INTRODUCTION
Inflammatory pseudotumor (IPT) is a rare benign lesion histologically characterized by the presence of a heterogeneous population of inflammatory cells, particularly plasma cells, macrophages and fibroblasts, as well as areas of fibrosis and necrosis. Pack et al[1] first described IPT of the liver in 1953. Despite numerous subsequent descriptions in the literature[2-5], IPT remains difficult to diagnose, causing major problems particularly for differential diagnosis especially that with malignant liver tumor. There are neither specific signs in imaging techniques, nor conclusive biochemical tests. Histological examinations are always required for confirmation of the diagnosis. The etiology of IPT remains obscure to date, especially in the absence of documented evidence of its having associations with any particular diseases apart from phlebitis[6] and Crohn’s disease[7] shown in a few reports.
For our study, we selected a middle-aged male patient with hepatic IPT and peripheral eosinophilia associated with autoimmune pancreatitis (AIP). AIP is a recently recognized disorder and new clinical entity associated with irregular narrowing of the pancreatic ducts and swelling of the parenchyma[8-10]. AIP is difficult to diagnose differentially from pancreatic cancer. When liver tumors are found in AIP, there is a possibility of a misdiagnosis with pancreatic cancer with liver metastasis. Thus, the documentation of this case may be of value for the prevention of misdiagnosis in similar cases.
CASE REPORT
A 59-year-old man was admitted with obstructive jaundice. He had no remarkable familial or personal medical histories. He had taken no drugs prior to admission. Physical examination on admission revealed no abnormal findings except for the jaundice.
Abnormal laboratory findings were as follows: erythrocyte sedimentation rate (ESR) 60 mm/hr, CRP 0.6 mg/dL, white blood cell count 10100/mm3 with 13.3% eosinophils (1343/mm3), serum IgE 1481 IU/mL, IgG 4145 mg/dL, total bilirubin 11.6 mg/dL, ALP 655 IU/L. Serum amylase, ALT and AST were not elevated. CEA and CA19-9 remained within normal range. Autoantibodies were negative except for rheumatoid factor. Antibodies for hepatitis virus and HIV were negative. Stool specimens showed no ova or parasites.
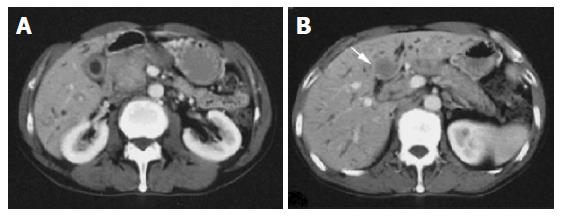
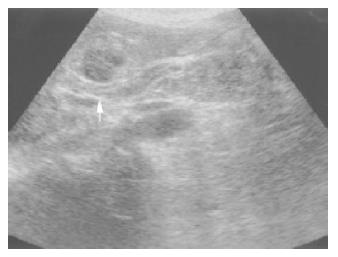
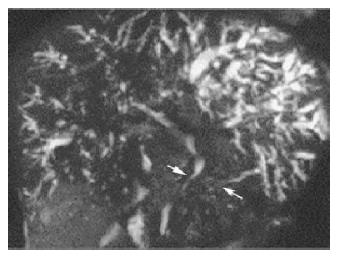
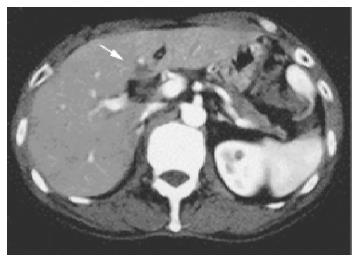
Ultrasonography and CT scan revealed dilatation of the intrahepatic bile duct, common bile duct stenosis with diffuse wall thickening, gallbladder wall thickening and swelling of the pancreas (Figure 1). Multiple liver masses were also demonstrated as low echogenic areas by ultrasonography (Figure 2), and they were not enhanced by contrast medium on CT. Magnetic resonance cholangiopancreatography (MRCP) revealed the stricture of the common bile duct and the markedly dilated intrahepatic bile duct (Figure 3). Endoscopic retrograde cholangiopancreatography (ERCP) was not successful, but the brush cytology specimens obtained from the distal bile duct and distal pancreatic duct contained considerable numbers of eosinophils though malignancy was not indicated by the cytology tests.
Figure 1 A: CT demonstrated dilatation of the intrahepatic bile ducts, swelling of the pancreatic head, thickening of the gallbladder wall; B: CT revealed the liver mass, which was not enhanced by contrast medium.
Figure 2 Ultrasonography demonstrated hypoechoic liver mass.
Figure 3 MRCP showed stricture of the common bile duct with marked dilatation of the intrahepatic bile duct and irregular narrowing of the pancreatic duct.
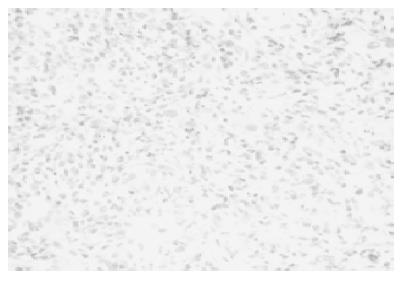
Percutaneous biopsy of the liver masses was performed under ultrasonographic guidance. Histology showed mixed inflammatory infiltrates composed of lymphocytes, histiocytes, plasma cells, and a prominent number of eosinophils. There was no evidence of epithelial, mesenchymal or lymphoid malignancy (Figure 4).
Figure 4 Biopsy specimens from the liver mass showed lymphocytes, histiocytes, plasma cells, and a number of eosinophils.
It did not contain normal liver tissue or malignant cells.
Prednisolone (40 mg/d) was administered orally. Four weeks later, the bile duct stricture, common bile duct wall thickening and gallbladder wall thickening improved remarkably. The liver masses resolved. The swelling of the pancreatic head also improved. The steroid dose was tapered gradually, and 6 mo later he took 5 mg of prednisolone. At this point, the biliary and pancreatic abnormal findings had completely disappeared (Figure 5), and the peripheral eosinophil count had also become normal (Figure 6).
Figure 5 CT revealed that the pancreatic swelling almost resolved 1 mo after the treatment.
Liver masses decreased.
Figure 6 MRCP demonstrated the normalized bile duct 6 mo after the treatment.
Two years after remission of the disorder, serum immunoglobulin IgG4 was assayed. The serum level of IgG4 remained markedly elevated to 1810 mg/dL. The patient, on a maintenance regimen of 5 mg of prednisolone, was in good health, and the features of the disorder remained resolved.
DISCUSSION
The clinical features of the present case were similar to those of pancreatic cancer with obstructive jaundice and liver metastasis. The liver masses were diagnosed as inflammatory pseudotumor (IPT) by histological examination of biopsy specimens. IPT is a benign lesion with the presence of a heterogeneous population of inflammatory cells, particularly plasma cells, macrophages and fibroblasts, as well as areas of fibrosis and necrosis. However, it is difficult to distinguish this lesion from malignant tumor. The diagnostic challenge of IPT is emphasized by the fact that most of the reported cases were diagnosed by surgical procedures including either wedge resection or lobectomy. In such intricate cases as the present one, careful attention must be paid so that misdiagnosis and resultant inappropriate treatment can be avoided.
The most conspicuous finding in the laboratory tests was the marked peripheral eosinophilia. This might suggest a diagnosis of hypereosinophilic syndrome (HES). HES is diagnosed when the following criteria are met: Persistent eosinophilia (1500 eosinophils/mm3) for at least 6 mo or death before 6 mo with signs and symptoms of HES disease; exclusion of other recognized causes of eosinophilia; organ system involvement or dysfunction attributable to eosinophil infiltration or not otherwise explained[11]. In the present case, the peripheral eosinophil count was lower than 1500/mm3, and the condition persisted for only 2 mo. Thus, a case like this should not be diagnosed as HES, despite the fact that possible causes of eosinophilia such as parasitic infection had been excluded. Nonetheless, it cannot be ruled out that eosinophilia might play a role in the pathogenesis of the clinical features presented.
Another conspicuous feature was obstructive jaundice caused by biliary stricture with common bile duct wall thickening. We first considered that this obstructive jaundice was associated with eosinophilic cholangitis (EC). Butler first reported EC in 1985, which was associated with obstructive jaundice, lymphadenopathy and peripheral eosinophilia, and showing marked eosinophilic infiltration in the gallbladder and cystic duct as well as mild infiltration in the liver[12]. Subsequently, a number of cases with similar conditions were reported under various nomenclatures, including primary biliary sclerosing cholangitis with eosinophilic infiltration and eosinophilic gastroenteritis with biliary involvement as well as EC. In our patient, an additional feature was the swelling of the pancreas, whereas pancreatic involvement was never observed in the reported cases with EC[13-21]. Furthermore, the mean age of the patients with EC was 34 years (range 20-48), while our patient was 59. Our patient showed marked hypergammaglobulinemia (IgG 4145 mg/dL), a finding not reported in EC cases except for one with mild serum IgG elevation of 1700 mg/dL[14]. Thus, it was hardly conceivable that the present case belonged to a category of eosinophilic cholangitis or the like.
It is in this regard that we think of autoimmune pancreatitis (AIP) for a disorder manifesting the aforesaid features. AIP is a recently recognized unique form of chronic pancreatitis with irregular narrowing of the pancreatic duct and swelling of the pancreatic parenchyma[8-10]. This condition was initially described as chronic pancreatitis associated with hypergammaglobulinemia by Sarles et al[18] in 1961. Since then, AIP has been described mainly in Japanese literature. Multiple definitions are given for this condition, including primary inflammatory sclerosis of pancreas, sclerosing pancreatitis, lymphoplasmacytic sclerosing pancreatitis, autoimmune sclerosing pancreatitis and sclerosing pancreato-cholangitis. As those terms indicated, the affected organs are not limited to the pancreas. The biliary tree is the most frequent location involved[19]. Okazaki et al[10] reported that obstructive jaundice was observed in 40% of their series. The increase of peripheral eosinophil count is often observed in AIP[9]. Furthermore, the age of AIP patients was reported to be an average of 59-61 years[10,20], clearly higher than that of eosinophilic cholangitis.
Hamano et al[21] reported that patients with AIP had high serum IgG4 concentrations, the finding of which is usually observed in only a limited number of conditions, including autoimmune skin diseases such as pemphigus vulgaris and pemphigus foliaceus. Recently, the IgG4 assay has been recognized as the most useful test for the diagnosis of AIP. The present case showed a high serum IgG4 concentration of 1810 mg (normal range <135 mg/dL).
Based on our findings, the diagnosis of this case was AIP with IPT, a condition not previously described in the literature. Recently, IPT was associated with an immune reaction[22,23]. It is entirely possible that IPT is associated with AIP.
To date, several treatment approaches, including antibiotics[24], nonsteroidal anti-inflammatory drugs[25], have been described for IPT of the liver. The spontaneous resolution of the lesion after a few months has been reported[26]. The prognosis of this lesion is considered favorable in the majority of cases. The liver mass of our patient was successfully treated with prednisolone. However, there had been a few cases having undergone surgical resection or liver transplantation[27-29]. Some of them could have avoided surgery if preoperative diagnosis was certainly made.
In conclusion, a case of hepatic IPT and peripheral eosinophilia in AIP was reported. The present case deserves to be documented. This is helpful in avoiding misdiagnosis and inappropriate treatment in similar cases.