INTRODUCTION
Acute mesenteric ischemia is an abdominal emergency due to inadequate tissue perfusion. Consequence of late diagnosis is a multi-system organ dysfunction syndrome, which may lead to death[1]. The mortality rate due to intestinal ischemia (Ii) ranges between 60-100%. Yet there is no non-invasive method to diagnose Ii in the early stage. There are several attempts to analyze a biochemical marker for the diagnosis of Ii; however, no suitable marker has been identified till date.
The measurement of plasma D (-)-lactate, amylin level, serum concentration of cytosolic beta-glucosidase (CBG) and diamine oxidase (DAO) may be useful to assess the intestinal injury[2-6]. Preliminary findings suggest that human intestinal fatty acid binding protein (hIFABP) may serve as a diagnostic marker for early intestinal mucosal damage[7,8]. The results suggest that plasma and peritoneal lactic acid (LA) are sensitive indicators in the early diagnosis of bowel ischemia in contrast to serum CK, which is not a useful indicator[9,10]. Glutathione-S-transferase (α-GST) monitoring is also a useful tool for the diagnosis of Ii[11].
Association of Alcohol dehydrogenase (ADH) (EC 1.1.1.1) levels with various pathological conditions has already been suggested[12-14]. It has been reported that endotoxemia occurs in Ii[15]. Gastrointestinal barrier dysfunction in Ii has also been reported[16-18]. Recently, involvement of endotoxin in up-regulation of ADH gene from liver reported by Mezey et al[19] (2003) along with the above-mentioned findings, prompted us to look for ADH levels in Ii. ADH is a major enzyme catalyzing the biological oxidation of ethanol in mammals[20]. Five protein subunits (α, β, γ, π and χ) combine into three classes of isoenzymes. The class IV (σ) ADH encoded by ADH7 is a key enzyme in the metabolism of retinol to retinoic acid (RA)[21,22]. Rat ADH3 and human class I (ADH) are analogous and mainly localized in liver. Rat ADH2 and human χ-ADH (class III) are analogous and ubiquitously found in all tissues. The correspondence between rat ADH-1 and human π-ADH (class II) is not clear. Human π-ADH has been detected exclusively in liver while rat ADH 1 is found in eye, stomach, lung and other rat organs but not in liver[23]. The wide tissue distribution of ADH is already established[23,24]. In an attempt of qualitative determination of ADH in animals suffering from Ii, we found significant increase in ADH enzyme activity. Thus further studies were therefore conducted to investigate the association of increase in ADH enzyme activity with SMA occlusion.
MATERIALS AND METHODS
Eight weeks old rats (Wistar strain) weighing between 150-200 g were fasted 18 h before conducting the experiments. Two groups were assigned, sham-operated control (n = 24) and one-hour ischemic test group (n = 24). Midline laprotomy was performed on both the groups after giving ether anesthesia. The control group of animals was sham-operated in order to trace the superior mesenteric artery (SMA) while in test group the SMA was flush ligated. Both the groups were kept under observation for 1 h.
After 1 h the blood was withdrawn from portal vein, right ventricle of heart, dorsal aorta (DA) and inferior vena cava (IVC). The serum was separated immediately and stored at -40 °C till the assay was performed. The serum glutamic acid pyruvate transaminase (SGPT) was assayed in serum sample drawn from portal vein and heart while alcohol dehydrogenase (ADH) was assayed in samples drawn at all points mentioned above within 24 h of blood withdrawal. Alcohol dehydrogenase assay was performed by the method of Skursky et al[25]. The substrate p-nitroso N, N dimethyl aniline (NDMA) was prepared in the laboratory[26]. Both kinetic as well as one-point incubation method were performed. Concentration of ADH is expressed in terms of μM of NDMA reduced per min per mL of serum.
The enzyme kinetic assays were carried out in 96 well flat bottom micro titer plates and 7.5 μL of serum was added to each well. To this 132.5 μL of substrate solution was added and the plates were read immediately at 440 nm in kinetic mode for 10 min on the kinetic reader Softmax Pro, Version 4 (Molecular devises, USA). SGPT assay was carried out using ‘Infinate GPT’ kit (Accurex Biomedical Pvt. Ltd, India).
Intestinal tissue of about 10 cm in length and a small part of liver were removed immediately after the end of the experiment and fixed in 10% formal saline. The sections were stained with hematoxylin/eosine for histopathological investigation. The grades of the injury to the intestine were defined as mentioned by Khurana et al[27] briefly: grade 0- normal. Grade 1- subepithelial blebs with small areas of apical epithelial loss; stroma and crypts normal; vessels are patent. Grade 2- total loss of villous epithelium but crypts and stroma are normal; vessels are patent. Grade 3- total villous loss with some crypts involved; stroma normal; vessels are patent. Grade 4- all crypts involved, stroma is normal, but vessels patent and remain patent. Grade 5- all Grade 4 changes with stromal necrosis and/or evidence of vessel thrombosis.
Student t test was used for statistical analyses of data using ‘Quick-stat’software.
RESULTS
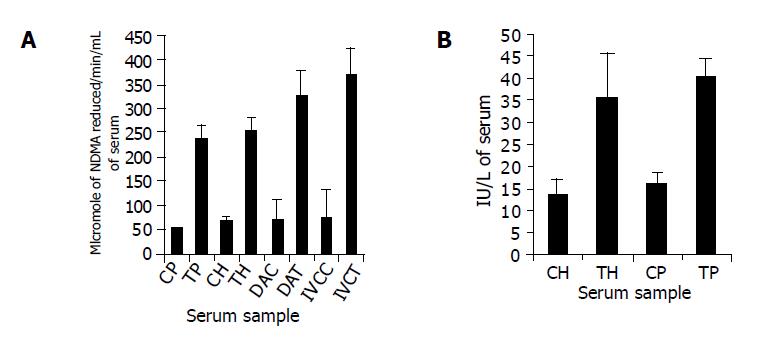
The levels of SGPT and ADH are depicted in (Figure 1). The values of SGPT in portal blood in test and control animals were 39.93±7.99 IU/L and 15.70±4.83 IU/L, respectively. SGPT levels in samples drawn from heart were 35.27±18.11 IU/L and 13.04±6.87 IU/L, respectively in test and control animals. The increase in SGPT levels in heart blood of test animals was not significant as compared to that in portal blood of test animals (P = 0.054).
Figure 1 Alcohol Dehydrogenase Assay By Skursky et al (A) and SGPT Assay (B) performed in heart blood and portal blood of 1 h ischemic test and sham operated control group.
A: Alcohol dehydrogenase assay performed by Skursky et al method. CH and TH represent the blood withdrawn from heart of control group and test group, respectively. CP and TP represent the blood withdrawn from portal vein of control group and test group, respectively. DAC and DAT represent the dorsal aorta blood sample from control and test group, respectively while IVCC and IVCT represent the blood sample from inferior vena cava of control and test group, respectively. The EU expressed in terms of micromoles of NDMA reduced per min/L of serum; B: SGPT assay. CH and TH represent the blood withdrawn from heart of control group and 1 h ischemic test group, respectively. CP and TP represent the blood withdrawn from portal vein of control group and 1 h ischemic test group, respectively.
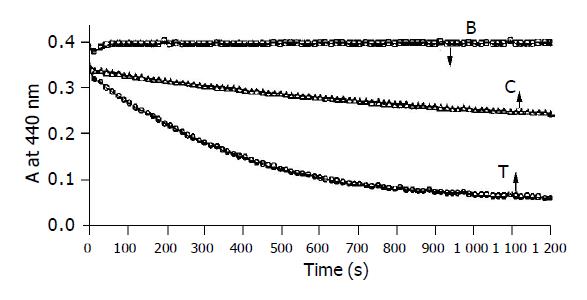
There was nearly 5 times increase in the levels of ADH in blood from portal vein, DA and IVC; however, heart blood showed a rise of about 4 times in test group as compared to control group. The levels of ADH in portal blood of test group and control group were 232.72±99.44 EU and 46.38±21.69 EU, respectively whereas those in heart blood of test and control were 250.85±95.14 EU and 65.37±30.55 EU, respectively. Similarly there was an increase in DA and IVC blood in test group (319.52±80.14 EU and 363.90±120.68 EU, respectively) as compared to control group (67.68±63.22 EU and 72.50±58.50 EU, respectively). The increase in ADH levels at all the points in test animals were highly significant as compared to control group (P<0.001). The kinetic data of blood sample taken from heart of the test animal and control animal also support this fact (Figure 2). Kinetic data also reconfirmed the finding that the levels of ADH at all the points mentioned above were significantly higher in test group as compared to control group.
Figure 2 Kinetic assay of Alcohol dehydrogenase from serum carried out at 30 °C.
(B) Showing practically no auto-reduction of NDMA in the absence of serum, (C) reduction of NDMA in the presence of serum drawn from the heart of control animal after 1 h of sham operation, (T) reduction of NDMA in presence of serum drawn from the heart of test animal after 1 h SMA occlusion.
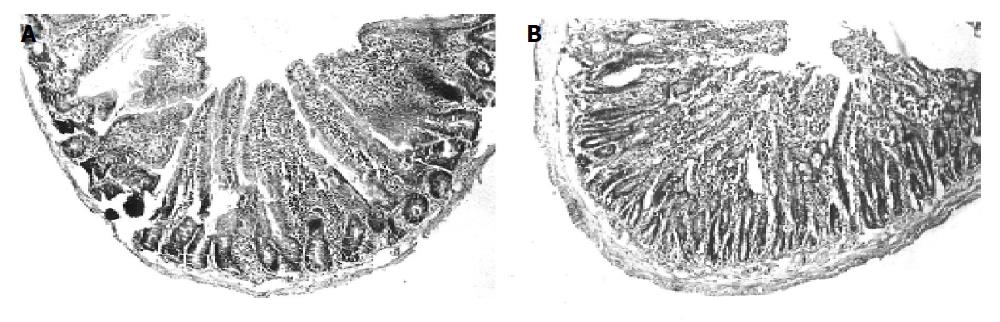
It was interesting to note that histological studies in liver tissue from control and test animal do not show any apparent damage. Grade three injuries were observed in intestines of the test animals those who had suffered 1-h SMA occlusion (Figure 3).
Figure 3 Histology of intestine from rats.
A: C- control sham operated group; 0 grade injury i.e. no damage to villi and crypts; B: t-test one-hour intestinal ischemic group; Total villous loss with some crypts involved; stroma normal. Vessels are patent (i.e. grade 3 injury).
DISCUSSION
Our data reveals highly significant increase in the levels of ADH at all points as mentioned above, as compared to control group. There is evidence to suggest that the barrier properties of the gastrointestinal mucosa are significantly in conditions like ischemia[16,17]. Measurement of plasma (or luminal) clearances of water-soluble molecules has proved to be a popular method for studying intestinal permeability18. Rise in ADH enzyme levels suggest that probably ADH leaks out from gastrointestinal lumen into the blood compartment due to an intestinal barrier dysfunction caused by Ii. Caglayan et al[28] (2002) studied ischemia-reperfusion in intestine in rabbits and estimated CK, LDH, AST and SGPT. They concluded that in ischemic condition there was a rise in AST, LDH and CK levels but there is no rise in the level of SGPT. With these findings they concluded that in ischemia of intestine, liver per se is not contributing into the increased levels of other enzymes and their primary source is injured intestine. It is a well-known fact that alcoholism causes liver damage, but Chrostek et al[29] has shown that in alcoholics the main source of serum ADH is gastrointestinal tract and not liver. Our study also reports significant increase in SGPT levels in portal blood without insignificant increase in heart blood in test animals. This is another supporting evidence that in our study, the intestine and not the liver is responsible for the rise of serum ADH levels in Ii. Data obtained from the histological findings also suggest that there was no damage of liver cells in the test animals.
Variation in SGPT levels in control heart blood and test heart blood was not found to be statistically significant. However, portal vein samples of the same animals showed significant increase of SGPT levels (P = 0.05). This can be explained as the portal blood represents diffuse drainage from the intestine allowing higher concentration of SGPT due to ischemic intestine while such blood that enters subsequently into liver and in body-circulation gets diluted and final concentration would vary according to blood volume of each animal.
It seems that residual level of ADH in normal animal is around 46 EU and the rise due to ischemic insult shoots up to 232 EU in heart blood. Comparatively the levels of ADH in heart, DA and IVC show higher levels than blood withdrawn from portal vein, which is contradictory to the argument, made in relation to SGPT. The DA blood represents the blood, which supplies blood to organs and tissues of lower part of the body while IVC blood represents peripheral blood circulation and collection of blood from all organs and tissues from distal part of the body. When closely compared, the levels of ADH obtained in test animals are 232.72±99.44 EU in portal blood, 250.85±95.14 EU in heart blood, 319.52±80.14 in DA blood and 363.90±120.68 in IVC blood, imply that point of collection of sample does not affect the level of detection of ADH. On the contrary the higher levels of ADH at different points highlight their utility towards diagnosis of Ii as only tissue damaged in this process was the intestine. However, this fact can be explained by hypothesizing that there may be some unknown compounds or factors, which might be getting released by intestinal tissue into the blood, which may interact with other tissues allowing additional ADH release in the blood. The endotoxemia occurs following severe injuries in the intestine in Ii[15]. It is also known that bacterial endotoxin up-regulates the ADH gene in liver[19]. However, we believe, the duration of 1-h ischemia is insufficient to up-regulate the ADH gene in liver and cause increased ADH enzyme in blood. The increase in ADH levels is equally significant in portal blood as well as all other points (Figure 1) in SMA-ligated test animals, which suggests that it cannot be due to up-regulation of ADH gene in the liver by endotoxin. The investigation of such factor will be an interesting approach but presently beyond the scope of this paper.
ADH is also termed as s-nitroso glutathione (GSNO) terminase involved in the elimination of aldehyde resulting from lipid peroxidation, thereby constituting a defense mechanism against cytotoxic aldehydes generated by lipid peroxidation[30]. Since there is generation of localized free radicals, nitric oxide, lipid peroxides and possible production of GSNO in ischemic intestine[31], it is surmised that the increased levels of ADH may be involved in neutralization of lipid peroxidation product and degradation of GSNO.
ADH1 and ADH4 are expressed throughout the gastrointestinal tract and exhibit retinol dehydrogenase activity, which is responsible for the generation of retinoic acid (RA). RA is an essential modulator of epithelial development, differentiation and aging of vascular smooth muscle cells[30]. Due to the occurrence of apoptosis and large amount of cell destruction of enterocytes in ischemia, the role of ADH in generation of enterocytes and preparedness of tissue for recovery cannot be neglected.
The significant increase in the levels of ADH in portal and heart blood along with similar increase in DA blood and IVC blood within 1 h of SMA occlusion without increase in SGPT in heart blood suggests that the origin of ADH is from ischemic intestine. This also rules out the possibility of direct pathophysiological involvement of liver. In fact, the histopathological examination of liver tissue from test samples reconfirms the above findings. Since IVC blood sample, which represents peripheral blood, has shown that increased levels of ADH in test animals confirms the potential of serum ADH in early diagnosis of Ii. Even in clinical settings femoral blood sample can be taken very effectively. Therefore, we feel its clinical utility is much more prominent; however, clinical trials are required in order to confirm this finding and utility in day to day clinical practices.
ACKNOWLEDGEMENTS
Authors acknowledge and thank the Department of Science and Technology, Ministry of Science and Technology, Government of India, for the financial supports to this work. We express our sincere thanks to Dr. Latey for his kind suggestion and help. We also thank Dr. Milind Mirashi (Cytochem laboratories, Pune) for his help in histological studies.
Assistant Editor Guo SY Edited by Gabbe M