Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.306
Revised: June 28, 2005
Accepted: July 1, 2005
Published online: January 14, 2006
AIM: To cost-effectively express the 23-ku pE2, the most promising subunit vaccine encoded by the E2 fragment comprising of the 3’-portion of hepatitis E virus (HEV) open reading frame 2 (ORF2) in plastids of tobacco (Nicotiana tabacum cv. SR1), to investigate the transgene expression and pE2 accumulation in plastids, and to evaluate the antigenic effect of the plastid-derived pE2 in mice.
METHODS: Plastid-targeting vector pRB94-E2 containing the E2 fragment driven by rice psbA promoter was constructed. Upon delivery into tobacco plastids, this construct could initiate homologous recombination in psaB-trnfM and trnG-psbC fragments in plastid genome, and result in transgene inserted between the two fragments. The pRB94-E2 was delivered with a biolistic particle bombardment method, and the plastid-transformed plants were obtained following the regeneration of the bombarded leaf tissues on a spectinomycin-supplemented medium. Transplastomic status of the regenerated plants was confirmed by PCR and Southern blot analysis, transgene expression was investigated by Northern blot analysis, and accumulation of pE2 was measured by ELISA. Furthermore, protein extracts were used to immunize mice, and the presence of the pE2-reactive antibodies in serum samples of the immunized mice was studied by ELISA.
RESULTS: Transplastomic lines confirmed by PCR and Southern blot analysis could actively transcribe the E2 mRNA. The pE2 polypeptide was accumulated to a level as high as 13.27 μg/g fresh leaves. The pE2 could stimulate the immunized mice to generate pE2-specific antibodies.
CONCLUSION: HEV-E2 fragment can be inserted into the plastid genome and the recombinant pE2 antigen derived is antigenic in mice. Hence, plastids may be a novel source for cost-effective production of HEV vaccines.
- Citation: Zhou YX, Lee MYT, Ng JMH, Chye ML, Yip WK, Zee SY, Lam E. A truncated hepatitis E virus ORF2 protein expressed in tobacco plastids is immunogenic in mice. World J Gastroenterol 2006; 12(2): 306-312
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.306
Hepatitis E is a water-borne disease caused by hepatitis E virus (HEV) in many tropical and subtropical areas. Over 50 outbreaks have been reported since its first epidemic in Delhi, India, documented in 1955 and 1956[1]. It accounts for more than 50% of acute viral hepatitis among young adults in developing countries with a fatality rate of 1-3% in non-pregnant patients and up to 20% in pregnant women[2]. Consequently, it is a serious threat to public health and has an urgent need of effective vaccines.
HEV appears as a 27-30-nm virus-like particle. It has a ≈ 7.5-kb linear, single-stranded, a positive-sense RNA made up of a short 5’-noncoding region, a 3’-poly(A) tail, and three overlapping open reading frames (ORFs)[3,4]. ORF1 extends approximately 5 kb from the 5’-end and encodes a polyprotein with functional motifs typical of RNA-dependent RNA polymerase, RNA helicase, papain-like cysteine proteinase and methyltransferase. ORF2 is made up of several dozens of base pairs downstream of the ORF1 and extends approximately 2 kb to the termination codon that is 200-300 bp from the 3’-poly (A) residues. ORF2 contains a consensus signal peptide sequence at its amino terminus and a capsid-like region similar to that seen with other virus capsid proteins. ORF3 partially overlaps the ORF1 and ORF2, and encodes a 123-aa polypeptide with the possible role of a cytoskeletal anchor site for the assembly of virus particles[5].
Since killed and attenuated vaccines for hepatitis E are not available due to a lack of culture system for HEV production, recombinant proteins represent the best hope for subunit vaccines. The ORF2-encoded protein has been shown to be the most promising candidate because the only neutralizing epitope identified to date is mapped to its carboxy-terminal region between aa 578-607[6]. To explore the possibility that these subunit vaccines could be produced through recombinant protein approach, ORF2 fragments have been expressed in prokaryotes[7], insect cells[8], animal cells[9], and transgenic plants[10]. Satisfactory accumulations of the ORF2 proteins in bacterial[7] and animal cells[9] have been achieved. However, if the vaccines are to be produced in such systems, a relatively higher expense may be encountered. In insect cells[11] and nuclear transformed tomatoes[10], the ORF2-encoded peptides are accumulated at much lower levels (1-2 mg/L and 48-61 ng/g fresh weight, respectively). Hence, a technique for cost-effective large-scale production of the ORF2 subunit vaccines plays an important role in the control of hepatitis E.
Plastids are unique organelles in plants. Each plant cell accommodates up to 10 000 copies of the plastid genome, indicating the potential of using transplastomic plants to express foreign proteins at elevated levels[12]. To obtain high-level accumulation of the ORF2-encoded pE2 peptide (aa 394-607), we inserted its corresponding cDNA sequence, the E2 fragment[7], into the plastid genomes of tobacco plants (Nicotiana tabacum cv. SR1). The E2 fragment was expressed satisfactorily at RNA and protein levels, and the plastid-produced pE2 was found to be antigenic in mice. Our results suggest that plastid transformation may be an economical alternative for large-scale production of edible HEV vaccines.
A polylinker (upper strand, 5’-CATGGCCGCGGGGGCCCGCTAGCAGGCCTG CGGCCGCATCGATGAGCT-3’; lower strand, 5’-CATCGATGCGGCCGCAGG CCTGCTAGCGGGCCCCCGCGGC-3’) was cloned in NcoI-SacI site of pVSR326 (GenBank accession no.: AF527485). The obtained pVSR326 derivative was digested by SacII and inserted with a (His)5 fragment (upper strand: 5’-GCGGGGTT CTCATCATCATCATCATGGTCCGC-3’; lower strand: 5’-GGACCATGATGATG ATGATGAGAACCCCGCGC-3’). The resultant plasmid was termed pMLVHisA.
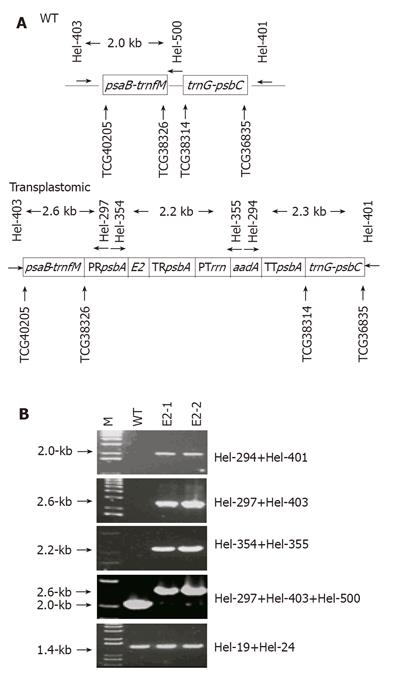
A 0.8-kb E2 fragment originally obtained from a conserved region near the 3’-terminal of the ORF2 in a Chinese HEV strain (GenBank accession no.: D11092) was released by BamHI-EcoRI digestion of pGEX-E2[13] and cloned in the corresponding site in pBluescript KS. Then the 0.8-kb fragment was removed from the resulting plasmid by SpeI-ClaI digestion before it was cloned into the NheI-ClaI site in pMLVHisA. This pMLVHisA derivative was digested by XbaI and KpnI to produce a cassette of “rice psbA promoter-E2-rice psbA terminator” which was then inserted into the corresponding site in pRB94[14]. The resulting construct, termed pRB94-E2, has two cassettes: “rice psbA promoter-E2-rice psbA terminator” for pE2 production, and “tobacco rrn promoter-aadA-tobacco psbA terminator” for expressing the spectinomycin-degrading aminoglycoside 3’-adenyltransferase. These two cassettes were flanked by a 1.5-kb trnG-psbC arm and a 1.9-kb psaB-trnfM arm (nt 36 835-38 314 and 38 326-40 205, respectively, in tobacco plastid genome, GenBank accession no.: Z00044) (Figure 1A). These two arms were for homologous recombination upon delivery of pRB94-E2 into chloroplasts.
Seeds of tobacco SR1 were germinated on MS medium (Sigma, St. Louis, USA) plus 3% sucrose and 0.6% agar (Sigma). After the seedlings were grown for 3-4 wk, the leaves were placed on petri-dishes with RMOP medium[15] for one day before being bombarded with a PDS-1 000/He Biolistic® Particle Delivery System (Bio-Rad).
DNA for plastid transformation was prepared using the High Purity Plasmid Maxiprep System (Marligen Bioscience Inc., USA). One microgram of pRB94-E2 plasmid was used to coat 1 mg of 0.6-µmol/L diametric tungsten particles (M13, Bio-Rad) according to the standard procedure. The coated particles were bombarded at 1 100 psi to tobacco leaves on petri-dishes. One milligram of the coated particle was used for each bombardment. The bombarded leaves were kept on the same petri-dishes for 24 h before being cut into ≈ 1 cm2 pieces and put on RMOP plus 500 µg/mL spectinomycin dihydrochloride. Spectinomycin-resistant shoots emerged in about a month, and their leaves were used as explants for subsequent rounds of regeneration until homoplasmy was achieved.
Primer pairs (Hel-297/Hel-403, Hel-294/Hel-401 and Hel-354/Hel-355) capable of differentiating WT and transplastomic genomes (Table 1 and Figure 1A) were used. In addition, Hel-19/Hel-24 was used for the amplification of a 1.4-kb fragment in the endogenous rbcL gene from both the WT and the transplastomic plants. Thirty-five cycles of PCR were performed using the Taq DNA polymerase (Promega). PCR products were separated on a 0.8% agarose gel for visualization under UV illumination after ethidium bromide staining.
| Name | Sequence (5’-3’) | Position |
| Hel-19 | atgtcaccacaaacagag | 57 595-57 6121 |
| Hel-24 | atccaaaacgtccactgc | 59 005-59 0021 |
| Hel-294 | agcccgtcatacttgaagctagac | 6 563-6 5872, 5 191-5 2153 |
| Hel-297 | ataccaatgtcaaccaagccagcc | 2 008-2 0323 |
| Hel-348 | gatgatcatagaagcccctttacc | 199-2222, 38 524-38 5471 |
| Hel-354 | tgcaagcacgatttggggagag | 1 806-1 8273 |
| Hel-355 | cagatcaatgtcgatcgtggctg | 6 856-6 8782, 4 900-4 9223 |
| Hel-399 | tggcaaaacaagatgttgcggag | 38 341-38 3631 |
| Hel-401 | gttctttaaattccgtgggtggtg | 36 683-36 7061 |
| Hel-403 | agaaccaatttcgggattgggcac | 40 312-40 3351 |
| Hel-720 | gtagaatgctagatgccc | 4 300-4 3173 |
| Hel-721 | agctttggcgagctagttg | 7 388-7 4062 |
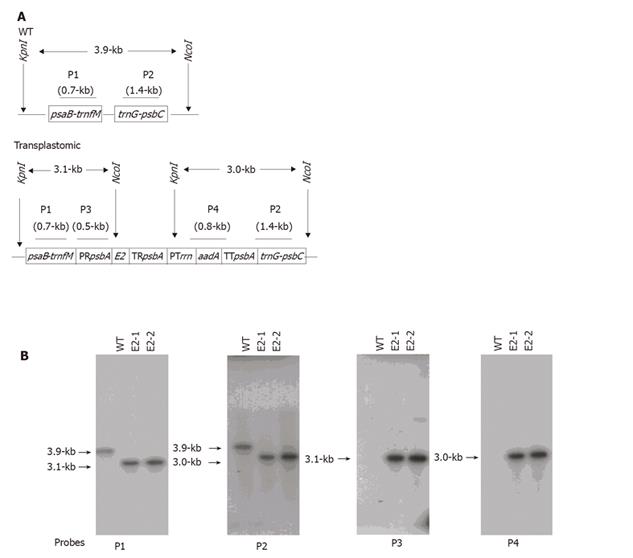
Genomic DNA was digested by NcoI and KpnI, separated on a 0.8% agarose gel and transferred onto a GeneScreen Plus® membrane (PerkinElmer), before separate hybridization to four 32P-labeled DNA probes (P1, P2, P3, and P4 in Figure 2A). Hybridization was performed overnight at 65°C in a buffer containing 250 mmol/L NaCl, 7% SDS and 125 mmol/L phosphate, pH 7.0. After hybridization, blots were washed twice (10 min each) at room temperature in 2 × SSC plus 0.5% SDS, and once at 65 °C for 15min in 0.2 × SSC plus 0.1% SDS. Blots were then exposed to X-ray.
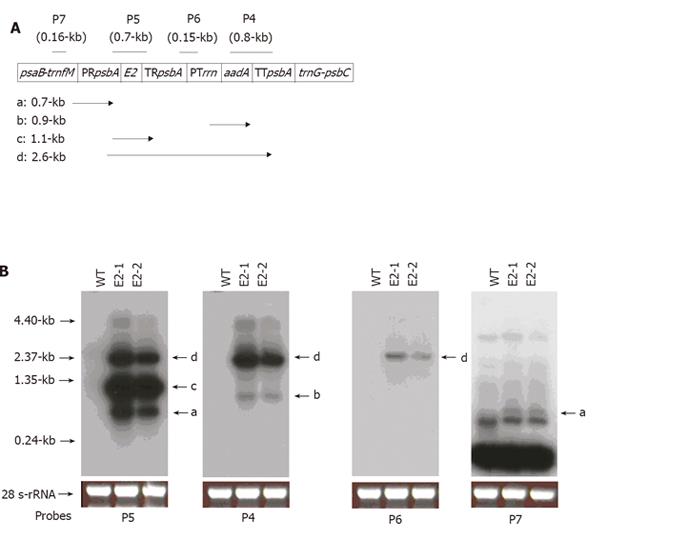
RNA samples were prepared from leaves of WT and transplastomic plants. Seven micrograms of the isolated RNA was separated on 1.2% agarose gel, blotted onto a membrane and hybridized to four 32P-labeled DNA probes (P4, P5, P6, and P7 in Figure 3A). The aadA-specific probes (P4) were the same as those used for Southern blot analysis, while “psbA-Prrn” (P6) and trnfM (P7) probes were made with templates generated by PCR using primer pairs of Hel-720/Hel-721 and Hel-348/Hel-399, respectively (Table 1). Hybridization and washing conditions were the same as in Southern blot analysis.
HEV antigen ELISA Kit and standard pE2 antigen were purchased from Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Beijing, China. Total soluble protein (TSP) from weighed WT and transplastomic plant tissues (leaves and seeds) was extracted with a non-denaturing buffer [50 mmol/L Tris-HCl (pH 8.5), 10 mmol/L sodium acetate, 100 MgCl2, 1 mmol/L EDTA and 1 mmol/L PMSF]. After protein quantification with the Bradford agent, 1 µg leaf TSP and 5 µg seed TSP were used for ELISA. Antigen concentrations expressed as nanogram pE2 per microgram TSP were calculated using an ELISA standard curve established with a serial dilution of the standard pE2 antigen. These concentrations were further converted to microgram pE2 per gram fresh tissue using the protein yield (µg TSP/g fresh weight) as multiplication factor. The average values for these factors from three independent assays were 20.95 ± 3.95 and 10.79 ± 1.40 mg TSP/g fresh seeds and leaves, respectively.
Four-week old BALB/c mice were divided into four groups. Each mouse in the first group was subcutaneously injected with 100 μL of PBS solution, the second group with 100 μg of TSP from WT plant, the third group with 100 μg of TSP from the E2-1 leaves, and the fourth group with 10 μg of TSP (equivalent to ≈ 1 μg pE2) from E. coli expressing the pE2 polypeptide. Injection was performed once a week for three consecutive weeks. One week after the last infection, serum samples were collected for pE2-specific antibody detection.
Serum samples were diluted five times with coating buffer (0.015 mol/L sodium carbonate, 0.025 sodium bicarbonate, pH 9.6). Fifty microliters of the diluted samples were added to a 96-well microplate and incubated at 4 °C overnight. After the coating buffer was removed, the wells were blocked with 5% non-fat milk in PBS supplemented with 0.5% Tween 20 for 2 h, which was followed by four washes with 200 μL PBS plus 0.5% Tween 20. Then 200 ng of the pE2 antigen (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.) diluted in 50 μL PBS plus 0.5% Tween 20 was added and incubated at 37 °C for 1 h. After the uncaptured pE2 was removed and the wells were washed five times, 100 μL of HRP-conjugated monoclonal anti-HEV antibodies (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.) were added. After incubation at 37 °C for 30 min, the antibody solution was removed and the wells were washed five times. Then 50 μL chromogen A and 50 μL chromogen B were added to the well and the plate was incubated at 37 °C for 2 h. After color development, 50 μL stop solution was added and absorbance at 450 nm (A450) was measured with equal volume of mixture of chromogen A and chromogen B as reference.
The vector pRB94 was reported to have an increased chloroplast-targeting efficacy[14]. Hence we used its backbone and made a construct pRB94-E2 for tobacco chloroplast transformation in this study.
Two shoots were obtained after culturing the bombarded leaf pieces in spectinomycin-containing RMOP medium for about a month. The shoots were selected for two more generations in the same RMOP medium. They were then transferred to grow in greenhouse after root development in a hormone-free MS medium. The resulting plants were named E2-1 and E2-2.
To confirm whether the spectinomycin-resistant E2-1 and E2-2 were transplastomic, PCR were performed with primer pairs that could only amplify DNA fragments that resulted from homologous recombination (Table 1 and Figure 1A) for which three pairs were used. The first pair (Hel-297/Hel-403) could produce a 2.6-kb product that resulted from recombination in the psaB-trnfM arm, the second (Hel-294/Hel-401) a 2.0-kb fragment as a result of recombination in the trnG-psbC arm, and the third (Hel-354/Hel-355) a 2.2-kb fragment from the rice psbA promoter to the aadA-coding sequence. All the three pairs produced PCR products of expected sizes from E2-1 and E2-2 DNA samples (Figure 1B), indicating that homologous recombination occurred and the E2- and aadA-expressing cassettes were co-integrated and stayed intact in the plastid genomes.
To confirm the transgene integration and to determine the homoplasmic status, DNA of both the WT and transplastomic plants in the greenhouse was digested by KpnI and NcoI, and subjected to Southern blot analysis with probes hybridizing to the psaB-trnfM arm (P1), trnG-psbC arm (P2), rice psbA promoter (P3), and aadA-coding region (P4). Restriction fragments and their lengths in kb for both the WT and transplastomic genomes are shown in Figure 2A. When probes P1 and P2 were used, the WT revealed a 3.9-kb band, while the transplastomic plants produced bands of 3.1- and 3.0-kb, respectively (Figure 2B). Absence of the 3.9-kb band in the transplastomic plants indicated homoplasmy. When probes P3 and P4 were used, the transplastomic plants showed the expected 3.1- and 3.0-kb bands, respectively (Figure 2B). These results supported the data from PCR (Figure 1) and confirmed that both E2- and aadA-expressing cassettes were transformed into the chloroplast genome, and the transplastomic plants achieved homoplasmy.
In the E2-1 and E2-2 plants, the transgenes were actively transcribed. When the blot bearing RNAs from both the WT and the transplastomic plants was hybridized with E2-specific probes P5 (Figure 3A), three bands (0.7-, 1.1- and 2.6-kb) were detected in the transplastomic plants (bands a, c, and d, Figure 3B), indicating that E2-containing transcripts were terminated at multiple locations. When the same blot was hybridized with aadA probes P4, a 2.6-kb band and a 0.9-kb band appeared (Figure 3B). The 2.6-kb band represented run-through transcripts initiated from the rice psbA promoter, since it could be detected by E2-specific probes P5, but not by trnfM-targeting probes P7 (Figure 3B). The 0.9-kb band was the aadA transcripts plus 0.1-kb 5’-UTR between the rrn promoter and aadA-coding fragment. To determine the initiation sites for the 0.7- and 1.1-kb transcripts, probe P7 was used. In this case, only the 0.7-kb band was revealed (band a, Figure 3B). Therefore, the 0.7-kb transcripts were initiated from trnfM, while the 1.1-kb transcripts were solely from the E2-expressing cassette, which consisted 0.2-kb psbA 5’-UTR, 0.8-kb E2 and the remaining polylinker sequences (≈ 0.1-kb).
To investigate if the pE2 antigen was produced in E2-1 and E2-2, TSP extracted from both plants was used in a pE2-specific ELISA assay. An ELISA standard curve was established using different amounts of standard pE2 and used for quantifying the pE2 expressed as amount of fresh pE2 per gram. As high as 13.27 and 0.46 μg of pE2 were detected in leaves and seeds, respectively (Table 2). In comparison with the concentrations (47.9 ng/g fresh leaf tissue and 61.2 ng/g fresh fruit) of the same pE2 antigen expressed in tomato nuclear transformants[10], this study achieved over 200-fold increase in leaf pE2 content (Table 2). This elevated level of pE2 accumulation faithfully demonstrated that plastid transformation was superior to nuclear engineering for the production of HEV vaccines.
| Plant | Tissue | pE2 | |
| (ng/µg TSP) | (µg/g fresh weight) | ||
| WT | Leaves | nd | nd |
| Seeds | nd | nd | |
| E2-1 | Leaves | 1.090 ± 0.182 | 13.273 ± 2.217 |
| Seeds | 0.015 ± 0.004 | 0.261 ± 0.060 | |
| E2-2 | Leaves | 0.630 ± 0.133 | 5.912 ± 1.251 |
| Seeds | 0.018 ± 0.005 | 0.460 ± 0.093 | |
To investigate if the plastid-derived pE2 proteins were antigenic, TSP from leaves of transplastomic plant E2-1 was subcutaneously injected into mice once a week for three consecutive weeks. One week after the last injection, serum samples were collected for antibody detection.
The pE2 polypeptides produced in plastids were antigenic when injected into four-week old BALB/c mice, according to ELISA that specifically detected pE2-reactive antibodies (Table 3). In addition, in consistent with reports from other groups[7,16], bacterially-expressed pE2 could also stimulate mice to produce the corresponding antibodies (Table 3). Therefore, plastids were an ideal compartment for the production of antigenic pE2 proteins.
| Group | A450 | |
| Expt A | Expt B | |
| PBS | 0.117 | 0.137 |
| WT TSP | 0.138 | 0.134 |
| E2-1 TSP | 0.387 | 0.779 |
| E. coli TSP | 1.367 | 2.62 |
The unique biological property of plastid-containing plant cells is the high ploidy degree of plastid genomes[17]. Unlike nuclei, plastids provide an environment where foreign gene activity is not subjected to positional effect and epigenetic silencing. Thus, plastid transformation has the potential to steadily express foreign proteins at elevated level. Compared to the pE2 content in nuclear transformed tomatoes[10], this study resulted in a dramatic increase in pE2 accumulation and demonstrated that plastid transformation was substantially superior to its nuclear counterpart for pE2 production. High-level pE2 expression in tobacco plastids did not affect growth rates, flowering, seed setting or any other morphological features (data not shown), indicating that no apparent pleiotropic effects occur on the transplastomic plants.
The pE2 can interact with one another to form homodimers that are strongly recognized by serum samples from hepatitis E patients[13] and highly immunogenic in monkeys[7] and rats[16]. These findings reveal two important aspects about the HEV subunit vaccines. The first aspect is that carboxy terminus of the ORF2-encoded major structural protein possesses good antigenicity, and the other aspect is that conformation of these subunit vaccines is crucial in antibody stimulation and in antibody-antigen interaction. Plastid-derived pE2 should therefore possess the right conformation in order to be functional.
Plastids do offer an ideal compartment for foreign proteins to have functional folding. Examples include plastid-expressed nontoxic B subunit of E. coli heat-labile enterotoxin[18] and human somatotropin[19]. The pE2 expressed in this study reacted with the pE2-specific conformational antibody in the HEV antigen ELISA kit. Such a reaction could be abolished in the presence of SDS and when heating was applied to denature the protein (data not shown), suggesting that the plastid-derived pE2 possesses functional conformation.
Antibodies cross-reacting with pE2 were detected in serum samples of mice immunized with plastid-derived pE2 in this study. This is consistent with reports that antigenic pE2 can be produced from bacteria[7,16], and confirms that plastids are a cheaper source for the production of HEV vaccine.
The feasibility of pE2 as a broad-type antigen against HEV has been manifested by several observations. First, with the exception of a Mexican isolate and a USA isolate, the 0.8-kb pE2-coding sequence used in this study shares more than 90% homology with the corresponding sequences in other HEV isolates[7]. Second, though at least four genotypes have been identified, HEV is antigenically conserved and so far only one serotype has been identified[1]. Third, all naive macaques infected with HEV genotypes 1, 2, 3, and 4 have been reported to produce antibodies to the ORF2 polypeptides, one of which is comprised of aa 458-607[20], shorter than the plastid-produced pE2 (aa 394-607) in this study. Hence, we may anticipate that the pE2 peptide in its proper conformation can stimulate antibodies against all the four reported genotypes.
Plastid-encoded genes are typically organized into polycistronic transcription units that give rise to overlapping RNAs. Though the significance of this complicated mode of expression is unknown, the intergenic sequences involved should not possess the typical function of strong terminators. The multiple bands detected with the E2-specific probes (P5) indicate that transcripts initiated from the rice psbA promoter could pass through the rice psbA terminator. This run-through transcription might actually contribute to the synthesis of the aadA-encoded protein, since aadA-specific probes revealed much less initiation of the aadA transcripts from the tobacco rrn promoter. The same rrn promoter was able to satisfactorily initiate aadA transcription, when the E2 fragment in the pRB94-E2 vector was replaced by the bacterial uidA gene (data not shown). Whether proximal DNA sequences influence activity of the rrn promoter is unclear at present.
While this study has proved the feasibility of expressing immunogenic pE2 in plastids, the tobacco plant is nevertheless inedible and a transgenic tobacco-based vaccine that can be administered by subcutaneous injection is a feasibility supported by the present work. For hepatitis B, an edible plant-based oral vaccine has been demonstrated in animals[21]. Introduction of the pE2-coding sequences into the plastid genomes of edible plants such as tomato, lettuce and carrot would thus be more desirable to test if an oral-based vaccine for hepatitis E could be realized. In tomatoes, foreign protein has been shown to express in fruit chromoplasts[14]. This example provides assurance for the production of edible vaccines through plastid transformation.
In conclusion, transplastomic tobacco plants expressing the antigenic HEV pE2 peptide at elevated level can be successfully generated and it is possible to produce pE2 in edible plant parts for the purpose of immunizing human beings against HEV infection.
The authors thank Professor Ralph Bock for providing the pRB94 chloroplast transformation vector, and Drs S. Leelavathi and V.S. Reddy for providing the pVSR326 plasmid and their help in the early stage of this study. The authors also thank the laboratory of Professor M.H. Ng (Department of Microbiology, University of Hong Kong) for the gift of the plasmid pGEX-E2.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Skidmore S. Overview of Hepatitis E Virus. Curr Infect Dis Rep. 2002;4:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 809] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Worm HC, van der Poel WH, Brandstätter G. Hepatitis E: an overview. Microbes Infect. 2002;4:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045-9053. [PubMed] |
| 6. | Schofield DJ, Glamann J, Emerson SU, Purcell RH. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol. 2000;74:5548-5555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Im SW, Zhang JZ, Zhuang H, Che XY, Zhu WF, Xu GM, Li K, Xia NS, Ng MH. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine. 2001;19:3726-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Zhang M, Emerson SU, Nguyen H, Engle RE, Govindarajan S, Gerin JL, Purcell RH. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001;20:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Jameel S, Zafrullah M, Ozdener MH, Panda SK. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J Virol. 1996;70:207-216. [PubMed] |
| 10. | Ma Y, Lin SQ, Gao Y, Li M, Luo WX, Zhang J, Xia NS. Expression of ORF2 partial gene of hepatitis E virus in tomatoes and immunoactivity of expression products. World J Gastroenterol. 2003;9:2211-2215. [PubMed] |
| 11. | Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, Purcell RH. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Kawasaki S, Ideta T, Okajima T, Tokuomi H, Suko S. [Headache, high fever and cognition disorder: tuberculous meningoencephalitis]. Nihon Rinsho. 1975;Spec No:724-75, 724-75. [PubMed] |
| 13. | Zhang JZ, Ng MH, Xia NS, Lau SH, Che XY, Chau TN, Lai ST, Im SW. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J Med Virol. 2001;64:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Ruf S, Hermann M, Berger IJ, Carrer H, Bock R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol. 2001;19:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A. 1993;90:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 517] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Li F, Riddell MA, Seow HF, Takeda N, Miyamura T, Anderson DA. Recombinant subunit ORF2.1 antigen and induction of antibody against immunodominant epitopes in the hepatitis E virus capsid protein. J Med Virol. 2000;60:379-386. [PubMed] |
| 17. | Bendich AJ. Why do chloroplasts and mitochondria contain so many copies of their genome. Bioessays. 1987;6:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 205] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Kang TJ, Loc NH, Jang MO, Jang YS, Kim YS, Seo JE, Yang MS. Expression of the B subunit of E. coli heat-labile enterotoxin in the chloroplasts of plants and its characterization. Transgenic Res. 2003;12:683-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Staub JM, Garcia B, Graves J, Hajdukiewicz PT, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol. 2000;18:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 275] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Zhou YH, Purcell RH, Emerson SU. A truncated ORF2 protein contains the most immunogenic site on ORF2: antibody responses to non-vaccine sequences following challenge of vaccinated and non-vaccinated macaques with hepatitis E virus. Vaccine. 2005;23:3157-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci U S A. 2001;98:11539-11544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |