Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.532
Revised: July 25, 2005
Accepted: October 10, 2005
Published online: January 28, 2007
AIM: To perform a long culture passage of H pylori without serum, taking into account its cytotoxicity and the presence of the probable new cytotoxic factor.
METHODS: One sample of H pylori 60190 (ATCC 49503) was grown on Brain Heart Infusion (BHI) agar containing 0.5% 2,6-di-O-methyl-β-cyclodextrin without any serum, being passaged 70-100 times every 3-4 d for approximately 2 h, while another sample of H pylori contained 70 mL/L fetal calf serum without 2,6-di-O-methyl-β-cyclodextrin. Their supernatant and extract after 16 h in culture were evaluated for changes in cell morphology and for cell viability using HeLa cells. Furthermore, the characteristics of the probable cytotoxic factor in the extract were examined on partial purification studies and its cytotoxicity was evaluated in various human cells.
RESULTS: The supernatant and the extract of the bacterium grown on serum-free medium had strong cytotoxicity compared with those grown on serum-containing medium. They irreversibly damaged HeLa cells without vacuolation that was altogether different from that of the bacterium when grown with serum. Their cytotoxicity was easily measured by cell viability assay. The probable cytotoxic factor partially purified and detected by chromatography had characteristics difference from that of vacuolating toxin and a broad cytotoxicity toward various cell lines.
CONCLUSION: Serum-free long culture method of H pylori makes its supernatant and its extract cytotoxic enough to be easily measured by cell viability assay. The probable cytotoxic factor has a unique characteristic and might be a new cytotoxin.
-
Citation: Ohno H, Murano A. Serum-free culture of
H pylori intensifies cytotoxicity. World J Gastroenterol 2007; 13(4): 532-537 - URL: https://www.wjgnet.com/1007-9327/full/v13/i4/532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.532
Irreversible degeneration and cell death were induced in gastric mucous epithelium infected with H pylori both in an in vitro and an in vivo study, including electron microscopic examination. These cells deteriorated without vacuolations, and biopsy specimens containing the organism obtained from gastric mucosa demonstrated a loss of microvilli and irreversible cell damage[1-4]. Even after eradication therapy of the bacterium, there were no significant differences in the histological scores and atrophic scores in the inflammation of the gastric body and the atrophy of the antrum[5-9]. However, the only known cytotoxic factor of H pylori is vacuolating toxin (Vac A), which causes vacuolations in assay cells 18-24 h after addition, but is a reversible and weak cytotoxin[10,11]. The clinical findings that the bacterium caused irreversible and severe destruction to the gastric lineage appear incompatible with the in vitro observations that H pylori produces only a reversible and comparatively weak cytotoxin that induces vacuolations. Whether there is any direct irreversible cytotoxic factor of H pylori remains to be determined. Although, so far, none of the studied factors met these requirements, there is some evidences that indicated cytoskeletal cell death or round-formed cell death differed from vacuolation-formed cell death[3,12,13].
To our best of knowledge, this is the first report about the cytotoxicity of H pylori after a long duration of culture without serum. We cultured H pylori 60 190, plating many passages without serum for approximately two years, considering that the cell growth environments should be as near as possible to the actual intragastric ones. Here, we showed serum-free long period culture made the supernatant and the extract of H pylori dramatically cytotoxic such that it induced irreversible decayed cell death easily measurable with a cell viability assay. Cell death induced by these samples showed a rapid development of the cell death process and was rarely accompanied by vacuolation. These observations might explain clinical findings and develop into a new field of the bacterium’s cytotoxicity.
The H pylori strain 60 190 was obtained from American Type Culture Collection. Brain Heart Infusion (BHI) was purchased from DIFCO Laboratories and 2,6-di-O-methyl-β-cyclodextrin was purchased from Wako Pure Chemical Industries Ltd.
H pylori 60190 (ATCC49503) was the source for purification. Bacterial cells were grown on Brain Heart Infusion (BHI) agar containing 5% fetal calf serum for 7-10 d, then transferred to BHI broth containing 2,6-di-O-methyl-β-cyclodextrin with gradual stepwise decreases, i.e., 5%, 2%, 1%, 0.5%, of the concentration for 2-4 d without serum and maintaining stable growth conditions, being passaged 100-120 times every 3-4 d. Then bacterial cells were scraped and cultured for 16 h at 37°C in the liquid medium of BHI containing 0.5% 2,6-di-O-methyl-β-cyclodextrin without serum in an ambient atmosphere containing 50 mL/L CO2, while agitating with a rotary shaker. Bacterial cells were collected by centrifugation at 12 000 g for 20 min at 4°C and suspended in 10 mmol/L Tris-HCl buffer (pH 7.7). The cells were washed once and sonicated at 54% effect for 30 s × 6 sets in Ultrasonic Disruptor (Tomy Seiko Co., Ltd.) and stored at -80°C overnight. Sonically disrupted cells were thawed and sonicated again under the conditions previously described and centrifuged at 100 000 g for 60 min at 4°C. The aliquots of the most upper layer were stored at -80°C until use.
HeLa cells (Cell Resource Center for Biomedical Research, Tohoku University) cultured in Eagle’s modified minimal essential medium containing 100 mL/L fetal bovine serum (MEM-FBS) were seeded into 96-well plates at a density of 104 cells per well. After 24 h, the cells were washed twice with PBS and challenged with 10 μL of aliquots of serial fraction or serial dilution of protein solution with 90 μL of MEM-FBS for a total volume of 100 μL per well. Cells were incubated at 37°C for 24 h and cell viability was evaluated by WST-1 assay using Cell Counting kits (DOJINDO Laboratories). Each determination was performed in triple wells.
Proteins in the bacterial extract were precipitated with a 700 g/L saturated solution of ammonium sulfate. After centrifugation at 12 000 g for 20 min, the pellets were resuspended in 10 mmol/L Tris-HCl buffer (pH 7.7) and dialyzed against the same buffer.
The anion exchange chromatography was performed on DEAE Sephacel (Amersham Pharmacia Biotech UK Ltd.) with 10 mmol/L Tris-HCl buffer (pH 7.7). The cation exchange chromatography was CM Sepharose Fast Flow (Amersham Pharmacia Biotech UK Ltd.) with 10 mmol/L Tris-HCl buffer (pH 5.8). The proteins were eluted with the same buffer containing a linear gradient of 0-0.3 mol/L NaCl and 0-1.0 mol/L NaCl, respectively. Size exclusion chromatography was performed on a Superose 12 HR 10/30 column (Pharmacia) with buffer containing 10 mmol/L Tris-HCl (pH 7.7) and 0.15 mol/L NaCl at a flow rate of 0.25 mL/min.
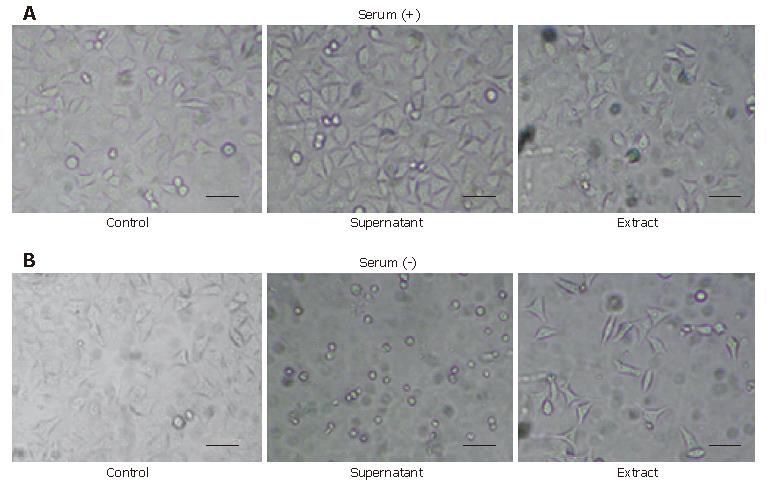
We cultured H pylori 60190 with or without serum for approximately two years. The serum-free culture method using 0.5% 2,6-di-O-methyl-β-cyclodextrin was less favorable for the bacterium and made it inclined to be easily autolysed in the culturing process. In this study, we defined the serum-free method to culture the bacterium using Brain Heart Infusion (DIFCO) and 0.5% 2,6-di-O-methyl-β-cyclodextrin without serum for approximately two years in semisolid medium, and the conventional method using Brain Heart Infusion and 70 mL/L serum without cyclodextrin in semisolid medium. After more than approximately 100 passages, both the supernatant and the extract of the bacterium cultured with the serum-free method induced characteristic morphological changes in HeLa cells, compared to the supernatant and the extract cultured using serum; the cells exposed to its supernatant showed a round-formed shape or small debris with a destroyed contour without vacuolation and the cells exposed to its extract showed an elongated or spindle-like shape with a decrease in cell number in 24 h, while the control cells exposed to the supernatant and the extract had scarcely changed, except for some vacuolations that were similar to the previously reported morphological change (Figure 1).
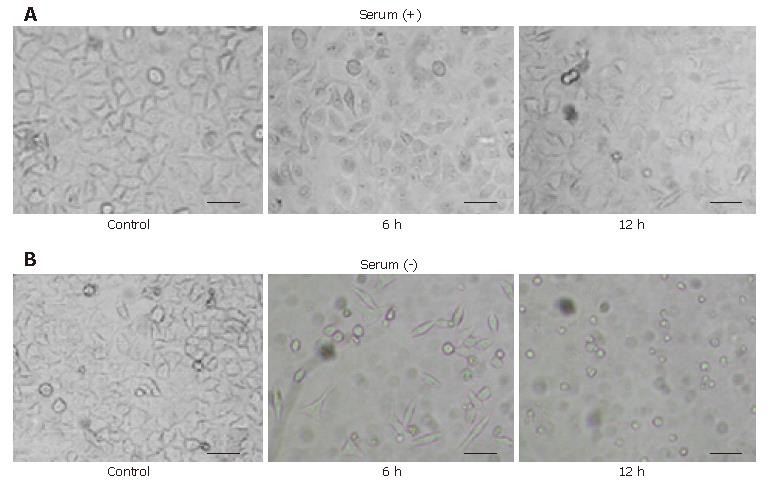
We observed that both HeLa cells exposed to the supernatant and the extract of the bacterium cultured without serum showed the round-formed change or round debris through the elongated and spindle-like shape without vacuolation. We examined whether HeLa cells exposed to the extract had undergone the same changes using a denser extract. We found that both of the HeLa cells exposed to the supernatant and the extract finally showed the round-formed or shrunken-formed change through the elongated or spindle-like form after 24 h. We compared the morphologic change of HeLa cells exposed to the extract cultured with serum and cultured for approximately one year without serum (Figure 2), and considered that both contained probably a similar cytotoxic factor.
The conventional search of cytotoxin in H pylori used the uptake of neutral red into the vacuole of sample-treated cells because the supernatant of the bacterium caused some vacuolations in cells[14], but scarcely any degeneration or remarkable decrease of the number of assay cells. No direct cell viability assay, therefore, was used in search of the cytotoxic factor of H pylori. However, we were able to successfully adopt this cell viability assay in place of the neutral red uptake method because of the radical cytotoxicity of the supernatant and the extract cultured without serum.
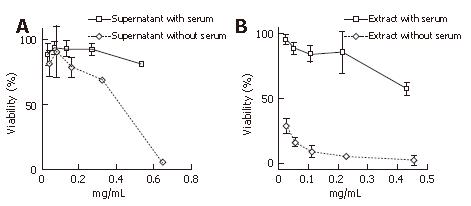
The cytotoxicity was determined with an assay using HeLa cell viability with WST-1 tetrazolium and a lactate dehydrogenase release assay. This cell viability assay revealed the intense cytotoxicity toward HeLa cells, as we predicted. Both the supernatant and the extract cultured without serum had a greater cytotoxicity toward HeLa cells than the ones cultured with serum. The extract, especially of that cultured without serum, had a far greater cytotoxicity than that cultured with serum (Figure 3). This cytotoxicity was thought to be irreversible because the viability assay used measured the intracellular enzyme in the dead or destroyed cells. No cell reversibility could be ascertained for certain when we removed the supernatant and the extract from the cells at 6 h and 12 h after the addition and replaced it with the control lysate for a further 24 h observation (data not shown).
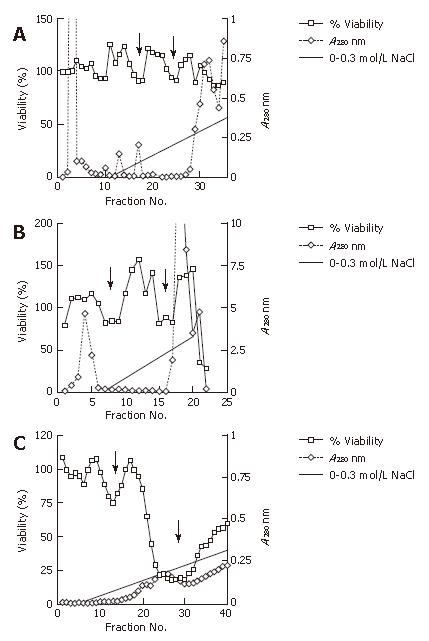
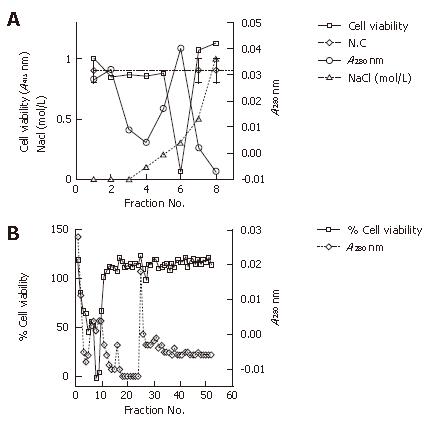
We pursued the growing cytotoxicity of the extract using a cell viability assay and anion exchange chromatography in accordance to the passage time without serum. The extract at the onset of culturing without serum showed two minor dips of cell viability at approximately 0.1 mol/L NaCl and 0.2 mol/L NaCl with gradient elution, respectively. The extract after approximately 6 mo of culture (approximately 50 passages) from the onset indicated clearer minor dips at the same concentration of the NaCl linear gradient elution; while approximately one year (approximately 100 passages) from its onset without serum, the extract had a major dip at 0.2 mol/L NaCl and a minor dip at 0.1 mol/L NaCl with far less elution protein. We found that the putative cytotoxic factor had an isoelectric point less than pH 7.7 due to the use of the buffer with pH 7.7 (Figure 4).
In addition, we examined the extract cultured approximately one year (approximately 100 passages) from its onset without serum using cation exchange chromatography and size exclusion chromatography. Cation exchange chromatography showed one clear dip at around 0.3 mol/L NaCl in the gradient elution that indicated that the putative cytotoxic factor had an isoelectric point of more than pH 5.8 due to the use of the buffer with pH 5.8. Size exclusion chromatography showed a sharp dip at around 37-45 ku, which was different from the molecular mass of the vacuolating toxin (87 ku) (Figure 5).
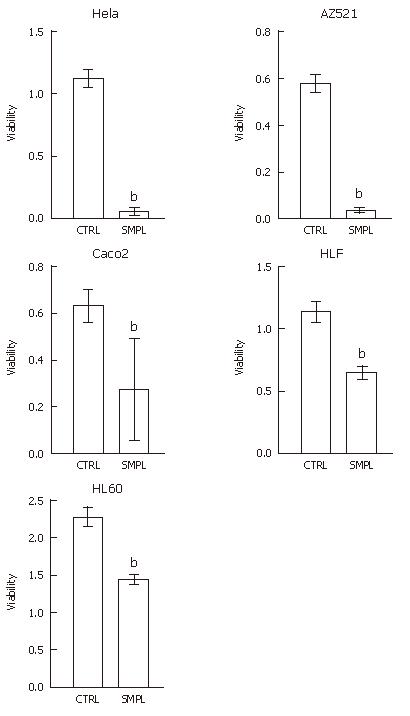
These extracts partially purified by anion exchange chromatography showed robust cytotoxicity toward HeLa cells and other human cells, including AZ521 (gastric carcinoma cells), HLF (hepatocellular carcinoma cells), Caco2 (colorectal carcinoma cells), and HL60 (promyeloblastic leukemia cells) (Figure 6).
This is probably the first report about serum-free long culture method of H pylori. We showed that the established strain, H pylori 60 190, could be converted to a far more cytotoxic strain with a serum-free long culture method that was altogether different from that obtained using conventional culture methods including serum. This cytotoxicity was acquired by the long process of culturing it without serum. Both the supernatant and the extract cultured without serum caused cytotoxicity toward HeLa cells that was distinctly different from the cytotoxicity of H pylori cultured using serum of the conventional method to help the feeble growth of H pylori. The conventional method using serum certainly may ultimately help the growth state[15] but was considered to be very much unlike the actual growing environment. 2,6-Di-O-methyl-β-cyclodextrin (CD) is considered to reduce the toxic effect of fatty acids as well as bovine serum albumin (BSA)[16,17]. Although blood and serum may contain growth-stimulatory factors required by the organism[14], the strain 60 190, we used, grew in media with CD without any serum, but did not grow in media without sera or CD as the other strains were reported[18].
The supernatant of the serum-free culture was reported to cause epithelial cytoskeletal disruption[13] after as little as 48 h culture, while 72 h culture decreased the apoptotic signaling of cells, as compared to serum-containing cultures[19]. Serum-free culture method may be less favorable to the organism but more useful with the research of its cytotoxic factors.
Most in vitro studies hitherto have used H pylori strains cultured using serum, and if any serum-free culture of the bacterium was used for a short time[10,14], it may have been that the supernatant caused the morphological cytotoxic change with vacuolations and without any remarkable cell viability loss or any decrease of assay cell number. However, we discovered the supernatant and the extract of the bacterium cultured for long periods of time without serum had a different cytotoxicity in both the cell morphology and the activity that scaled up with the passage duration for a year or more, Furthermore, the shape of the organism cultured in serum-free medium had seemed to be more cocoid form than that would be in serum-containing medium.
In addition, our chromatography study showed that putative cytotoxic factor had an isoelectric point between pH 5.8 to 7.7, with a molecular mass of approximately 37-45 ku, which was different from that of the vacuolating toxin (87 ku). The anion exchange chromatography study appeared to indicate two cell-viability dips, a minor dip and a major dip. Further investigation will be required to determine the number of possible factors involved.
This experimental method might represent a state closer to that of the actual intragastric state, rather than that obtained by using conventional serum-using studies. The experimental method reported herein may also produce results more closely related to clinical observations, which would redress the conventional concept that H pylori has only a weak and reversible cytotoxin. H pylori might, in fact, be more insidious and more fierce than the researchers had ever conceived.
Our result may provide a new approach to the cytotoxin research and elucidate a pathological mechanism in H pylori using this new method, which is different from that of the vacuolating toxin, the only presently known cytotoxin.
We thank Ohno M and Tamanoi I for constructive comments.
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
| 1. | Tricottet V, Bruneval P, Vire O, Camilleri JP, Bloch F, Bonte N, Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Taniguchi Y, Ido K, Kimura K, Satoh K, Yoshida Y, Takimoto T, Kihira K, Ookawara S, Mato M. Morphological aspects of the cytotoxic action of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6 Suppl 1:S17-S21. [PubMed] |
| 3. | Wang XM, Kojima T, Satoh K, Taniguchi Y, Tokumaru K, Saifuku K, Seki M, Kihira K, Ido K, Uchida JY. The value of LYM-1 cells for examining vacuole formation and loss of cell viability induced by culture supernates of Helicobacter pylori. J Med Microbiol. 1997;46:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, Dixon MF. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 207] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Yamada T, Miwa H, Fujino T, Hirai S, Yokoyama T, Sato N. Improvement of gastric atrophy after Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2003;36:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tucci A, Poli L, Tosetti C, Biasco G, Grigioni W, Varoli O, Mazzoni C, Paparo GF, Stanghellini V, Caletti G. Reversal of fundic atrophy after eradication of Helicobacter pylori. Am J Gastroenterol. 1998;93:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Forbes GM, Warren JR, Glaser ME, Cullen DJ, Marshall BJ, Collins BJ. Long-term follow-up of gastric histology after Helicobacter pylori eradication. J Gastroenterol Hepatol. 1996;11:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Annibale B, Aprile MR, D'ambra G, Caruana P, Bordi C, Delle Fave G. Cure of Helicobacter pylori infection in atrophic body gastritis patients does not improve mucosal atrophy but reduces hypergastrinemia and its related effects on body ECL-cell hyperplasia. Aliment Pharmacol Ther. 2000;14:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Satoh K, Kimura K, Takimoto T, Kihira K. A follow-up study of atrophic gastritis and intestinal metaplasia after eradication of Helicobacter pylori. Helicobacter. 1998;3:236-240. [PubMed] |
| 10. | Cover TL, Halter SA, Blaser MJ. Characterization of HeLa cell vacuoles induced by Helicobacter pylori broth culture supernatant. Hum Pathol. 1992;23:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Yahiro K, Niidome T, Hatakeyama T, Aoyagi H, Kurazono H, Padilla PI, Wada A, Hirayama T. Helicobacter pylori vacuolating cytotoxin binds to the 140-kDa protein in human gastric cancer cell lines, AZ-521 and AGS. Biochem Biophys Res Commun. 1997;238:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136-15141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Bebb JR, Letley DP, Rhead JL, Atherton JC. Helicobacter pylori supernatants cause epithelial cytoskeletal disruption that is bacterial strain and epithelial cell line dependent but not toxin VacA dependent. Infect Immun. 2003;71:3623-3627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570-10575. [PubMed] |
| 15. | Shahamat M, Mai UE, Paszko-Kolva C, Yamamoto H, Colwell RR. Evaluation of liquid media for growth of Helicobacter pylori. J Clin Microbiol. 1991;29:2835-2837. [PubMed] |
| 16. | Hazell SL, Graham DY. Unsaturated fatty acids and viability of Helicobacter (Campylobacter) pylori. J Clin Microbiol. 1990;28:1060-1061. [PubMed] |
| 17. | Khulusi S, Ahmed HA, Patel P, Mendall MA, Northfield TC. The effects of unsaturated fatty acids on Helicobacter pylori in vitro. J Med Microbiol. 1995;42:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli PF, Abate L, Esposito E, de Gregorio L, Aziz J. Growth of Helicobacter pylori in media containing cyclodextrins. J Clin Microbiol. 1993;31:160-162. [PubMed] |
| 19. | Shibayama K, Doi Y, Shibata N, Yagi T, Nada T, Iinuma Y, Arakawa Y. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun. 2001;69:3181-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |