activate the immune system. Therefore, any tumor antigen-specific T cell will have its first antigen encounter with tumor cells or resting dendritic cells. Because there is no co-stimulation, either situation will drive the T cells into anergy or apoptosis and, eventually, tumor tolerance[47]. A conceivable mechanism for the induction of tumor-specific immunity is that expression of the viral fusogenic membrane proteins, apoptotic cells or exosomes of fused cells[4849] might release danger signals resulting in a more efficient presentation of tumor antigens and activation of T cells. This is in concert with recent reports demonstrating that apoptotic tumor cells, but not malignant cells in necrotic tumors, can provoke an anti-tumor immune response[50], if the tumor cells were killed in a caspase-3-dependent manner[51]. In addition, the xenogenization of tumor cells by presentation of viral antigens on the cell surface in conjunction with MHC class I molecules might contribute to the induction of a tumor-specific immune response[5253].
In this study we demonstrated that enhanced fusion function of RSV-F by co-expression of RSV-G does not significantly enhance in situ tumor vaccination in the two tested syngeneic tumor models. However, our data indicate that the vaccination effect critically depends on tumor cell apoptosis, which be can be further enhanced by combination with chemotherapy and viral oncolysis[3237]. Overall, these findings provide insight into improved tumor vaccination strategies relying on the intratumoral expression of viral fusogenic membrane proteins.
COMMENTS
Background
In 1994, Polly Matzinger presented a new theory called the danger model, suggesting that a specific immune response develops as a result of danger detection rather than discrimination between self and non-self antigens. According to the danger model, the immune surveillance system fails to detect tumor antigens because transformed cells do not send any danger signals. Danger signals are thought to act by stimulating dendritic cells to mature so that they can present foreign antigens and stimulate T cells. Dying mammalian cells have also been found to release danger signals. In the absence of such signals there is often no immune response or tolerance may develop.
Research frontiers
Linardakis et al demonstrated in a syngeneic murine B16 melanoma model that the expression of fusogenic membrane protein G from vesicular stomatitis virus (VSV-G) can enhance the efficacy of a weak allogeneic vaccine. Hoffmann et al demonstrated previously that expression of viral fusogenic membrane proteins can induce apoptosis in tumor cells.
Innovations and breakthroughs
In two syngeneic subcutaneous murine colon cancer models we demonstrated that intratumoral injection of a replication-defective adenovirus encoding respiratory syncytial virus fusion protein (RSV-F) alone (Ad.RSV-F) or together with the attachment glycoprotein RSV-G (Ad.RSV-F/G) leads to a significant growth reduction of both the vector-treated and contralateral untreated tumor. Treatment response was associated with a strong tumor-specific CTL response and significantly improved survival. Intratumoral injection of Ad.RSV-G or a soluble RSV-F encoding adenovirus (Ad.RSV-Fsol) had no significant anti-neoplastic effect, suggesting the therapeutic effect is not mediated by intrinsic immunological properties of the viral proteins. Although in vitro transduction of colon cancer cell lines with Ad.RSV-F/G resulted in about 8-fold larger syncytia than with Ad.RSV-F, the in vivo outcome was not significantly different. Transduction of murine colon cancer cell lines with Ad.RSV-F or Ad.RSV-F/G caused apoptotic cell death, in contrast to Ad.RSV-G or Ad.RSV-Fsol, suggesting an importance of the mode of cell death. Our results provide insight into improved tumor vaccination strategies relying on the intratumoral expression of viral fusogenic membrane proteins.
Applications
This strategy might be applicable for the treatment of human cancer.
Peer review
This is a good study designed to elucidate the relevance of the size of syncytia or the way tumor cells die on the therapeutic outcome after tumor vaccination using intratumoral expression of viral fusogenic membrane proteins. The results are informative and potentially helpful for human cancer treatments.