Published online Dec 28, 2007. doi: 10.3748/wjg.v13.i48.6529
Revised: September 2, 2007
Accepted: September 6, 2007
Published online: December 28, 2007
AIM: To investigate the effects of ZK1916784, a low calcemic analog of calcitriol on intestinal inflammation.
METHODS: Acute and chronic colitis was induced by dextran sodium sulfate (DSS) according to standard procedures. Mice were treated intraperitoneally with ZK1916784 or placebo and colonic inflammation was evaluated. Cytokine production by mesenterial lymph node (MLN) cells was measured by ELISA. Immunohistochemistry was performed to detect intestinal dendritic cells (DCs) within the colonic tissue, and the effect of the calcitriol analog on DCs was investigated.
RESULTS: Treatment with ZK191784 resulted in significant amelioration of disease with a reduced histological score in acute and chronic intestinal inflammation. In animals with acute DSS colitis, down-regulation of colonic inflammation was associated with a dramatic reduction in the secretion of the proinflammatory cytokine interferon (IFN)-γ and a significant increase in intereleukin (IL)-10 by MLN cells. Similarly, in chronic colitis, IL-10 expression in colonic tissue increased 1.4-fold when mice were treated with ZK191784, whereas expression of the Th1-specific transcription factor T-beta decreased by 81.6%. Lower numbers of infiltrating activated CD11c+ DCs were found in the colon in ZK191784-treated mice with acute DSS colitis, and secretion of proinflammatory cytokines by primary mucosal DCs was inhibited in the presence of the calcitriol analog.
CONCLUSION: The calcitriol analog ZK191784 demonstrated significant anti-inflammatory properties in experimental colitis that were at least partially mediated by the immunosuppressive effects of the derivate on mucosal DCs.
- Citation: Strauch UG, Obermeier F, Grunwald N, Dunger N, Rath HC, Schölmerich J, Steinmeyer A, Zügel U, Herfarth H. Calcitriol analog ZK191784 ameliorates acute and chronic dextran sodium sulfate-induced colitis by modulation of intestinal dendritic cell numbers and phenotype. World J Gastroenterol 2007; 13(48): 6529-6537
- URL: https://www.wjgnet.com/1007-9327/full/v13/i48/6529.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i48.6529
Inflammatory bowel disease (IBD) is a chronic immune-mediated disease of the gastrointestinal tract of still unknown etiology, most commonly involving inflammation of the terminal ileum and colon. The disease is characterized by deregulated immune responses, and in particular, CD4+ T cells that produce large amounts of proinflammatory cytokines [such as interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor (TNF-α)] have been shown to play a central role[1]. Besides genetic factors that are thought to predispose individuals to develop IBD[2], the environment seems to contribute to the disease[3,4]. There is reason to believe that vitamin D may be an environmental factor that affects IBD, as vitamin D deficiency has been linked to ulcerative colitis and Crohn's disease even when the disease is in remission[5,6]. Additionally, vitamin D deficiency has been shown to accelerate the development of IBD symptoms among IL-10 knockout mice that develop spontaneous enterocolitis[7]. Also, calcitriol treatment reduces the ability of IL-10 knockout mice to produce and to respond to TNF-α[8], and it has recently been shown that application of a calcitriol analog reduces intestinal inflammation in a 2,4,6-trinitrobenzene sulfonic acid colitis model[9].
Calcitriol is the activated form of vitamin D3 that binds to a nuclear receptor protein. In the CD4+CD45RBhigh transfer model of colitis, in which T- and B-cell deficient recipient mice develop IBD symptoms when injected with CD4+CD45RBhigh T cells, lymphocytes from vitamin D receptor (VDR) knockout mice increase colitis severity in recipient mice compared to similar cells from wild-type animals[10]. Also, VDR knockout mice develop a more severe colitis in response to dextran sodium sulfate (DSS) treatment than wild-type mice, which can be decreased in severity by dietary or rectally administered calcitriol[11]. To underline the importance of calcitriol in IBD, the VDR gene has been mapped to a region on chromosome 12 that has been shown to be linked to susceptibility for Crohn's disease, which suggests an influence of calcitriol on the intestinal immune system[12].
The immunoregulatory effects of the cellular receptor for calcitriol are complex and not yet finally understood. However, as the VDR was discovered in resting and activated lymphocytes[13], it has been suggested that calcitriol might be involved in immunoregulation. In particular, CD4+ T cells that are known to play a major role in chronic colitis express VDR, and are among the identified targets of activated vitamin D[14,15]. Indeed, calcitriol is able to inhibit T-cell proliferation and secretion of different proinflammatory cytokines in vitro[13,16], and to suppress the development of several experimental models of chronic inflammatory diseases, such as rheumatoid arthritis, experimental autoimmune encephalomyelitis and diabetes in vivo[7,10,17-19]. Also, the presence of calcitriol significantly inhibits cell proliferation of T lymphocytes obtained from patients with ulcerative colitis[20,21]. In mice treated with calcitriol, increased production of TGF-β1 and IL-4 was observed[22], cytokines that are associated with the inhibition of T cell effector function in a murine model of colitis[23]. Furthermore, calcitriol has been found to inhibit dendritic-cell (DC) maturation, which leads to reduced alloreactive capacity[24] and enhanced generation of regulatory CD4+CD25+ T cells[25].
Calcitriol selectively regulates the immune response without compromising host ability to fight infection[17], which makes it an attractive target compared to standard immunosuppressive medication in patients with IBD. However, calcitriol has clear dose-limiting hypercalcemic effects that interfere with its systemic clinical use due to strong influence on calcium homeostasis and the risk of associated side effects. Recently, ZK191784, a new low-calcemic calcitriol derivative, has been identified that inhibits lymphocyte proliferation and suppresses secretion of proinflammatory cytokines by monocytes[26]. The compound has shown immunosuppressive activity in a murine contact hypersensitivity model when given systemically at concentrations that do not cause hypercalcemic effects, thereby suggesting a possible therapeutic application as an immunosuppressive agent[26].
The aim of our study was to investigate whether this modified calcitriol analog might have potential therapeutic value in the treatment of acute and chronic intestinal inflammation, using the DSS model of colitis.
Female Balb/c mice were obtained from Charles River (Germany) and were used for the experiments at 6-8 wk of age and 20-22 g body weight. Animals obtained food and water ad libitum. The local Institutional Review Board approved the animal studies.
The calcitriol analog ZK191784 was synthesized as previously described[26], dissolved in ethanol at a concentration of 1 × 10-2 mol/L and kept at -20°C until use. The following antibodies were used in the study (all purchased from BD Pharmingen, Heidelberg, Germany): anti-CD3, anti-CD11c, anti-CD28, anti-CD16/CD32, anti-MHC-II, anti-CD40, anti-CD80, and anti-CD86.
DSS (molecular mass 40 000) was purchased from ICN (Eschwege, Germany). Acute colitis was induced by feeding 3% DSS over 7 d[27]. Treatment with ZK191784 (100 μg/kg per day orally) or vehicle was either started on day -3 before DSS administration and maintained through to d 7 (“pretreatment”), or started on the first day of DSS application through d 7 (“treatment”). Mice were sacrificed on d 8. For induction of chronic colitis, mice received four cycles of DSS treatment. Each cycle was followed by a period of 10 d water without DSS. Treatment was performed before the first and before the third cycle of DSS for 7 d each (100 μg/kg per day orally). The animals were killed on d 8 after completion of the fourth cycle.
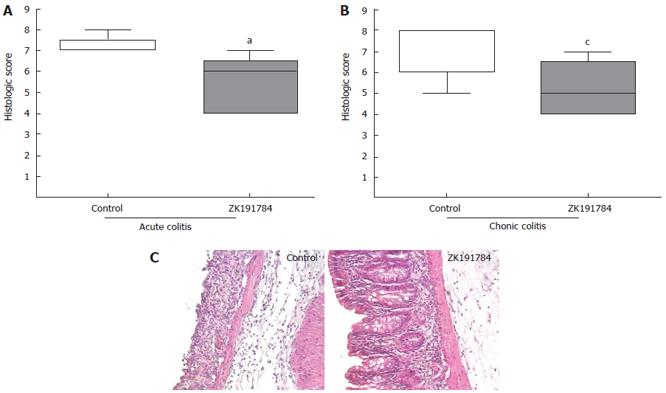
From the distal third of the colon, 1 cm of tissue was harvested from each animal, embedded in paraffin, stained with hematoxylin/eosin after sectioning, and used for histological analysis. To quantify the tissue damage, a scoring system was used as described previously[27]. Thereby, three sections, each obtained at a distance of 100 μm were evaluated. Mice were scored individually, each score representing the mean of three sections. Two independent investigators, blinded to the treatment group, performed histological examination. The total histological score represented the sum of the epithelium and infiltration score, and ranged from 0 to 8[27].
MLN cells (pooled from each group of mice) were collected in cold cell-culture medium [RPMI-1640, 10% fetal calf serum (FCS), 100 U/mL penicillin and 100 μg/mL streptomycin; GIBCO-BRL, Eggenstein, Germany] and β-mercaptoethanol (3 × 10-5 mol/L; Sigma). Tissues were mechanically disrupted and the cell suspensions were filtered through a cell strainer (70 μm). Tissue-culture plates were coated in part with anti-CD3 (2.5 μg/well), and 2 × 105 cells/well were incubated in 200 μL complete medium containing 10 U/mL IL-2 (Proleukin; Chiron) and in part with soluble anti-CD28 (1 μg/mL) for 24 h. Cytokine levels were measured in the supernatant by ELISA (all from Endogene, Woburne, MA, USA), according to the manufacturer’s instructions.
RNA was extracted from colonic tissue using the RNeasy kit (Qiagen, Hilden, Germany) and transcribed (Promega, Mannheim, Germany). Quantification of cytokine mRNA was performed using a light cycler (Roche, Molecular Systems, Mannheim, Germany). The following primer pair was used for amplification of T-beta: 5'-AGGCTGCCTGCAGTGCTTC-3'and 5'-CTCGCCTGGTGAAATGTGC-3', annealing temperature at 62°C, and 3 mmol/L MgCl2. For IL-10 amplification, the following primer pair was used: 5'-TCCTTAATGCAG GACTTTAAGGGTTACTTG-3'and 5'-GACACCTTG GTCTTGGAGCTTATTAAAATC-3', annealing temperature 62°C, and 3 mmol/L MgCl2. For standardization, β-actin was amplified using the following primer pair: 5'-TGGAATCCTGTGGCATCCATGAAAC-3' and 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'.
Bone-marrow-derived DCs (BM-DCs) were generated as described previously[28]. Briefly, bone marrow was flushed from femurs and tibiae of mice, and cells cultured for 10 d with PRMI (RPMI, 10% FCS, 1% penicillin/streptomycin, 1% l-glutamine, and 0.1% 2-mercaptoethanol) containing 200 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech/Tebu, Germany). Subsequently, BM-DCs were incubated with different concentrations of ZK191784 (10, 100 and 1000 nmol/L) and stimulated overnight with 1 μg/mL LPS (Sigma, Germany). Supernatants were harvested for ELISA and BM-DCs were used for fluorescence-activated cell sorting (FACS) analysis.
For isolation of CD11c+ DCs, spleen and MLNs were excised from ZK191784- or vehicle-treated animals (100 μg/kg per day orally, for 5 d), injected with 100 U/mL collagenase IV in RPMI (Worthington Biochemicals, Lakewood, NJ, USA) and incubated for 30 min at 37°C. After incubation, organs were mechanically dissociated. In the case of the spleen, red blood cells were removed by ACK lysis. CD11c+ DCs were positively selected from single-cell suspensions from the spleen and MLNs using CD11c microbeads and a magnetic cell separation column, according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The resulting DC preparations were > 95% CD11c-positive. Isolated DCs were stimulated overnight with 5 μg/mL phosphothioate-stabilized CpG-ODN with the following sequence: ODN1668 5'-TCCATGACGTTCCTGATGCT-3' (Metabion, Martinsried, Germany). Supernatants were harvested for ELISA and cells were used for FACS analysis.
Cells were analyzed by FACS using two-color staining. Briefly, isolated lymphocytes were incubated with 20 μg/mL of anti-CD16/CD32 mAb and 10% FCS to block Fc receptors, and stained with both fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated mAbs. The cells were washed and analyzed by flow cytometry using an EPICS-XL MCL Coulter. For immunohistochemistry, intestinal tissue samples were snap-frozen in liquid nitrogen embedded in optimal cutting temperature medium and 5- to 10-μm cryostat sections were cut. Incubation with primary antibodies was followed by incubation with biotinylated polyclonal anti-rat IgG or anti-hamster IgG (both Dianova, Germany) mAbs as secondary antibodies. Tissue was stained using the avidin/biotin complex immunoperoxidase kit according to the manufacturer's instructions (Vector Laboratories) and developed with 3-amino-9-ethylcarbazol. Sections were counterstained with hematoxylin/eosin.
Statistical analysis was performed using the Mann-Whitney U test for unpaired samples (histological score, cytokine levels) and Student’s t test (cytokine levels of primary DCs and BM-DCs). Differences were considered statistically significant at P < 0.05 (labeled). For graphical analysis of the data, box plots were used. The box stretches from the lower hinge to the upper hinge and contains the middle half of the scores in the distribution. The median is shown as a line across the box, and the largest value below the upper hinge and the smallest value above the lower inner fence are drawn additionally, indicating the distribution.
As shown previously, vitamin D deficiency leads to severe colitis in IL-10 knockout mice[7]. However, limitations are involved in the study of cytokine-deficient animals, as intestinal inflammation in these mice may not represent a normal immune response, due to the lack of important regulatory cytokines. Therefore, we decided to investigate the effects of ZK191784 in the DSS models of acute and chronic colitis, as results in both models are not impaired by cytokine deficiency.
Administration of ZK191784 to mice before and during induction of acute colitis ameliorated colitis, as demonstrated by a significant reduction in the histological score by 25% compared to control mice (Figure 1A: control, 7.2 ± 0.44 vs ZK191784, 5.4 ± 1 34; P < 0.05), and reflected by a less severe inflammatory infiltrate and reduced epithelial damage, as seen in histological sections of the intestine (Figure 1C). Whether animals were pretreated with the calcitriol analog before induction of colitis or whether application of ZK191784 was started at the time of colitis induction made no difference in the ameliorating effect of the compound. Also, when animals were treated sequentially with the calcitriol analog during induction of chronic colitis, intestinal inflammation improved as well by 27.1% (Figure 1B: control, 7. 2 ± 1. 2 vs ZK191784, 5.25 ± 1. 9; P < 0.05). As demonstrated previously[26], the calcitriol compound did not increase serum levels of calcium in a significant manner, as compared to serum calcium levels in control animals (data not shown).
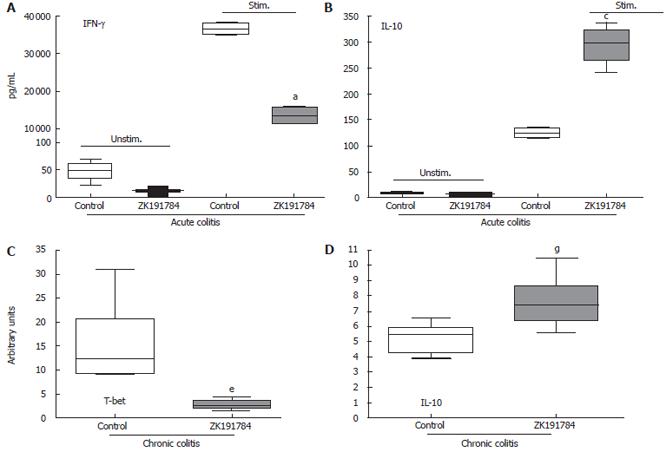
In order to further characterize the differences in colitis severity between ZK191784-treated and control animals, we compared the cytokine secretion in both groups. Therefore, MLNs of diseased animals were harvested at the end of the experiment, and levels of pro- and anti-inflammatory cytokines within the supernatant of MLN cells were measured. The secretion of the proinflammatory cytokines IFN-γ (Figure 2A) and IL-6 (data not shown) by isolated cells was dramatically suppressed by treatment with ZK191784 in acute colitis compared with control mice (IFN-γ, -62.7%; IL-6, -47.4%, P < 0.05), whereas IL-10 secretion increased significantly by 2.3-fold (Figure 2B). Similarly, in chronic colitis, spontaneous IFN-γ secretion was suppressed by ZK191784 (control, 46.5 ± 8.4 pg/mL; ZK191784, 4.2 ± 1.2 pg/mL, P < 0.05). However, in chronic colitis we were not able to detect a significant difference in the secretion of IL-6 or IL-10 by MLN cells derived from ZK191784- or vehicle-treated animals (data not shown).
Th1 cytokines play a proinflammatory role in chronic DSS-induced colitis[29]. Therefore, we evaluated the effects of ZK191784 administration on expression of the Th1-specific transcription factor T-beta in the colonic tissue, using real-time PCR. As shown, the levels of T-beta expression were significantly reduced to 18% compared with those in the control group (P < 0.0001, Figure 2C). Additionally, even if we were not able to detect a significant difference in the secretion of the anti-inflammatory cytokine IL-10, the mRNA-levels of these cytokines increased slightly by 1.4-fold in ZK191784-treated animals (P = 0.00025, Figure 2D).
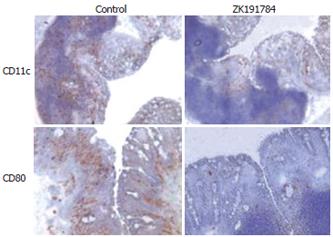
Vitamin D3 is known to interact with antigen-presenting cells and to prevent their activation[30], therefore, we were interested to see whether administration of the calcitriol compound ZK191784 influenced the activation of intestinal DCs within the inflamed colonic mucosa. As shown in Figure 3, immunohistochemical analysis for CD11c+ DCs within the colonic lamina propria revealed a dramatic decrease in cell numbers in intestinal tissue derived from mice treated with ZK191784, as compared to that from control mice. Additionally, when staining for the activation marker CD80 was performed, almost no positive cells were found in the colonic mucosa of calcitriol-treated animals, whereas as expected, in control animals, large numbers of infiltrating CD80+ cells were detected. No changes were observed concerning the numbers of CD3+ T cells (data not shown).
So far, our results indicated that the positive effects of ZK191784 could be mediated by an influence of the compound on antigen-presenting DCs. It has previously been shown that calcitriol inhibits IL-12 production by human monocytes[31]. To determine whether this capacity was shared by ZK191784, the calcitriol analog was tested for its ability to inhibit the secretion of proinflammatory cytokines by BM-DCs that were stimulated with LPS overnight in the presence of different concentrations of ZK191784.
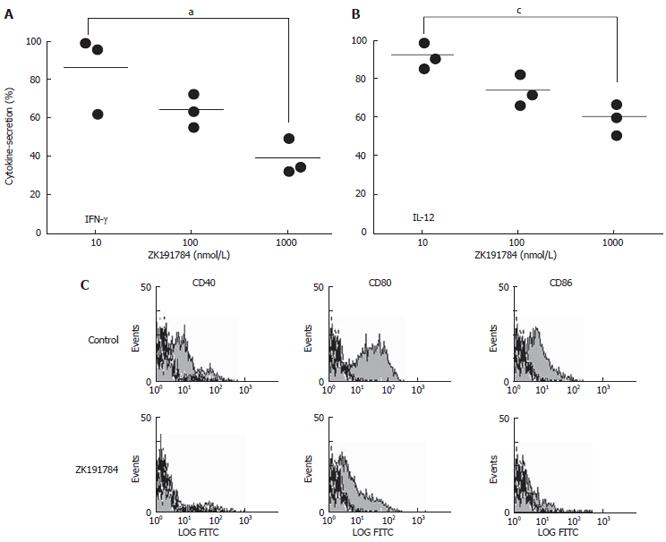
The secretion of IL-10 was not influenced by the presence of the compound (data not shown). However, ZK191784 was able to significantly inhibit the production of the proinflammatory cytokines IFN-γ and IL-12 by 60% and 40%, respectively, in a dose-dependent manner, after stimulation of cells with LPS (Figure 4A and 4B; IFN-γ, P = 0.0227; IL-12, P = 0.0069). Additionally, FACS analysis revealed reduced expression of costimulatory molecules (CD40, CD80 and CD86) after stimulation on the surface BM-DCs exposed to ZK191784 (Figure 4C). Therefore, the presence of the calcitriol analog within cell cultures seems to inhibit the activation of in vitro-derived BM-DCs.
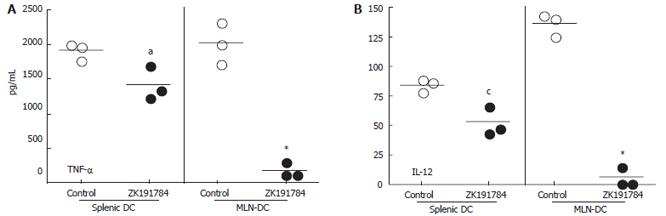
To further evaluate the effects of ZK191784 on DC in vivo, mice were treated with the calcitriol analog or vehicle, and primary CD11c+ DCs were isolated from the spleen and MLNs. FACS analysis revealed no difference in the expression of costimulatory molecules in DCs from different treatment groups. However, after stimulation with CpG in vitro, primary splenic and mesenteric DCs isolated from mice treated with ZK191784 secreted significantly lower levels of proinflammatory cytokines than those derived from control mice (Figure 5A and 5B). TNF-α secretion from splenic DCs from ZK191784-treated animals was reduced to 74% (P = 0.0364) and IL-12 production was decreased to 62%, as compared to control DCs (P = 0.0136). The effect was even more dramatic when secretion of proinflammatory cytokines from mucosal MLN DCs was evaluated. We were able to show that TNF-α and IL-12 levels from ZK191784-treated mice were reduced to 9.0% (P = 0.0005) and 4.4% (P < 0.0001), respectively, compared to cells from control animals. The results suggest that the in vivo effects of the calcitriol analog ZK191784 are more dramatic at mucosal sites of the intestine than in systemic lymphatic tissue.
In our study we extended previous data about the importance of calcitriol in colitis as we demonstrated that administration of ZK191784, a less calcemic analog of calcitriol, ameliorated not only acute but also chronic DSS-induced colitis. In contrast to previous studies, in which the influence of vitamin D deficiency was investigated in IL-10 knockout mice, our results were not obtained in cytokine-deficient animals, but in normal Balb/c mice. Additionally, our data suggested that the effects of the compound were at least partly due to its influence on DCs in the intestinal mucosa, as systemic administration of ZK191784 inhibited the activation of DCs within the spleen, MLNs and intestinal lamina propria by down-modulation of proinflammatory cytokines and inhibition of costimulatory molecule expression on the surface of DCs.
Calcitriol is known to act directly on T cells because vitamin D3 inhibits the proinflammatory transcription factor nuclear factor (NF)κB, and subsequently impairs the expression of proinflammatory cytokines TNF-α and IFN-γ[20]. TNF-α is known to be an important mediator of inflammation in IBD patients[32], and several TNF-α-blockers are effective in IBD[33]. Therefore, any treatment that targets the TNF-α pathway and Th1-associated cytokines is likely to be a possible treatment for intestinal inflammation. Indeed, stimulated splenic and mesenterial DCs produced lower amounts of TNF-α when mice were treated with the calcitriol analog, and secretion of the proinflammatory cytokines IFN-γ and IL-6 by MLN cells was dramatically reduced after ZK191784 administration. On the other hand, protein levels of the anti-inflammatory cytokine IL-10 increased in acute colitis and mRNA levels of these cytokines were also enhanced in the chronic colitis model after application of ZK191784.
In vitro experiments have demonstrated that calcitriol renders DCs in a perpetual state of immaturity, as it down-regulates the expression of MHC class II and costimulatory molecules[34,35] and decreases the capacity of myeloid human DCs to induce Th1-cell development[36]. Also, it inhibits IL-12 production by both macrophages and DCs via inhibition of NFκB activation and transcriptional repression of the IL-12p40 gene[31,36]. The proinflammatory cytokine IL-12 is involved in the pathogenesis of colitis[37], as administration of anti-IL-12 substantially reduces the severity of intestinal inflammation, and mice treated with IL-12 during the application of DSS develop a more severe acute colitis as compared to controls[38]. Therefore, inhibition of IL-12 secretion by antigen-presenting cells can also be an important way to ameliorate disease.
However, so far, it is unclear whether mucosal DCs are also targets for the immunomodulatory activity of calcitriol. Our results demonstrate that infiltrating activated CD11c+ DCs into the intestinal lamina propria were dramatically reduced in number after systemic administration of ZK191784. Additionally, the reduced expression of costimulatory molecules by DCs, accompanied by the inhibition of proinflammatory cytokine secretion, may contribute substantially to decrease DC-dependent T-cell activation within the intestinal mucosa and could largely account for the immunosuppressive properties of this compound on acute and chronic colitis development in our model.
Interestingly, recent data have suggested that in vivo macrophages, DCs and epithelial cells are a potential source of calcitriol[39,40]. Therefore, local calcitriol production may keep antigen-presenting cells in an immature state in the healthy intestinal mucosa, and support the suppression of mucosal Th1 cytokine secretion and T-cell proliferation. This mechanism would help to establish tolerance by inhibition of unnecessary inflammatory responses. It is possible that vitamin D deficiency, either due to malnutrition or malabsorption in patients with IBD, is an additional piece of the puzzle that helps to disturb the normal immunoregulation in the gut. Our data support this hypothesis, as administration of the calcitriol analog was able to ameliorate symptoms of DSS colitis by inhibition of activation of mucosal DCs, as demonstrated by lack of expression of costimulatory molecules that would otherwise drive the inflammatory process.
Another recent study suggests an essential function for DCs in programming lymphocyte homing and microenvironmental positioning[41]. The authors were able to demonstrate that calcitriol that was processed by antigen-presenting DCs upregulated the expression of the chemokine receptor CCR10 on the surface of responding T cells, and enabled them to migrate to the skin. On the other hand, the expression of the gut homing adhesion molecule α4β7 and the intestinal chemokine receptor CCR9 on the T-cell surface was suppressed. Therefore, it is possible that, in addition to changes in the phenotype and number of intestinal DCs, ZK191784 can act via DCs on T cells within the gut by up-regulation of skin homing chemokine receptors and down-modulation of gut homing molecules. This may reduce the number of infiltrating T cells within the gut during colitis and ameliorate severity of intestinal inflammation. Further studies are under way to investigate the expression of chemokine receptors on T cells in response to the calcitriol analog ZK191784.
The data presented by us and others point to a crucial role of calcitriol-regulated processes in IBD. As no interference by vitamin D compounds with the ability of animals to act defensively against opportunistic infections has so far been shown[17], calcitriol may be an attractive treatment strategy compared to other immunosuppressive reagents used in IBD patients. However, so far, development of hypercalcemia limits calcitriol administration. The new calcitriol analog ZK191784 that was used in our study has previously been shown to have reduced calcemic activity and therefore, a more favorable therapeutic profile than calcitriol.
In conclusion, we believe that we showed for the first time that ZK191784, a low-calcemic calcitriol analog, significantly ameliorated acute and chronic DSS-induced colitis, most likely due to inhibition of DC activation that prevented development of proinflammatory pathogenic T cells. This less hypercalcemic calcitriol analog is therefore an attractive immunomodulatory agent with few side effects, which, either alone or in combination with other drugs, may have therapeutic applications in the treatment of IBD.
Chronic IBD in patients still has unknown etiology. However, it is thought that a dysregulated response of antigen-presenting DCs towards bacterial and food antigens within the gut plays a role within the disease process. Currently, treatment of disease consists of immunosuppressive drugs such as steroids and azathioprine, which have significant side effects. We investigated the effects of a calcitriol analog with few side effects for the treatment of murine colitis. Additionally, we were able to show that the calcitriol analog influenced the phenotype and number of DCs within the gut.
Besides genetic factors that are thought to predispose individuals to develop IBD, the environment seems to contribute to the disease. There are reasons to believe that vitamin D may be an environmental factor that affects colitis, since vitamin D deficiency has been linked to IBD, even when the disease is in remission. Additionally, vitamin D deficiency has been shown to accelerate the development of colitis symptoms among mice that develop spontaneous enterocolitis. It has been shown that calcitriol selectively regulates the immune response without compromising the host’s ability to fight infection, which makes it an attractive therapeutic option compared to standard immunosuppressive medication in patients with chronic colitis. However, calcitriol has clear dose-limiting hypercalcemic effects that interfere with its systemic clinical use, due to a strong influence on calcium homeostasis and the risk of associated side effects.
We were able to show that treatment with ZK191784, a low-calcemic calcitriol analog, resulted in significant amelioration of murine colitis in acute and chronic intestinal inflammation. The down-regulation of colonic inflammation was associated with a dramatic reduction in the secretion of proinflammatory cytokines and a significant increase in anti-inflammatory mediators by cells within local lymph nodes. Additionally, lower numbers of infiltrating activated DCs were found in the colon in mice that were treated with the calcitriol analog, and the secretion of proinflammatory cytokines by mucosal DCs was inhibited in the presence of the calcitriol analog.
We were able to show, as far as we are aware, for the first time that a low-calcemic calcitriol analog significantly ameliorated acute and chronic murine colitis. Inhibition of DC activation prevented development of proinflammatory pathogenic T cells. This calcitriol analog is therefore an attractive immunomodulatory agent with few side effects, which, either alone or in combination with other drugs, may have therapeutic applications in the treatment of IBD.
IBD: Inflammatory bowel disease, chronic recurrent colitis in patients with an autoimmune background. DCs: Dendritic cells, cells that capture foreign antigens and present them to lymphocytes, therefore inducing an immune reaction.
This paper by Strauch et al investigated the properties of ZK191784, a derivative analog of calcitriol (activated form of vitamin D3), with potentially fewer side effects and similar inhibition of T-cell function, to regulate colitis in an experimental mouse model. The results suggest that treatment with this inhibitor decreased the severity of acute and chronic colitis in this model.
S- Editor Zhu LH L- Editor Kerr C E- Editor Lu W
| 1. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1348] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 2. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3905] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 3. | Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S-11S. [PubMed] |
| 4. | Andus T, Gross V. Etiology and pathophysiology of inflammatory bowel disease--environmental factors. Hepatogastroenterology. 2000;47:29-43. [PubMed] |
| 5. | Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn's disease. Can J Gastroenterol. 2003;17:473-478. [PubMed] |
| 6. | Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, Hara K, Ookubo K, Yamao K, Kato M. Risk factors for vitamin D deficiency in patients with Crohn's disease. J Gastroenterol. 2004;39:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648-2652. [PubMed] |
| 8. | Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005;35:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Daniel C, Radeke HH, Sartory NA, Zahn N, Zuegel U, Steinmeyer A, Stein J. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. J Pharmacol Exp Ther. 2006;319:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Bemiss CJ, Mahon BD, Henry A, Weaver V, Cantorna MT. Interleukin-2 is one of the targets of 1,25-dihydroxyvitamin D3 in the immune system. Arch Biochem Biophys. 2002;402:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 488] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974-4980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 867] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 16. | Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3). Arch Biochem Biophys. 2000;377:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Cantorna MT, Hullett DA, Redaelli C, Brandt CR, Humpal-Winter J, Sollinger HW, Deluca HF. 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation. 1998;66:828-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Van Etten E, Decallonne B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C. Analogs of 1alpha,25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem. 2003;88:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Stio M, Martinesi M, Bruni S, Treves C, d'Albasio G, Bagnoli S, Bonanomi AG. Interaction among vitamin D(3) analogue KH 1060, TNF-alpha, and vitamin D receptor protein in peripheral blood mononuclear cells of inflammatory bowel disease patients. Int Immunopharmacol. 2006;6:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Stio M, Bonanomi AG, d'Albasio G, Treves C. Suppressive effect of 1,25-dihydroxyvitamin D3 and its analogues EB 1089 and KH 1060 on T lymphocyte proliferation in active ulcerative colitis. Biochem Pharmacol. 2001;61:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314-5319. [PubMed] |
| 23. | Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2710] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 24. | Penna G, Giarratana N, Amuchastegui S, Mariani R, Daniel KC, Adorini L. Manipulating dendritic cells to induce regulatory T cells. Microbes Infect. 2005;7:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004;89-90:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Zügel U, Steinmeyer A, Giesen C, Asadullah K. A novel immunosuppressive 1alpha,25-dihydroxyvitamin D3 analog with reduced hypercalcemic activity. J Invest Dermatol. 2002;119:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Schölmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2380] [Cited by in RCA: 2515] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 29. | Obermeier F, Kojouharoff G, Hans W, Schölmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 263] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Adorini L, Giarratana N, Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin Immunol. 2004;16:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol. 2000;30:498-508. [PubMed] |
| 32. | Ganesan S, Travis SP, Ahmad T, Jazrawi R. Role of tumor necrosis factor in Crohn's disease. Curr Opin Investig Drugs. 2002;3:1297-1300. [PubMed] |
| 33. | Rutgeerts PJ, Targan SR. Introduction: anti-TNF strategies in the treatment of Crohn's disease. Aliment Pharmacol Ther. 1999;13 Suppl 4:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Griffin MD, Kumar R. Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem. 2003;88:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 37. | Tozawa K, Hanai H, Sugimoto K, Baba S, Sugimura H, Aoshi T, Uchijima M, Nagata T, Koide Y. Evidence for the critical role of interleukin-12 but not interferon-gamma in the pathogenesis of experimental colitis in mice. J Gastroenterol Hepatol. 2003;18:578-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Kojouharoff G, Hans W, Obermeier F, Männel DN, Andus T, Schölmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Hewison M, Gacad MA, Lemire J, Adams JS. Vitamin D as a cytokine and hematopoetic factor. Rev Endocr Metab Disord. 2001;2:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 465] [Article Influence: 25.8] [Reference Citation Analysis (0)] |