Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5226
Revised: July 6, 2007
Accepted: August 10, 2007
Published online: October 21, 2007
AIM: To investigate the effects of propolis on bacterial translocation and ultrastructure of intestinal morphology in experimental obstructive jaundice.
METHODS: Thirty Wistar-Albino male rats were randomly divided into three groups, each including 10 animals: groupI, sham-operated; group II, ligation and division of the common bile duct (BDL); group III, BDL followed by oral supplementation of propolis 100 mg/kg per day. Liver, blood, spleen, mesenteric lymph nodes, and ileal samples were taken for microbiological, light and transmission electron microscopic examination on postoperative 7th d after sacrification.
RESULTS: The mean number of villi per centimeter and mean mucosal height of the propolis group were significantly different in the BDL group (P = 0.001 and 0.012, respectively). The electron microscopic changes were also different between these groups. Sham and BDL + propolis groups had similar incidence of bacterial translocation (BT). The BDL group had significantly higher rates of BT as compared with sham and BDL + propolis groups. BT was predominantly detected in MLNs and the most commonly isolated bacteria was Escherichia coli.
CONCLUSION: Propolis showed a significant protective effect on ileal mucosa and reduced bacterial translocation in the experimental obstructive jaundice model. Further studies should be carried out to explain the mechanisms of these effects.
- Citation: Sabuncuoglu MZ, Kismet K, Kilicoglu SS, Kilicoglu B, Erel S, Muratoglu S, Sunay AE, Erdemli E, Akkus MA. Propolis reduces bacterial translocation and intestinal villus atrophy in experimental obstructive jaundice. World J Gastroenterol 2007; 13(39): 5226-5231
- URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5226
The cytotoxicity of bile salts and the toxicity of high levels of intracellular bilirubin are suspected as mediators of some of the systemic consequences of obstructive jaundice. Recent data suggest that more complex mechanisms involving changes in gut flora, mucosal integrity, and macrophage-immune system interactions may be responsible for the complications of obstructive jaundice[1].
The gastrointestinal tract is not only a passive organ of nutrient absorption, but it additionally displays important endocrine, immunologic, metabolic, and barrier functions[2]. Bacterial translocation is the migration of bacteria or bacterial products from the intestinal lumen to mesenteric lymph nodes or other extraintestinal organs and sites[3]. Obstructive jaundice impairs intestinal barrier function leading to bacterial and endotoxin translocation in experimental and clinical studies. It affects the three levels of gut barrier globally; the immune barrier, the biological barrier, and and the mechanical barrier[2]. Moreover, biliary obstruction in the rats results in a significant depression of the reticuloendothelial system (RES) phagocytic function, which may cause impaired systemic bacterial clearance and is associated with decreased survival following E. coli endotoxemia[4].
Propolis is a natural product collected by honey bees from various plant sources. It has antibacterial, antifungal, scolicidal, antiinflammatory, antioxidant, immunomodulatory, antiviral and anticarcinogenic properties[5-13].
According to these properties, we planned to use propolis for determining the effects on bacterial translocation and the ultrastructure of intestinal morphology in experimental obstructive jaundice.
Thirty Wistar-Albino male rats, weighing 250 ± 25 g, were housed under constant temperature (21°C ± 2°C) individually in wire cages with a 12 h light-dark cycle. Twelve hours before anesthesia, animals were deprived of food, but had free access to water two hours before anesthesia. No enteral or parenteral antibiotics were administered at any time. The rats that died during the experiment were excluded from the experiment and no new rat was included. The procedures in this experimental study were performed in accordance with the National Guidelines for The Use and Care of Laboratory Animals and approved by Animal Ethics Committee of Ankara Research and Training Hospital.
Rats were randomly divided into three groups, each including 10 animals: groupI, sham-operated; group II, ligation and division of the common bile duct (BDL); group III, BDL followed by oral supplementation of propolis 100 mg/kg per day (Balparmak LTD, Istanbul, Turkey) with a nasogastric tube that was inserted daily and taken off after propolis supplementation. The first dose of propolis was given on postoperative d 1 and continued until the rats were sacrificed. Animals were sacrificed on postoperative d 7 by high-dose diethyl ether inhalation. Liver, blood, spleen, mesenteric lymph nodes, and ileal samples were taken for microbiological, light and transmission electron microscopic examination.
There is no standard dose for propolis based on previous experimental studies. In previous studies the dose ranged between 30-200 mg/kg per day[14,15]. We gave propolis at a dose of 100 mg/kg per day to each rat.
Animals were anesthetized by intramuscular injection of 30 mg/kg ketamine hydrochloride (Ketalar®; Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylasine (Rompun®, Bayer, Istanbul, Turkey). Midline laparotomy was performed under sterile conditions. In the sham-operated group (groupI) the common bile duct (CBD) was freed from the surrounding soft tissue and was manipulated without ligation and transection. In group II and III, CBDs of the rats were identified, double ligated with 5-0 silk, and divided between the ligatures. The same surgeon performed all procedures. The abdominal incisions were closed in two layers with continuous 3-0 silk sutures. Animals were allowed to feed after the operation.
The mesenteric lymph nodes, spleen and liver were chopped with sterile instruments under aseptic conditions. Then the tissue samples were weighed and placed in tubes containing 1.5 mL broth (thioglycollate, Oxoid, UK) and homogenized. Subsequently, 0.01 ml tissue samples were inoculated on blood agar (Oxoid, UK) and Levine Eosine Methylene Blue (EMB) agar (Oxoid, UK). Plates were incubated at 37°C for examination of bacterial growth. The growth of bacteria in quantitative culture was observed at 24 and 48 h.
One mL blood samples taken from inferior vena cava of rats were inoculated on the media of aerobic and anaerobic blood cultures. The aerobic and anaerobic blood cultures were observed by incubation in BACTEC 9240 blood culture system (Becton Dickinson, USA) at 37°C for seven days. Samples taken from the blood culture bottle, which gives a positive signal, was cultured by inoculating on blood agar and EMB agar. The subcultures were inoculated at 37°C under aerobic and anaerobic conditions and examined at 24 and 48 h.
For light microscope analyses, tissue samples from the terminal ileum were obtained from all animals. In order to avoid mucosal suffering, the intestinal lumen was carefully cannulated and gently washed with normal saline solution before the sampling. The ileal samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm by Leica RM 2125 RT, and stained with hematoxylin and eosin (HE) for routine light microscopic examination. Histopathological examinations were performed by a pathologist who was blinded to the study design and photographs were taken with Nikon Eclipse E 600. The number of villi per centimeter (V/cm) and the total mucosal thickness (measured from the tip of the villus to the muscularis mucosa) were assessed in all groups. The mucosal thickness was measured in a minimum of 20 well-preserved villi in each randomly selected sample from each tissue block.
For transmission electron microscopic (TEM) analyses, samples were fixed with phosphate buffered (pH: 7.3) 2.5% glutaraldehyde and 2% PFA mixture solution for 2 h at room temperature. They were washed with phosphate-buffered saline solution (PBS) (pH: 7.3) and were fixed with 1% osmium tetraoxide for 2 h as secondary fixation. After washing, they were embedded in Araldite 6005 and were cut with Leica EM FCS (Vien-Austria) ultramicrotome. 1 μm semi-thin sections were stained with Toluidin blue-Azur II to select the region of interest for the following procedures. 60-70 nm thin sections were stained with uranil acetate and lead citrate. They were examined and photographed using a LEO 906 E TEM (80 kV-Oberkochen-Germany).
Differences between the numbers of positive cultures from the groups were evaluated by Chi-square test and P values of less than 0.05 were considered to be significant. Scores of total mucosal thickness and number of villi per centimeter were presented as mean ± SD and compared by One-Way ANOVA or Kruskal-Wallis variance analysis. If the P values of the variance analyses were statistically significant, differences between groups were analysed with the Mann-Whitney U test. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS), version 13.0 for Windows (SPSS Inc., Chicago, USA). P < 0.05 was considered to be statistically significant.
All rats were sacrificed on postoperative d 7. Two rats from group II (BDL group) and one from group III (BDL + propolis group) died in the early postoperative period probably due to anesthesia.
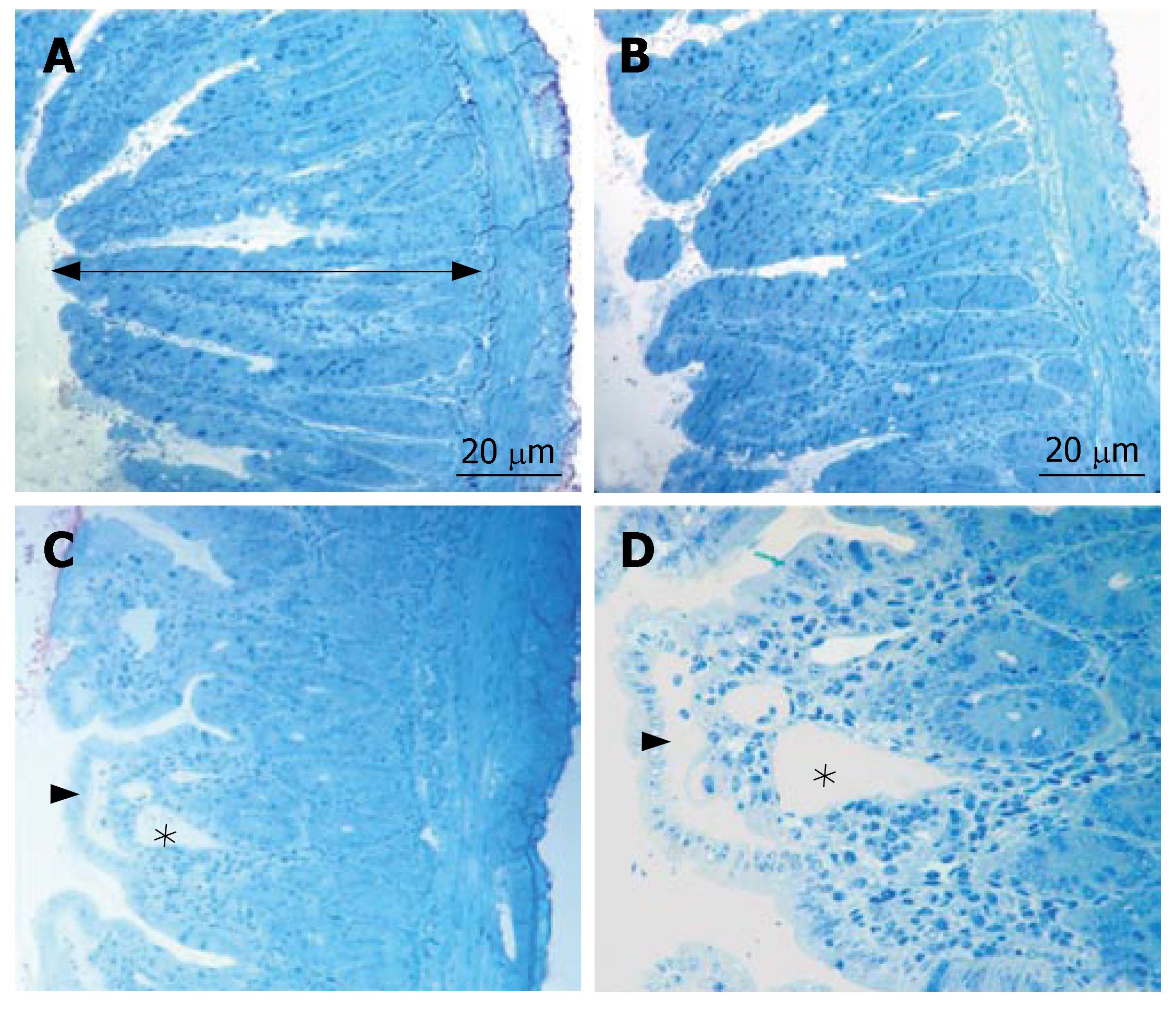
In all specimens of the sham group, the histologic features showed regular appearence of ileal tissue. When we evaluated the specimens systematically, including assessment of villous architecture, surface and crypt epithelia, lamina propria constituents and submucosal structures, no alteration was found in the sham group (Figure 1A). Non specific morphological abnormalities were evident in the intestinal mucosa of the BDL group. The specimens of the BDL group presented villous blunting associated with reduced mucosal thickness. We indicated subepithelial edema mostly located at the tip of the villi, but also extended throughout the villus, with the epithelial layer moderately lifted from the lamina propria. We observed that the crypts were generally preserved. The number of villi per centimeter (V/cm) (villus density) was decreased in the BDL group (Figure 1B). In group III, the subepithelial edema still existed, but villous blunting was not evident. Further, the crypts generally appeared to be preserved (Figure 1C). Although the number of villi per centimeter and the height of the mucosa were higher in the sham group, there was no statistically significant difference between sham and propolis groups (P > 0.05). On the other hand, there was a statistically significant difference between the BDL group and other groups (P < 0.05). The mean number of villi per centimeter and mean mucosal height of the groups are given in Table 1 and Table 2.
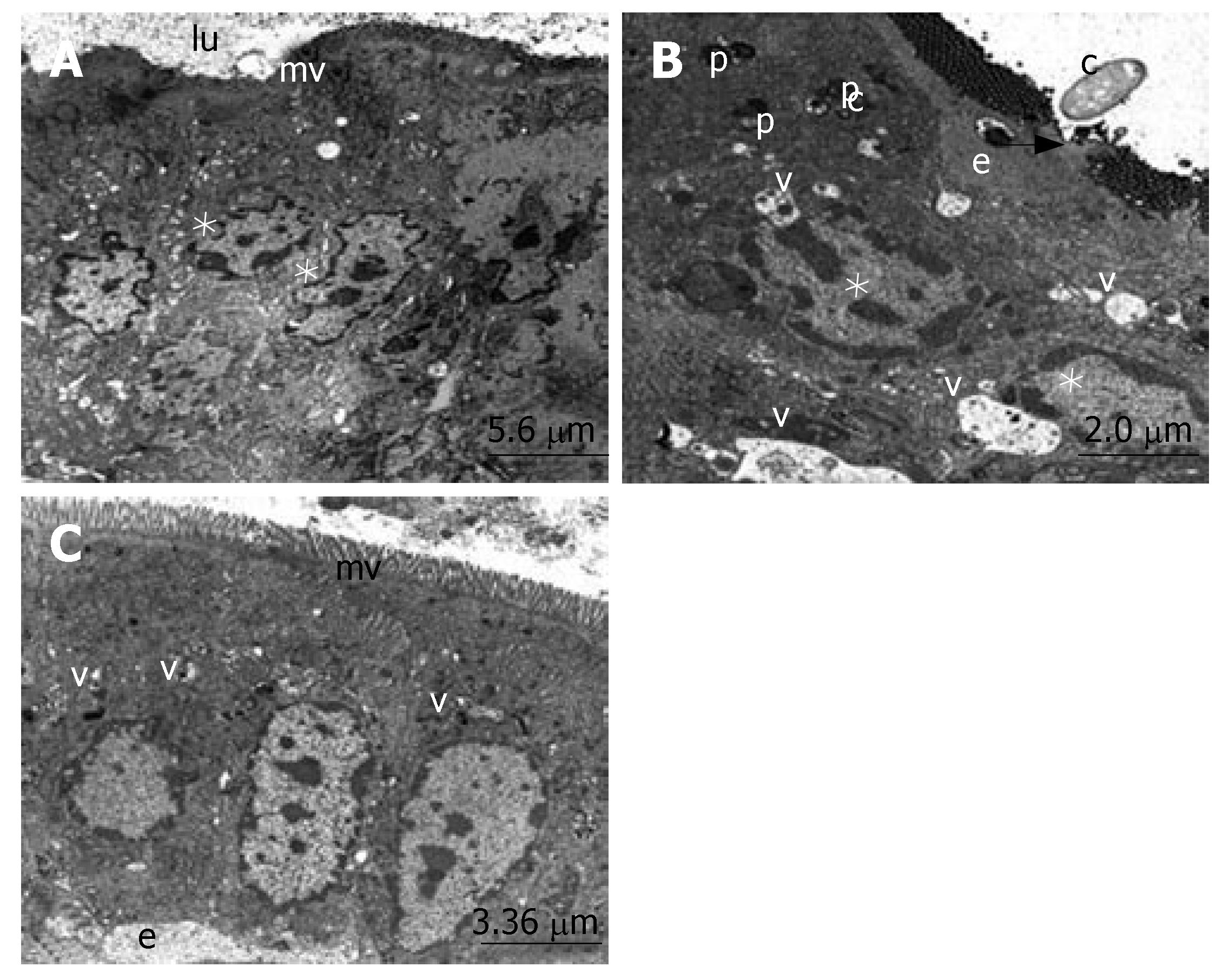
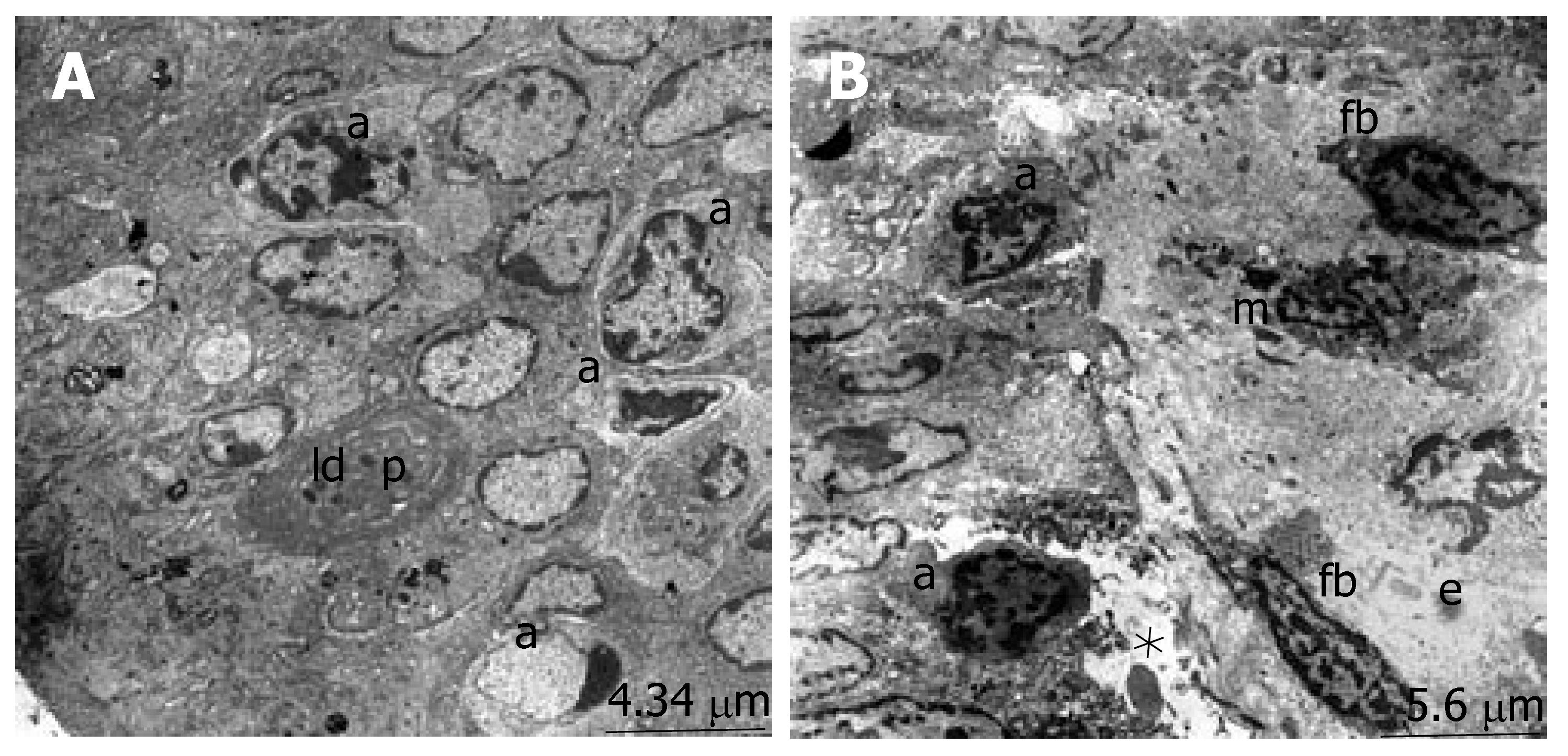
The ultrastructure of the ileum was observed by electron microscopy. The intestinal surface epithelium showed regular architecture with a large number of microvilli exposed to the lumen and the enterocytes, which are tightly bound by junctional complexes, showed the regular architecture of the small intestine surface epithelium in the sham group (Figure 2A). When we evaluated the micrographs of group II, we observed the desquamation of the epithelial tissue, cytoplasmic vacuoles with the inclusion of lipid droplets and phagosomes. The nucleus of the enterocytes were flattened possibly due to both apical surface and subepithelial edema. Ultrastructural findings indicating the invasion of candida, especially located in the desquamated epithelial, were detected in many areas (Figure 2B). The number of apoptotic cells, characterized by cytoplasmic condensation and nuclear fragmentation, was qualitatively increased. The phagosomes, including lipid droplets, were observed in the cytoplasm of the epithelial cells. The infiltration of macrophages and fibrocytes were evident. Basal membranes were separated due to subepithelial edema (Figure 3). Structure of the enterocytes and microvilli were regular in the propolis group. The junctional complexes were in common apppearance and adjoining outer membranes of surface absorptive cells were close to each other. The cytoplasmic vacuoles were present but they were smaller than the vacuoles in the BDL group. The nuclei of surface absorptive epithelial cells were regular, in accordance with the regression of the apical surface and subepithelial edema. Findings supporting the invasion of candida were not evident (Figure 2C).
The rates of bacterial translocation (BT) to liver, spleen, MLNs, and blood for all groups are summarized in Table 3. Sham and BDL + propolis groups had similar incidence of BT. The BDL group had significantly higher rates of BT as compared with sham and BDL + propolis groups. Only BT rates to spleen were not statistically different between the BDL and BDL + propolis groups. BT was predominantly detected in MLNs and the most commonly isolated bacteria was E. coli.
| Groups | Liver | Spleen | MLNs | Blood |
| Sham (GroupI) | 0/10 (0%) | 0/10 (0%) | 1/10 (10.0%) | 0/10 (0%) |
| BDL (Group II) | 6/8 (75.0%) | 4/8 (50.0%) | 7/8 (87.5%) | 4/8 (50%) |
| BDL + Propolis (Group III) | 2/9 (22.2%) | 2/9 (22.2%) | 3/9 (33.3%) | 0/9 (%) |
| P values | ||||
| Ivs II | 0.002 | 0.023 | 0.002 | 0.023 |
| II vs III | 0.044 | > 0.05 | 0.036 | 0.029 |
| Ivs III | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
Propolis is a resinous material collected by bees from various plants. Once collected, this material is enriched with salivary and enzymatic secretions of bees. Propolis is used by bees to cover hive walls, fill cracks or gaps and embalm killed invader insects. Hundreds of chemical compounds have been identified from propolis. Propolis contains a variety of flavonoids, phenols, alcohols, terpenes, sterols, vitamins and amino acids. Antimicrobial properties of propolis seem attributable mainly to the flavonoids, pinocembrin, galangin, and pinobanksin. Pinocembrin also exhibits antifungal properties. Flavonoids are well-known plant compounds that have antioxidant, antibacterial, antifungal, antiviral, and antiinflammatory properties[16-18].
Bacterial translocation is defined as the phenomenon by which bacteria, their products, or both bacteria and products, cross the intestinal barrier. The first site encountered by the microorganisms or products undergoing translocation is the mesenteric lymph node. Subsequently, extension to the liver, spleen, and systemic circulation may occur. The mechanisms which are proposed to promote bacterial translocation are small bowel bacterial overgrowth, immune deficiency states, and physical damage to the intestinal mucosa and vasculature that causes increased permeability. Certain enteric organisms such as E. coli, Proteus mirabilis, Klebsiella pneumoniae, seem to undergo translocation more easily. When any of the three mechanisms that promote translocation becomes severe, prolonged, or combined with other mechanisms, bacterial translocation may lead to sepsis and death in experimental animals[19].
Obstructive jaundice is a common clinical entity complicated by intestinal failure and endotoxemia, leading to high morbidity and mortality rates[2]. Kuzu et al[20] collected peritoneal swab, mesenteric lymph node, portal venous blood, liver wedge biopsy, and bile samples for culture in patients with obstructive jaundice, and they demonstrated translocation of enteric bacteria despite common use of preoperative antibiotics. Welsh et al[21] demonstrated a reversible impairment in gut barrier function in patients with cholestatic jaundice by using the lactulose/mannitol permeability test and concluded that these data might directly identify an important underlying mechanism contributing to the high risk of sepsis in jaundiced patients.
Obstructive jaundice globally affects three levels of gut barrier: the immune barrier, the biological barrier, and the mechanical barrier. Obstructive jaundice depresses Kupffer cell clearance capacity and natural killer cell activity, reduces T cells in intestinal epithelium, alters intestinal mucosal immunity and deprives the gut from biliary secretory IgA and from other specific and nonspecific antibodies contained in bile that inhibit adhesion of enteric bacteria on the intestinal wall. Bile salts exert bacteriostatic properties, and therefore their absence from the intestinal lumen results in quantitative and qualitative disruption of the indigenous microflora. Absence of intraluminal bile also deprives the gut from their trophic effect resulting in intestinal atrophy[2]. The cellular alterations of the mechanical barrier are associated with significant disturbances of intestinal oxidative status, with increased lipid peroxidation, protein oxidation and oxidation of non-protein and protein thiols. These biochemical changes are indicative of high oxidative stress in the intestine after biliary obstruction and represent another significant parameter of intestinal injury leading to barrier failure[22].
Propolis has antibacterial, antioxidant and anti-inflammatory properties. There are a number of studies documenting the antimicrobial functions of propolis, its extracts, and constituents. This is a broad spectrum activity against Gram-positive (S. aureus, S. pyogenes, S. viridens, D. pneumoniae, and C. diphtheria) and Gram-negative (E. coli, K. pneumoniae, P. vulgaris, P. aeruginosa, S. typhi, S. paratyphi-A, S. paratyphi-B, and S. flexneri) rods and cocci, Helicobacter pylori, Tryponosoma cruzi, Toxoplasma gondii, Trichomonas vaginalis, Candida, Saccharomyces, Cryptococcus, Mycobacteria, as well as viruses (HIV, Herpes viruses, influenza viruses, adenovirus, poliovirus type 2)[5]. Propolis also possesses potent antimicrobial activity, providing an alternative therapy against infections caused by resistant strains such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium[23]. Although the chemical composition of propolis varies depending on the site of its collection, antimicrobial properties seem attributable mainly to the flavonoids pinocembrin, galangin, and pinobanksin. Pinocembrin also exhibits antifungal properties. Other active compounds are ester of coumaric acid and caffeic acids. Prenylated p-coumaric and diterpenic acids possess antibacterial and cytotoxic activities. Caffeoylquinic acid derivatives show immunomodulatory and hepatoprotective actions and furofuran lignans inhibit the growth of some bacteria[24].
Propolis has been used in the treatment of cutaneous lesions such as burns, wounds, and ulcers. Morales et al[25] used a hypoallergic formula of propolis and obtained a very satisfactory evolution and cicatrization in cases of wounds with and without infection. A fast cure, shorter treatment period, and less septic complications were also obtained. The cicatrization was evident between the 4th and 5th d by the early formation of granulation tissue. The antimicrobial capacity was evident with a fast regression of the septic component of the supurated wounds. Propolis exerts a wound healing effect by minimizing acute inflammatory exudate, stimulating macrophage activity, promoting collagen production, and stimulating epithelialization. In our present study, the mean number of villi per centimeter and mean mucosal height of the propolis group were significantly different from the BDL group (P = 0.001 and 0.012, respectively). The electron microscopic changes were also different between these groups (Figures 1-3). We conclude that enhancement of wound healing capacity by the use of propolis might be the reason for decreased atrophy of intestinal mucosal villi.
Propolis also has immunomodulatory activity. In an experimental study, a water-soluble derivative of propolis (WSDP) and its components stimulated macrophages thus influencing specific and nonspecific immune defence mechanisms[13,26]. In another study, it was shown that WSDP treatment induced extensive proliferation of nucleated cells in the spleen and bone marrow, which are mainly macrophages and hematopoietic cells[17].
Ara et al[27] found that intraperitoneal administration of CAPE reduced tissue levels of malondialdehyde and myeloperoxidase, but increased levels of glutathione in the ileum after bile duct ligation. Additionally, CAPE decreased interleukin-1α, interleukin-6, tumor necrosis factor-α, and intestinal mucosal injury, but the effect of CAPE on bacterial translocation was not revealed. In this study, although bacterial translocation in CAPE-treated rats was lower than in the control group, the difference was not significant.
In our study, bacterial translocation was reduced significantly. The BDL group had significantly higher rates of BT as compared with sham and BDL + propolis groups (P < 0.05). Propolis may reduce bacterial translocation by enhancing mucosal barrier function, supporting generalized immune function, and reducing bacterial overgrowth. As we mentioned before, these three mechanisms are the main mechanisms responsible for bacterial translocation.
In conclusion, propolis showed a significant protective effect on ileal mucosa and reduced bacterial translocation in an experimental obstructive jaundice model. Further studies should be carried out to explain the mechanisms of these effects.
This work is financially supported by Balparmak Pazarlama Koll. Sti., Istanbul, Turkey. We thank Dr. M Tahir Oruc for accompanying us during designation of the study.
S- Editor Zhu LH L- Editor Lutze M E- Editor Yin DH
| 1. | Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57:316-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Assimakopoulos SF, Vagianos CE, Charonis A, Nikolopoulou VN, Scopa CD. Intestinal failure in obstructive jaundice. World J Gastroenterol. 2005;11:3806-3807. [PubMed] |
| 3. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Holman JM, Rikkers LF. Biliary obstruction and host defense failure. J Surg Res. 1982;32:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol. 1998;36:347-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 848] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 6. | Scazzocchio F, D'Auria FD, Alessandrini D, Pantanella F. Multifactorial aspects of antimicrobial activity of propolis. Microbiol Res. 2006;161:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Lu LC, Chen YW, Chou CC. Antibacterial activity of propolis against Staphylococcus aureus. Int J Food Microbiol. 2005;102:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kismet K, Kilicoglu B, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Salih B, Akkus MA. Evaluation on scolicidal efficacy of propolis. Eur Surg Res. 2006;38:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Hu F, Hepburn HR, Li Y, Chen M, Radloff SE, Daya S. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J Ethnopharmacol. 2005;100:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, Endo Y, Sato T, Satoh S, Tadano T. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull. 2006;29:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286-7292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Shimizu K, Ashida H, Matsuura Y, Kanazawa K. Antioxidative bioavailability of artepillin C in Brazilian propolis. Arch Biochem Biophys. 2004;424:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Orsolić N, Knezević AH, Sver L, Terzić S, Basić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Basnet P, Matsushige K, Hase K, Kadota S, Namba T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol Pharm Bull. 1996;19:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Sugimoto Y, Tarumi T, Kaneko Y, Isayama S, Kawai N, Sugimoto H, Yamada H, Kamei C. Effect of propolis extract on D-galactosamine-induced hepatic injury in rats. Biol Pharm Bull. 1999;22:1237-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Dimov V, Ivanovska N, Manolova N, Bankova V, Nikolov N, Popov S. Immunomodulatory action of propolis. Influence on anti-infectious protection and macrophage function. Apidologie. 1991;22:155-162. [DOI] [Full Text] |
| 17. | Orsolic N, Tadic Z, Benkovic V, Horvat A, Lojkic M, Basic I. Radioprotective effect of a water-soluble derivate of propolis in mice. Mellifera. 2004;4:45-52. |
| 18. | Schimdt JO. Chemical composition and application. Bee products: Properties, Application and Apitherapy. New York: Plenum Press 1997; 15-26. |
| 19. | Lichtman SM. Bacterial [correction of baterial] translocation in humans. J Pediatr Gastroenterol Nutr. 2001;33:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kuzu MA, Kale IT, Cöl C, Tekeli A, Tanik A, Köksoy C. Obstructive jaundice promotes bacterial translocation in humans. Hepatogastroenterology. 1999;46:2159-2164. [PubMed] |
| 21. | Welsh FK, Ramsden CW, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;227:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand. 2004;180:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Kilic A, Baysallar M, Besirbellioglu B, Salih B, Sorkun K, Tanyuksel M. In vitro antimicrobial activity of propolis against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Ann Microbiol. 2005;55:113-117. |
| 24. | Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73 Suppl 1:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 364] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Morales WF, Garbarino JL. Clinical evaluation of a new hypoallergic formula of propolis in dressings. Bee products: Properties, Application and Apitherapy. New York: Plenum Press 1997; 101-105. |
| 26. | Orsolić N, Basić I. Immunomodulation by water-soluble derivative of propolis: a factor of antitumor reactivity. J Ethnopharmacol. 2003;84:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Ara C, Esrefoglu M, Polat A, Isik B, Aladag M, Gul M, Ay S, Tekerleklioglu MS, Yilmaz S. The effect of caffeic acid phenethyl ester on bacterial translocation and intestinal damage in cholestatic rats. Dig Dis Sci. 2006;51:1754-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |