Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.181
Revised: July 28, 2005
Accepted: August 25, 2005
Published online: January 14, 2006
Gastric cancer is the second most frequent cancer in the world, accounting for a large proportion of all cancer cases in Asia, Latin America, and some countries in Europe. Helicobacter pylori (H pylori) is regarded as playing a specific role in the development of atrophic gastritis, which represents the most recognized pathway in multistep intestinal-type gastric carcinogenesis. Recent studies suggest that a combination of host genetic factors, bacterial virulence factors, and environmental and lifestyle factors determine the severity of gastric damage and the eventual clinical outcome of H pylori infection. The seminal discovery of H pylori as the leading cause of gastric cancer should lead to effective eradication strategies. Prevention of gastric cancer requires better screening strategies to identify candidates for eradication.
-
Citation: Ando T, Goto Y, Maeda O, Watanabe O, Ishiguro K, Goto H. Causal role of
Helicobacter pylori infection in gastric cancer. World J Gastroenterol 2006; 12(2): 181-186 - URL: https://www.wjgnet.com/1007-9327/full/v12/i2/181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.181
Helicobacter pylori (H pylori) is a Gram-negative, microaerophilic bacterium which expresses an abundant amount of urease. Infection with this bacterium is a worldwide phenomenon. Prevalence increases with age, but differs quite dramatically among populations[1-4]. In the USA, prevalence is less than 20% at 20 years old and approximately 50% at 50 years[2]. In Japan, it is less than 20% under 20 years, increasing to a plateau of 70-80% at 40 years[3], while in Korea, it is 50% at 5 years and 90% at 20 years[4].
The epidemiological data suggest that H pylori gastritis is associated with gastric carcinogenesis[5,6]. H pylori colonizes the gastric mucosa and elicits both inflammatory and immune lifelong responses, including the release of various bacterial and host-dependent cytotoxic substances[7]. Pathological and clinical studies have convincingly proved the etiological role of H pylori in the development of chronic gastritis[8] and peptic ulcer[9]. Moreover, H pylori infection has been recognized as a risk factor for both the diffuse and intestinal types of gastric cancer[10], and the bacterium itself is classified as a class I carcinogen by the World Health Organization and International Agency for Research on Cancer Consensus Group[11].
H pylori strains carrying the cytotoxin-associated gene A (cagA) gene are strongly associated with an increased risk of gastric adenocarcinoma[12]. Recent studies suggest that the severity of gastric damage and eventual clinical outcome of H pylori infection are determined by a combination of host genetic and bacterial virulence factors[13-17]. cagA is divided into two major subtypes, the East Asian and Western types[18]. The grade of gastric atrophy (and therefore gastric cancer risk) is higher in patients with East Asian cagA-positive strains than in those with cagA-negative or Western cagA -positive strains. Of interest is that atrophy grade varies even among patients with East Asian cagA-positive strains[19], and that most H pylori-infected subjects in fact develop no significant disease, remaining asymptomatic throughout their lives. The reasons for this are not explained by bacterial virulence factors alone; rather, genetic factors of the host should also be considered to play a role in H pylori-induced outcomes.
Here, we discuss recent developments in gene-environment interaction and the importance of H pylori eradication in the prevention of gastric cancer.
H pylori has been associated with the location of gastric cancers, specifically those of the body and antrum. No association is seen with the location of cardiac tumors[20]. H pylori gastritis is characterized by severe, acute and chronic inflammation which would last for decades if not treated[21]. Such persistent inflammation likely has serious biological implications. For example, activated neutrophils generate reactive oxygen and nitrogen species, which are mutagenic and carcinogenic [22,23]. Atrophic gastritis, intestinal metaplasia and dysplasia are known to be precancerous. Uemura et al reported that gastric cancer developed in 36 (2.9%) of 1 246 infected and none of 280 uninfected patients[24]. Among their patients with H pylori infection, those with severe gastric atrophy, corpus-predominant gastritis and intestinal metaplasia were at a significantly higher risk for gastric cancer. Direct associations between H pylori infection and gastric cancer have been examined for many regions and ethnic groups[25]. In their retrospective analysis of a nested case-control study, Wang et al reported that the risk of death from gastric cancer in the H pylori -positive cohort was 1.985 times to the -negative control cohort (95% CI, 1.0026-3.9301), and that the OR of H pylori infection for gastric cancer was 4.467 (95% CI, 1.161-17.190)[26]. The high incidence of infection in patients with gastric cancer has been confirmed for both the intestinal and diffuse types, and is particularly strong in the former[27]. However, while H pylori appears to play a role in the initial step as a causative agent for chronic gastritis, the development of gastric cancer is multi-factorial.
Khanna et al in India reported a lower prevalence of H pylori infection in gastric cancer patients than in the healthy controls, without significance, and suggested that H pylori may not be responsible for gastric cancer[28]. On the other hand, Enroth et al showed that relative risk estimates for the association between H pylori and gastric cancer risk are to some extent determined by the diagnostic method used to detect H pylori infection[29]. The loss of serological markers of H pylori infection following the onset of severe atrophy and intestinal metaplasia is a well-known phenomenon, not least in Japan[30].
While some studies have reported no association with gastric cancer, the summary statistic derived from a meta-analysis indicated no doubt as to the existence of an association[25].
Recent studies have suggested that patients infected with cagA-positive strains of H pylori are at a significantly higher risk for gastric cancer than those carrying cagA-negative strains[31-36]. CagA protein, encoded by the cagA gene, is one of the most studied virulence factors of H pylori and is a highly immunogenic protein. The cagA gene is one of several genes of a pathogenicity island (PAI) called the cag PAI. The cag PAI contains 31 genes, 6 of which are thought to be encoded by a putative type IV secretion system. Although H pylori cagA-positive isolates from the USA and Japan induce similar IL-8 and apoptosis levels[37], the grade of gastric atrophy (and gastric cancer risk) is higher in patients with the East Asian cagA-positive strains than in those with cagA-negative or Western cagA-positive strains[18]. In Asian populations, however, almost all infected subjects harbor cagA-positive strains, raising legitimate questions about the relevance of this virulence factor as a risk determinant in such populations.
Held et al reported that although patients with antibodies to CagA at greatest risk of gastric cancer, risk is still significantly higher in those with cagA-negative H pylori infections than in uninfected persons[38]. Anti-CagA responses correlate with neutrophil infiltration, a low anti- H pylori IgG titer or combined with H pylori seronegativity was closely associated with non-cardia gastric cancer, independently of ethnicity[40]. A meta-analysis of the relationship between CagA seropositivity and gastric cancer showed that infection with cagA-positive H pylori increased the risk for gastric cancer over that with H pylori infection alone[41]. Because antibodies to CagA remain positive for longer than IgG antibodies to H pylori [42,43], the risk for gastric cancer based on H pylori IgG antibody might be underestimated[35,44].
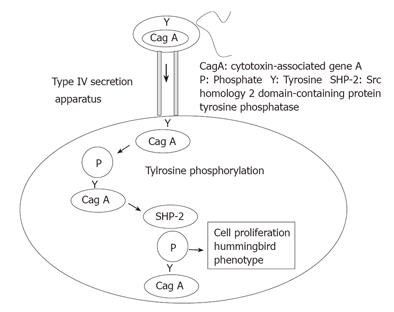
CagA is delivered into epithelial cells by the cag type IV secretion system, then phosphorylated on tyrosine residues and wired to the eukaryotic signal transduction pathway, which plays a major role in H pylori -host cell interactions and pathogenesis (Figure 1)[45-48]. In the injected gastric epithelial cell, CagA induces cellular spreading and elongation, termed the hummingbird phenotype, and this is thought to play a crucial role in the pathogenesis of cagA-positive H pylori infection. This CagA-dependent morphological transformation of gastric epithelial cells requires src homology 2 domain-containing protein tyrosine phosphatase-2 ( SHP-2)[49]. SHP-2 plays a key role in the intracellular signaling elicited by a number of growth factors, hormones and cytokines[50,51]. East Asian-type CagA exhibits stronger SHP-2-binding activity than Western-type CagA[19]. CagA.SHP-2 signaling may induce apoptosis and elevate the epithelial cell turnover associated with cagA-positive H pylori infection [26-32]. Extra cycles of DNA replication would increase the chance of genetic mutation leading to abnormal proliferation.
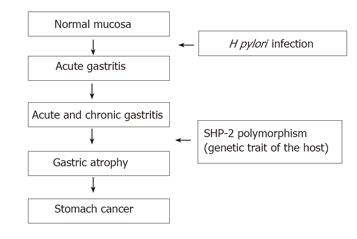
We have reported the association of a frequent single nucleotide polymorphism (SNP, JST057927, G-to-A) in the PTPN11 gene that encodes SHP-2 with gastric atrophy and gastric cancer[52]. We found that this polymorphism increased the risk of gastric atrophy and gastric cancer among H pylori -seropositive Japanese subjects. Carriage of the G allele of PTPN11 increased the risk of atrophy whereas the A/A genotype was protective against it. The SHP-2-binding activity of CagA influences the its virulence in the induction of gastric atrophy, the precursor lesion of gastric cancer. PTPN11 G/A polymorphism may constitute a genetic trait of the host predisposing to atrophy among those infected (Figure 2). CagA.SHP-2 complex formation may induce abnormal proliferation and movement of gastric epithelial cells, cellular changes that may eventually lead to gastric atrophy and gastric carcinoma. Against this, however, several groups have reported that SHP-2 is not involved in CagA action[53,54]. The resolution of this controversy is awaited.
High mucosal levels of cytokines in H pylori-infected patients have been reported, including IL-8, IL-6, IL-1B, TNF-A, MIP 1α and IL-2[55-62]. Host cytokine gene polymorphisms IL-1B, IL-1RN, TNF-A and IL-10 are suggested to be part of the genetic background predisposing patients to noncardia gastric cancer in response to H pylori[13,15,17,63]. IL-1B and TNF-A are functional polymorphisms that affect the production of IL-β and TNF-α, which inhibit gastric acid secretion[64,65]. The IL-1B gene encoding IL-1β is highly polymorphic, and several diallelic polymorphisms have been reported, two in the promoter region at positions -511 and -31, representing C-T and T-C transitions, respectively. Several studies have shown that these two polymorphisms are in near-total linkage disequilibrium[13,66]. They have been shown to significantly affect gastric mucosal IL-1β production in response to H pylori infection[67,68], and it is this higher production of IL-1β which most likely mediates their effect on gastric acid secretion. Zambon et al have reported that among host genetic factors contributing to H pylori disease outcome, IFN-G AA favors cagA-positive H pylori infection, TNF-A TT favors duodenal ulcer, while IL-10 TT favors intestinal metaplasia and noncardia gastric cancer [69]. In Japan and Korea, however, these associations appear less clear[70,71].
A joint World Health Organization/Food and Agriculture Organization Expert Consultation concluded that salt and salt-preserved food probably increase the risk of gastric cancer[72]. Substantial evidence from ecological, case-control and cohort studies suggest that cancer risk may also increase with a high intake of some traditional salt-preserved foods and salt per se, and that this risk could be decreased with a high intake of fruits and vegetable[73,74]. Other established non-dietary factors include cigarette smoking[75]. Tsugane et al have documented that the consumption of salted food (pickled vegetables and miso soup) appears to increase the risk of H pylori infection[76]. Salted food intake has been shown to act synergistically to promote the development of gatric cancer in Mongolian gerbils treated with N-methyl-N-nitrosourea (MNU)[77], and a synergistic enhancing effect between salted food intake and H pylori infection has also been reported in a case-control study in Korea[78]. Motani et al [79] reported that smoking and a high intake of miso soup were associated with noncardia cancer regardless of H pylori infection, and also a strong association between cagA-positive H pylori and noncardia cancer. Although H pylori infection is clearly an important risk factor for gastric cancer, smoking cessation and dietary modification may be practical strategies for the prevention of non-cardia gastric cancer among both H pylori -positive and -negative subjects.
Conclusive proof for a preventive effect of H pylori eradication on gastric carcinogenesis will never be available, because doing so would require the inclusion of individuals in a placebo trial in which the end point is gastric cancer; for not only practical but also ethical and economic reasons, no such study will ever be performed. The alternative is randomized controlled studies that are designed to examine the regression of preneoplastic conditions, such as intestinal metaplasia and gastric atrophy, as surrogate end points of eradication treatment success. One such study is a prospective, randomized, placebo-controlled, population-based primary prevention study of 1630 healthy carriers of H pylori infection from Fujian Province, China, recruited in July 1994 and followed up until January 2002[80]. A total of 988 participants did not have precancerous lesions (gastric atrophy, intestinal metaplasia, or gastric dysplasia) on study entry. Patients were randomly assigned to receive H pylori eradication treatment by a 2-wk course of omeprazole 20 mg, a combination product of amoxicillin and clavulanate potassium, 750 mg, and metronidazole 400 mg, all twice daily (n = 817); or placebo (n = 813). Among the 18 new cases of gastric cancer that developed, no overall reduction was observed in participants who received H pylori eradication treatment (n = 7) compared with those who did not (n = 11). In a subgroup of patients with no precancerous lesions on entry, no patient developed gastric cancer during a follow-up of 7.5 years after H pylori eradication treatment compared with those who received placebo (0 vs 6; P = 0.02). Although the incidence of gastric cancer development at the population level was similar between participants receiving H pylori eradication treatment and those receiving placebo over 7.5 years in a high-risk region of China, eradication of H pylori significantly decreased the development of gastric cancer in the subgroup of H pylori carriers without precancerous lesions.
A second study was conducted in Hong Kong[81]. The authors randomized 435 subjects into placebo and eradication groups, the latter of whom received a one-week course of anti-H pylori therapy of OAC (omeprazole 20 mg, amoxicillin 1g, and clarithromycin 500 mg twice a day). Clearance of H pylori infection at 5 years was confirmed by histology in 164 (74.5%) who had received the eradication therapy versus only 20 (9.3%) subjects in the placebo group. Ten subjects developed invasive gastric cancer during the 5-year follow-up period, four in the eradication and six in the placebo group. Overall progression of gastric intestinal metaplasia (IM), defined as a surrogate marker of cancer, was seen in 52.9% of subjects. Eradication of H pylori was significantly associated with a decrease in the risk of IM progression. Patients assigned to receive OAC had a significantly lower risk of progression compared with those who received placebo (OR for progression 0.63; 95% CI, 0.43-0.93). When those in the OAC group with eradication were compared with those in the placebo group with persistent infection, the OR of histological progression was further reduced to 0.48 (95% CI, 0.32-0.74). Although this intervention study failed to demonstrate an effect on gastric cancer risk, eradication of H pylori was protective against the progression of a premalignant gastric lesion, namely IM.
Another study was reported from Mexico[82]. A total of 316 CagA-positive subjects were randomized into placebo (n = 155) and eradication groups (n = 161), who received 20 mg of omeprazole, 1 g of amoxicillin and 500 mg of clarythromycin, all twice a day for 1 week. Endoscopy was performed at baseline and at 6 weeks and 1 year, with seven biopsies from each endoscopy reviewed by two pathologists. Cure rates in the eradication group were 79.2% and 75.7% at 6 weeks and 1 year, respectively, compared with respective placebo rates of 2.9% and 1.9% (P < 0.001). Outcome measures were both a consensus “worst biopsy” diagnosis and a weighted index score that incorporated the degree of severity of preneoplasia, with changes in these outcomes compared over time. No significant change in the worst biopsy diagnosis was observed between groups (improvement/worsening: placebo, 19.4%/10.5%; treatment, 22.5%/8.3%; P = 0.74). The change in index score was favorably greater in the treatment than in the placebo subjects (intention-to-treat analysis, P = 0.03). These studies of intermediate biomarkers provide circumstancial evidence that H pylori eradication diminishes the risk of gastric cancer.
Following partial gastrectomy, the mucosa of the residual stomach usually undergoes severe changes, such as gastric atrophy, intestinal metaplasia and dysplasia[83,84]. The 1996 Maastricht Consensus Report strongly recommended eradication in infected patients who had undergone gastrectomy for early gastric cancer[85]. Definite proof of the merit of eradication awaits the completion of a large randomized trial using cancer as the outcome. Nevertheless, the evidence now available supports a conclusion for eradication.
Effective H pylori eradication along with a natural decrease in infection due to improved living conditions have resulted in declining gastric cancer rates in Western countries, although this still remains a significant cause of morbidity and mortality in other parts of the world. While the genes in cag PAI are the most strongly virulence-related among those reported to date, other genes have also been reported as candidates that determine outcome in H pylori -infected persons[86,87]. Studies that identify further bacterial as well as host genetic factors that place the patient at greatest risk of disease progression may enhance our approach to better screening strategies, and will improve the control of H pylori infection in affected subjects.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Feldman RA, Eccersley AJ, Hardie JM. Epidemiology of Helicobacter pylori: acquisition, transmission, population prevalence and disease-to-infection ratio. Br Med Bull. 1998;54:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 499] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 3. | Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760-766. [PubMed] |
| 4. | Youn HS, Ko GH, Chung MH, Lee WK, Cho MJ, Rhee KH. Pathogenesis and prevention of stomach cancer. J Korean Med Sci. 1996;11:373-385. [PubMed] |
| 5. | Sipponen P, Marshall BJ. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am. 2000;29:579-92, v-vi. [PubMed] |
| 6. | Plummer M, Franceschi S, Muñoz N. Epidemiology of gastric cancer. IARC Sci Publ. 2004;311-326. [PubMed] |
| 7. | Peterson WL, Graham DY. H pylori. Gastrointestinal and liver Disease. Pathophysiology, diagnosis, management. 6th ed. Philadelphia: WB Saundes 1998; 604-619. |
| 8. | Cave DR. Chronic gastritis and Helicobacter pylori. Semin Gastrointest Dis. 2001;12:196-202. [PubMed] |
| 9. | Cohen H. Peptic ulcer and Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 11. | Infection with Helicobactor pylori. In: IARC monographs on the evaluation of the carcinogenic risks to humans. Vol. 61. Schistosomes, liver flukes, and Helicobactor pylori. Lyon, France: International Agency for Research on Cancer 1994; 177-241. |
| 12. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] |
| 13. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1675] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 14. | El-Omar EM, Chow WH, Rabkin CS. Gastric cancer and H. pylori: Host genetics open the way. Gastroenterology. 2001;121:1002-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 16. | Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Yamaoka Y, El-Zimaity HM, Gutierrez O, Figura N, Kim JG, Kodama T, Kashima K, Graham DY. Relationship between the cagA 3' repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M. Association between diversity in the Src homology 2 domain--containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004;189:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Whiting JL, Hallissey MT, Fielding JW, Dunn J. Screening for gastric cancer by Helicobacter pylori serology: a retrospective study. Br J Surg. 1998;85:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3552] [Article Influence: 122.5] [Reference Citation Analysis (3)] |
| 22. | Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1729] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 23. | Tamir S, Tannenbaum SR. The role of nitric oxide (NO.) in the carcinogenic process. Biochim Biophys Acta. 1996;1288:F31-F36. [PubMed] |
| 24. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 25. | Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103-1107. [PubMed] |
| 27. | Caputo S, Mosca F, Persi A, Ettaro G, Scaringi S, Russo G, Piazza D. [Helicobacter pylori and gastric cancer. The incidence of infection in personal experience]. Minerva Chir. 2002;57:649-655. [PubMed] |
| 28. | Khanna AK, Seth P, Nath G, Dixit VK, Kumar M. Correlation of Helicobacter pylori and gastric carcinoma. J Postgrad Med. 2002;48:27-28. [PubMed] |
| 29. | Enroth H, Kraaz W, Rohan T, Nyrén O, Engstrand L. Does the method of Helicobacter pylori detection influence the association with gastric cancer risk. Scand J Gastroenterol. 2002;37:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Kikuchi S, Crabtree JE, Forman D, Kurosawa M. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults. Research Group on Prevention of Gastric Carcinoma Among Young Adults. Am J Gastroenterol. 1999;94:3455-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 33. | Brenner H, Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G. Individual and joint contribution of family history and Helicobacter pylori infection to the risk of gastric carcinoma. Cancer. 2000;88:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Maeda S, Yoshida H, Ogura K, Yamaji Y, Ikenoue T, Mitsushima T, Tagawa H, Kawaguchi R, Mori K, Mafune Ki. Assessment of gastric carcinoma risk associated with Helicobacter pylori may vary depending on the antigen used: CagA specific enzyme-linked immunoadsorbent assay (ELISA) versus commercially available H. pylori ELISAs. Cancer. 2000;88:1530-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Ando T, Peek RM, Lee YC, Krishna U, Kusugami K, Blaser MJ. Host cell responses to genotypically similar Helicobacter pylori isolates from United States and Japan. Clin Diagn Lab Immunol. 2002;9:167-175. [PubMed] |
| 38. | Held M, Engstrand L, Hansson LE, Bergström R, Wadström T, Nyrén O. Is the association between Helicobacter pylori and gastric cancer confined to CagA-positive strains. Helicobacter. 2004;9:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Ando T, Perez-Perez GI, Kusugami K, Ohsuga M, Bloch KC, Blaser MJ. Anti-CagA immunoglobulin G responses correlate with interleukin-8 induction in human gastric mucosal biopsy culture. Clin Diagn Lab Immunol. 2000;7:803-809. [PubMed] |
| 40. | Tatemichi M, Hamada GS, Nishimoto IN, Kowalski LP, Iriya K, Rodrigues JJ, Tsugane S. Ethnic difference in serology of Helicobacter pylori CagA between Japanese and non-Japanese Brazilians for non-cardia gastric cancer. Cancer Sci. 2003;94:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Sörberg M, Engstrand L, Ström M, Jönsson KA, Jörbeck H, Granström M. The diagnostic value of enzyme immunoassay and immunoblot in monitoring eradication of Helicobacter pylori. Scand J Infect Dis. 1997;29:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Klaamas K, Held M, Wadström T, Lipping A, Kurtenkov O. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int J Cancer. 1996;67:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle PR, Stremmel W. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Dig Dis Sci. 1997;42:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 46. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 47. | Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999;96:14559-14564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 592] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 48. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 967] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 49. | Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 982] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 50. | Tartaglia M, Niemeyer CM, Shannon KM, Loh ML. SHP-2 and myeloid malignancies. Curr Opin Hematol. 2004;11:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Tang TL, Freeman RM, O'Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 272] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Goto Y, Ando T, Yamamoto K, Tamakoshi A, El-Omar E, Goto H, Hamajima N. Association between serum pepsinogens and polymorphismof PTPN11 encoding SHP-2 among Helicobacter pylori seropositive Japanese. Int J Cancer. 2006;118:203-208. [PubMed] |
| 53. | Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 54. | Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Zambon CF, Basso D, Navaglia F, Germano G, Gallo N, Milazzo M, Greco E, Fogar P, Mazza S, Di Mario F. Helicobacter pylori virulence genes and host IL-1RN and IL-1beta genes interplay in favouring the development of peptic ulcer and intestinal metaplasia. Cytokine. 2002;18:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, Plebani M. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res. 1996;26:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [PubMed] |
| 58. | Holck S, Nørgaard A, Bennedsen M, Permin H, Norn S, Andersen LP. Gastric mucosal cytokine responses in Helicobacter pylori-infected patients with gastritis and peptic ulcers. Association with inflammatory parameters and bacteria load. FEMS Immunol Med Microbiol. 2003;36:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Bontems P, Robert F, Van Gossum A, Cadranel S, Mascart F. Helicobacter pylori modulation of gastric and duodenal mucosal T cell cytokine secretions in children compared with adults. Helicobacter. 2003;8:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150-1156. [PubMed] |
| 61. | Ando T, Kusugami K, Ohsuga M, Ina K, Shinoda M, Konagaya T, Sakai T, Imada A, Kasuga N, Nada T. Differential normalization of mucosal interleukin-8 and interleukin-6 activity after Helicobacter pylori eradication. Infect Immun. 1998;66:4742-4747. [PubMed] |
| 62. | Ando T, Kusugami K, Ohsuga M, Ina K, Ichiyama S, Nada T, Ohta M. Mucosal macrophage inflammatory protein-1alpha levels are increased in Helicobacter pylori infection. J Clin Gastroenterol. 1998;27 Suppl 1:S144-S149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 64. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 65. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Hamajima N, Matsuo K, Saito T, Tajima K, Okuma K, Yamao K, Tominaga S. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn J Cancer Res. 2001;92:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 68. | Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, Gerhard M, Prinz C. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 69. | Zambon CF, Basso D, Navaglia F, Belluco C, Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Lee KA, Ki CS, Kim HJ, Sohn KM, Kim JW, Kang WK, Rhee JC, Song SY, Sohn TS. Novel interleukin 1beta polymorphism increased the risk of gastric cancer in a Korean population. J Gastroenterol. 2004;39:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | World Health Organization. Diet, nutrition, and the prevention of chronic diseases. WHO technical report series 916. Geneva: World Health Organization 2003; . |
| 73. | World Cancer Reserch fund, American Institute for cancer Reserch. Food, nutrition and the prevention of cancer: a global perspective. Washington: American Institute for Cancer Reserch 1997; . |
| 74. | Kono S, Hirohata T. Nutrition and stomach cancer. Cancer Causes Control. 1996;7:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | International Agency for Research on Cancer. Tobacco smoking and Tobacco smoke. IARC Monographs on the evaluation of the carcinogenic risks to Humans, 83. Lyon: International Agency for Research on Cancer 2004; . |
| 76. | Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K. Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res. 1994;85:474-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1046] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 81. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 82. | Ley C, Mohar A, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, Johnstone I, Parsonnet J. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double-blind, placebo-controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 83. | Langhans P, Bues M, Bünte H. Morphological changes in the operated stomach under the influence of duodenogastric reflux. Clinical follow-up over 20 years. Scand J Gastroenterol Suppl. 1984;92:145-148. [PubMed] |
| 84. | Weinstein WM, Buch KL, Elashoff J, Reedy T, Tedesco FJ, Samloff IM, Ippoliti AF. The histology of the stomach in symptomatic patients after gastric surgery: a model to assess selective patterns of gastric mucosal injury. Scand J Gastroenterol Suppl. 1985;109:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 674] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 86. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A. 2000;97:7533-7538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 87. | Ando T, Wassenaar TM, Peek RM, Aras RA, Tschumi AI, van Doorn LJ, Kusugami K, Blaser MJ. A Helicobacter pylori restriction endonuclease-replacing gene, hrgA, is associated with gastric cancer in Asian strains. Cancer Res. 2002;62:2385-2389. [PubMed] |