INTRODUCTION
Chronic duodenitis is a common disease which might be a separate entity or accompanied with duodenal ulceration and also a common clinical symptom of dyspepsia[1,2]. Chronic duodenitis could be divided into three types according to their endoscopic, histological, and etiologic findings[3]. Histologically chronic duodenitis was divided into two principal forms: primary (non-specific duodenitis, NSD) form which is usually restricted to the duodenal bulb and the first part of duodenum[4], and secondary (specific) form which has been found in association with various other disorders such as Crohn’s disease, sarcoidosis, aspiriningestion and stress[3]. The diagnosis of NSD is frequently made both endoscopically and histopathologically and many research articles have documented it[3-13]. Ultrastructural morphological study of NSD with scanning electron microscope has been seldom reported. Therefore, we carried out the present study on the ultrastructural changes of non-specific duodenitis.
MATERIALS AND METHODS
Materials
Multiple biopsies from 44 patients selected from the patients who underwent routine upper gastrointestinal endoscopy for a variety of reasons including epigastric pain or discomforts, heartburn etc. were taken from the mucosa duodenal bulb. Patients with active duodenitis and duodenal ulcers were excluded from this study. Specimens for SEM were obtained from 12 of 44 patients, five women and seven men with an age range of 19-62 years and an average age of 37.0±12.9 years. No attempt was made to match the morphologic changes with the clinical symptoms.
Methods
Biopsies were obtained through endoscopic biopsy forceps directly at the endoscopically abnormal mucosa in each area or randomly at the site if the mucosa appeared normal from the duodenal bulb. At each endoscopy two pinch biopsies on the same area were obtained from the duodenal bulb mucosa. One was for SEM, the other for light microscopy. Each biopsy specimen was then oriented.
The material used for light microscopy was immediately fixed in 40 g/L formaldehyde, dehydrated in a graded series of ethyl alcohol solution, embedded in paraffin and cut into 3-4 μm thick sections. These specimens were stained with hematoxylin-eosin, Warthin-Starry silver (for the identification of the H pylori infection) and then examined with light microscopy. All histological evaluations were performed blindly regardless of the endoscopical diagnosis by one pathologist. According to the extent of lesions, non-specific duodenitis was divided into normal (as control group), mild, moderate and severe degrees respectively referring to the Whitehead’s classification and literature[1-3,5-7].
A total of 12 specimens (three from each degree of NSD diagnosed and graded by histology) selected for SEM were placed immediately into 25 g/L glutaraldehyde in phosphate buffer, pH 7.4. After post-fixation and mucus removal, tissues were dehydrated in a graded series of acetone, critical point dried using liquid carbon dioxide as an exchange medium. After mounted onto stubs by means of quick drying silver paint, the specimens were sputter coated with gold palladium to a thickness of approximately 25 nm and examined under a JEOL JSM-30 scanning electron microscope at 20 kV.
RESULTS
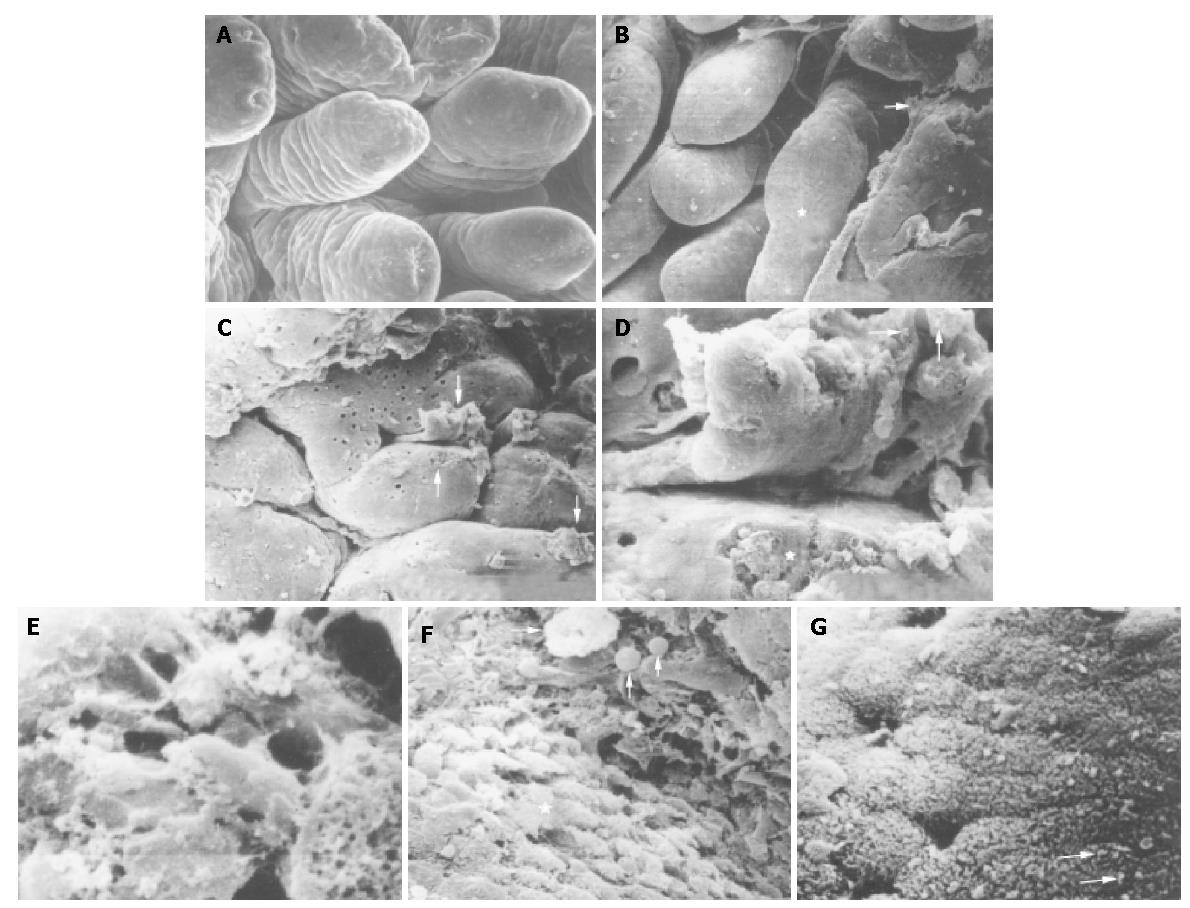
The morphologic changes of mucosal biopsies from the duodenal bulb showed various patterns. Ultrastructural alterations in NSD were restricted to the surface epithelial cells. Corresponding to the degrees of histological and endoscopical examination, NSD was also divided into the normal, mild, moderate and severe degrees. The details were as follows. The specimens taken from the normal duodenal bulb mucosa showed the villi in uniformed shapes and sizes such as leaf-like or fingerlike villous patterns with no surface exudates and intraluminal cells adhered to the surface (Figure 1A). The majority of villi (over 90%) were less than 0.2 mm. The epithelial cells were related to five to seven adjacent cells. Each cell surface was therefore five-seven sided but the sides were not equal in length. There were no gaps between adjacent cells. Mild degree of NSD had deformed and enlarged villi and its surface epithelial cells exhibited minor alterations with some exudates on the surface of the duodenal mucosa (Figure 1B). The villi were adhered and integrated. Parts of villi were greater than 0.2 mm but less than 0.3 mm. Moderate degree of NSD, had broader, swollen, lodging, shorter and flattened villi with convoluted or cerebroid patterns, and the minority of villi were greater than 0.4 mm (Figure 1C). Some areas of the duodenal mucosa were covered with mucus, which was mass or band-like secreted from the crypts. The most distinctive morphologic alteration was the ulcer-like defect on the surface of epithelial cells of villi (Figure 1D). The edge of the defect was abrupt and the bottom was rugged. In the defect there were inflammatory cells just as there were inflammatory cells and intraepithelial cells found in light microscopy. Epithelial cells around the defect were relatively normal. Severe degree of NSD had subtotal or total villous atrophy and severe erosion, in large areas of the mucosa covered with mucous exudates and hemorrhage and accompanied desquamation of epithelial cells (Figure 1E).
Figure 1 Photomicrograph of scanning electron microscopy of non-specific duodenitis.
A: Villi in uniformed shapes of leaf-like or fingerlike pattern of normal duodenal bulb mucosa. ×200; B: Deformed and integrated villi (asterisk) and exudates (arrow) in degree II of NSD. ×100; C: Broadened, flattened, convoluted or cerebroid villi, masses or band-like mucus (arrows), ulcer-like defect on the surface of a villus (long arrow) in degree II of NSD. ×100; D: Ulcer-like defect (asterisk) and a few of H pylori (arrows) in mucus. ×2500; E: Mucus and severe erosion on the surface of mucosa in degree III of NSD. ×500; F: Macrophages (long arrow) and inflammatory cells (arrows) on the surface of mucosa and gastric metoplasia cells (asterisk). ×2200; G: H pylori in S-shape on the microvilli of epithelial cells (arrows). ×3200.
In degrees I-III of NSD, there were cells on the epithelial surface of the villi which had the SEM features of lymphocytes, red blood cells and a few macrophages, neutrophils (Figure 1F). The epithelial cells of gastric metaplasia (manifested as replacement of the enterocytes of the villi by cells resembling the mucus-secreting surface cells of the stomach) with dome-shaped surface (Figure 1F) could also be found on the mucosa of three out of twelve patients (25%, 3/12). They were loosely arranged, and there were gaps between adjacent cells and a single cell loss or desquamation could be seen (Figure 1F). These might be the results of inflammatory edema due to inflammatory cells related to these gaps and the epithelial cell surface. A few bacteria were found on the microvilli of epithelial cells mainly on gastric metaplasia type, occasionally on intestinal type and luminal surface of the mucus (Figures 1D, G). Bacteria were found on the surface of five out of the twelve patients with NSD. According to the bacterial morphologic features curved in their long axis or S-shaped type found in SEM and based on the result of Warthin-Starry silver staining, the bacteria belonged to H pylori. No H pylori L-form was found in this study as reported by Wang et al[14]. The incidence of H pylori on the mucosa of NSD in this limited sample was 41.67% (5/12), which was higher than that from literature reports[8,13] and lower than that from the report of Qian et al[15]. H pylori infection was not found in non-ulcer dyspepsia patients[13]. This difference might result from different methods. Of course the most sensitive and accurate method was PCR for the diagnosis of H pylori infection[16].
DISCUSSION
Endoscopic and histological changes of inflammation often occurring in the mucosa of duodenal bulb in association with peptic ulcer and similar change in the absence of frank ulceration are termed non-specific duodinitis and common duodenitis, chronic duodenitis, peptic duodenitis, gastroduodenitis, etc.
There are different methods of classification of NSD, including clinical, endoscopic and histologic classifications. As for grading the NSD according to SEM there are few reports in the literature, except for a few papers which describe the surface morphological characteristics of villi, and the bacteria on the epithelial surface of gastroduodenal mucosa[7,17,18]. The grading of NSD by light microscopy is based on the inflammatory cell counts[6,7], whereas the grading of NSD by scanning electron microscopy is based on the surface morphologic alterations of the duodenal bulb mucosa as revealed in this study.
The controversy as to whether NSD is a separate entity or whether it is only a stage of ulcer disease has been confused by the contradictory observations that in some patients duodenitis progressed to duodenal ulceration while in others it did not. Scott et al[6] did contrast study of 16 pairs of patients with and without duodenal ulcer, which exactly matched for grade of duodenitis and the results supported the similarity of the two conditions. Our another study of NDS with inflammatory cell counts of lamina propria showed a significant change in degrees I-III of NSD compared with the normal. This article shows that NSD could also be divided into the normal and degrees I-III by SEM. These results could confirm the opinion that NSD is a separate entity disease based on ultrastructural morphology.
As its name implies, the cause of NSD is not very clear. There is evidence that the condition could be a part of the duodenal ulcer. Because of the coexistence of DU and duodenitis in surgical specimens, some authors[3,6,11] suggest that increased acid secretion and ethanol might have an important role in the pathogenesis of NSD based on the histological changes and the response to cimetidine therapy which would initially cause duodenitis. After prolonged exposure duodenal ulcer, H pylori infection could also play a key role in the pathogeneosis for gastroduodenal disease[8,19-22] and is an independent risk factor of chronic inflammation in duodenal bulb[8]. Since H pylori was first isolated in 1983, H pylori related diseases have become the hot spot of gastroenterological studies[17,23-26]. This organism infection not only relates to gastroduodenitis and most peptic ulcer[13,20,24,26] but also closely relates to gastric carcinomas and mucosa-associated lymphoid tissue lymphoma. H pylori previously named Compylobacter Pylori, has a lot of characteristics such as the typical s-shape, the corkscrew-like movement and the powerful urease enzyme. These allow a rapid movement through the mucous layer to adhere directly to the membranes of the surface mucous cells. Under certain unfavorable conditions H pylori could transform into L-form by which it can escape the body’s immune response[14]. Some metabolic products of the bacteria have chemotactic properties that could cause an intense inflammation and some products of H pylori have immunosuppressive effects on prolonging the infection[14]. White blood cells are attracted towards the chemotaxins, adhere to the vascular endothelium, emigrate between the endothelial cell junctions by amoeboid movement through venule into the mucosal surface[27] as indicated by the photomicrograph in this study. H pylori could be phagocytozed by invading polymorphonuclear leukocytes, macrophages and worsened inflammatory response[7,19,27], inducing several ultrastructural alterations such as edema of mucosa, architectural distortion of villi, epithelial damage, erosion, necrotic lesion and ulcer-like defect on the epithelium. These observations clearly demonstrate that pathological alterations in the ultrastructural level are induced mainly by H pylori with the result of disrupted mucosal barrier of duodenal bulb. H pylori was found on either gastric meterplasia epithelial cells or intestinal type cells in our study which differs from Steer’s[18] report that H pylori only existed on the surface of the gastric-type epithelial cells. The diagnosis of H pylori with SEM should be one of the gold standards. As for gastric metaplasia it might be normal. However, most observers consider it as a sign of severe duodenitis. As to the relationship between H pylori and gastric metaplasia, some authors[18,25] hold that H pylori closely relates to gastric metaplasia, others suggest that H pylori has no major role in the development of gastric metplasia in duodenal bulb, which is more common in DU patients[13,23,24,26,28]. The present study indicates that NSD is a separate entity disease caused by multiple factors such as toxic dietary components, hyperacidity, ethanol, nonsteroidal anti-inflammatory drugs and especially H pylori infection[3,8,22]. If the pathogenetic factors remain, duodenal ulceration may ensue on the basis of severe inflammation, desquamation, erosion, necrosis and the ulcer-like defect on the epithelial surface of duodenal bulb. The detailed relations between NSD, H pylori infection and gastric metaplasia still remain to be studied.
In conclusion, SEM is of value as an aid in the diagnosis of mucosal biopsies of duodenum and can improve the diagnostic criteria of NSD.