Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6348
Revised: July 23, 2005
Accepted: July 29, 2005
Published online: October 28, 2005
AIM: To provide the useful information for the diagnosis of liver cirrhosis by observing the morphology of peripheral hepatic vessels and the hemodynamics of microbubble arrival time in these vessels.
METHODS: Twenty-one subjects including 5 normal volunteers and 16 patients (liver cirrhosis, n=10; chronic hepatitis, n=6) were studied by contrast-enhanced coded phase inversion harmonic sonography (GE LOGIQ 9 series) using a 6-8 MHz convex-arrayed wide-band transducer. The images of peripheral hepatic artery, portal and hepatic vein were observed in real-time for about 2 min after intravenous injection of Levovist. The time when microbubbles appeared in the peripheral vessels (microbubble arrival time) was also recorded. The morphologic changes of peripheral hepatic vasculature were classified as marked, slight, and no changes based on the regularity in caliber, course, ramification, and the delineation of vessels compared to normal subjects.
RESULTS: The microbubble arrival time at peripheral artery, portal, and hepatic vein was shorter in cirrhotic patients than in chronic hepatitis patients and normal subjects. The marked, slight and no morphologic changes of peripheral hepatic vasculature found in 5 (5/6, 83.3%), 1 (1/6, 16.7%), and 0 (0/6, 0%) liver cirrhosis patients, respectively, and in 1 (1/10, 10%), 6 (6/10, 60%), and 3 (3/10, 30%) chronic hepatitis patients, respectively. There was a significant difference between the two groups (P<0.001).
CONCLUSION: Evaluation of the hemodynamics and morphology of peripheral hepatic vasculature by contrast-enhanced coded pulse inversion harmonic sonography can provide useful information for the diagnosis of liver cirrhosis.
- Citation: Zheng RQ, Zhang B, Kudo M, Sakaguchi Y. Hemodynamic and morphologic changes of peripheral hepatic vasculature in cirrhotic liver disease: A preliminary study using contrast-enhanced coded phase inversion harmonic ultrasonography. World J Gastroenterol 2005; 11(40): 6348-6353
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6348
Ultrasonography (US) is widely used for the purpose of screening for liver tumors. However, it has a limited value for the diagnosis of liver cirrhosis due to its low sensitivity and specificity[1].
With the advent of ultrasound contrast agent and associated ultrasonic technologies, drastic improvements have been achieved in the diagnosis and treatment of hepatic tumors[2]. However, application of contrast-enhanced US in the evaluation of diffused liver diseases is rare. Recently, using spectral Doppler US with time-intensity analysis software to measure the arrival time of microbubble agent, Levovist, in hepatic vein, Albrecht et al[3] found a much earlier onset of microbubble enhancement in cirrhotic patients than in normal controls or non-cirrhotic patients, and stressed that this method is a sensitive non-invasive test for liver cirrhosis. However, this technique is based on the Doppler US that has limitations in detecting slow blood signals in small vessels. Furthermore, it is not easy to perform continuous spectral Doppler tracing.
Microbubble-specific technologies, such as phase/pulse inversion harmonic imaging (PIHI) and coded harmonic angio, can display microbubble signals in blood vessels on a gray-scale background in real time[4]. Theoretically, microbubble arrival time can be measured using such gray-scale harmonic imaging techniques by direct visualization of microbubble enhancement in hepatic vessels, that is both hemodynamics and morphology of hepatic vessels can be evaluated simultaneously. Bang et al[5] recently used the pulse inversion imaging technique to assess the arrival time of microbubbles in hepatic vein and compared it with the spectral Doppler tracing method[3]. They noticed that there is no significant difference between these two methods.
On the assumption that the evaluation of hepatic vascular morphology when observing the hemodynamics of microbubble arrival time in hepatic vessels may provide more information for the diagnosis of liver cirrhosis, we conducted a study to observe the morphology of and microbubble arrival time in peripheral hepatic vessels in patients with liver cirrhosis and chronic hepatitis using contrast-enhanced coded harmonic angio mode, which is a real-time vascular imaging technique that combines PIHI with coded technology. The purpose of this preliminary study was to evaluate whether it is possible to provide useful information for the diagnosis of liver cirrhosis by observing the morphology and hemodynamics of hepatic vessels using contrast-enhanced coded harmonic angio technique.
This study consisted of 5 normal volunteers (4 men and 1 woman; age range 28-38 years, mean age 31 years) and 16 patients (12 men and 4 women; age range 18-73 years, mean age 54 years). Among the 16 patients, 10 had chronic hepatitis (hepatitis C, n=7; hepatitis B, n=3), 6 had liver cirrhosis (hepatitis C-related cirrhosis, n=3; hepatitis B-related cirrhosis, n=3). In addition, 4 patients (3 with cirrhosis and 1 with chronic hepatitis) were accompanied with hepatocellular carcinoma (HCC) with 1 (n=3) or 2 (n=1) nodules (less than 4 cm in diameter) in the right (n=3) or left (n=1) liver lobe. The diagnosis of chronic hepatitis (n=9) and liver cirrhosis (n=1) was made by histology in 10 patients. Other patients were diagnosed on the basis of clinical, biochemical and imaging findings on US (n=6), CT (n=5), MRI (n=3) and/or angiography (n=2). Normal subjects had no history or clinical signs of liver diseases. The Institutional Ethics Board of our university approved the study protocol and informed consent was obtained from all subjects.
In this study, we used Levovist (Schering, AG, Berlin, Germany) as a contrast agent, an intravenous microbubble agent consisting of 99.9% D-galactose and 0.1% palmitic acid. In brief, 2.5 g Levovist suspension at a concentration of 400mg/mL was prepared according to the manufacturer’s introductions. The suspension was injected via a 21- gauge cannula placed in the antecubital vein at a speed of 1 mL/s, and followed by a 10 mL normal saline flush.
Contrast-enhanced US study was carried out using GE LOGIQ 9 series (General Electric Medical System, Milwaukee, WI). A M7C convex-arrayed wide-band transducer with a frequency of 6-8 MHz was used. All subjects were fasted for at least 5 h before examination. Before contrast-enhanced study, fundamental B-mode imaging was performed to screen for any local lesions. Patients who had multiple (more than two nodules) and large (larger than 4 cm in diameter) nodules of solid occupying lesions were excluded in this study. The scanning section was selected at the right liver lobe through an intercostal approach. A non-tumor section was chosen to avoid the interference of the tumor for patients with HCC. Color Doppler imaging was also performed before the contrast study to ascertain the location and course of different liver vessels (especially the portal and hepatic vein) for the reference of identifying these vessels on contrast-enhanced images.
Contrast-enhanced US was then studied using coded harmonic angio mode with the mechanical index (MI) of 0.4-0.5, dynamic range of 72 decibels, main gain of 35-40 G and frame rate of 6-7 Hz. The scanning depth was maintained within 6-8 cm from the body surface. Single focus point was set at 1-2 cm beneath the liver surface. After the administration of Levovist, an inner time-meter was switched on. All the subjects were asked to breathe gently during examination. Real-time scanning with no background display was used to observe the vessel images of hepatic vasculature from the level equal to the origin of the third order branches of portal vein (segment level) to the liver surface, for about 2 min after injection. Simultaneously, the time when microbubbles appeared in the hepatic artery, portal and hepatic vein (microbubble arrival time) in the area described above was measured.
Real-time data were recorded on S-VHS videotapes and stored in the cine memory of the machine, which could load more than 2 000 frames of images (about 5 min in real time), and then saved to the hard disk for later review. After the examination, the dynamic contrast-enhanced images were replayed in the cine loop derived from the hard disk frame and series pictures were printed in the time sequence for later analysis.
Contrast-enhanced US studies were performed by the same operator using the same protocol in order to minimize the variations from the operators and condition settings. Another two authors reviewed the series pictures and evaluated the results without any knowledge of the subjects’ clinical data.
Referring to the evaluation method used in hepatic venography[6] and transhepatic portography[7], the morphologic changes of peripheral hepatic vasculature in patients with chronic liver disease were classified as marked, slight and no changes based on the regularity in caliber, course, ramification, and the delineation of vessels compared to normal subjects.
The peripheral vascular morphologic changes were compared among different groups using chi-square test, while the microbubble arrival time at different hepatic vessels was compared using Mann-Whitney test. P<0.05 was considered statistically significant.
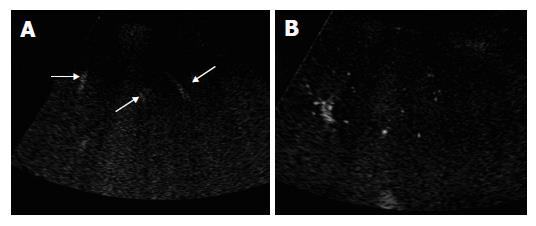
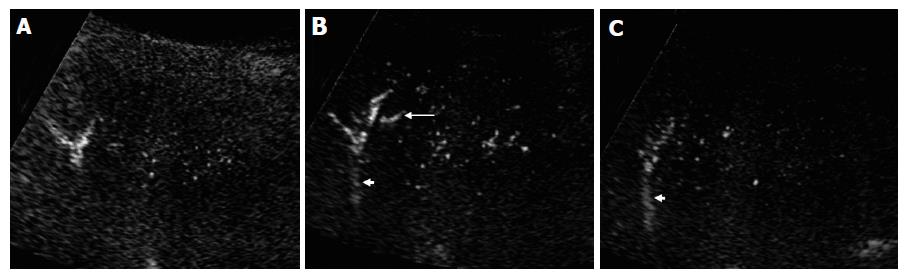
The peripheral artery could not be seen on coded harmonic angio mode before contrast, while the relative large portal and hepatic veins could be visualized during respiration (Figure 1A). After injection of contrast medium, microbubbles in the peripheral hepatic artery were displayed as small bright dots (Figure 1B). When enhanced by the contrast medium, the portal and hepatic vein became more echogenic than they were on pre-contrast images (Figure 2). In some subjects, the microbubble arrival time at different vessels could not be measured because of inadequate section showing no target vessels, or interference with deep breath of the subjects.
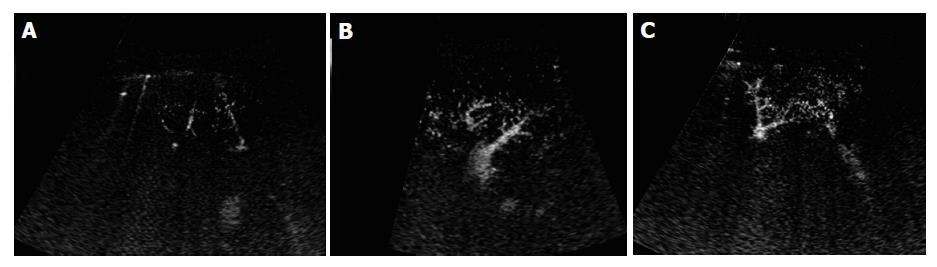
In the normal subjects, microbubbles appeared in the peripheral hepatic artery in about 16-28 s after Levovist injection. Arterial vessels were thin and regular in the early arterial phase in which only intraheaptic arterial vessels were visualized on contrast-enhanced images (Figure 3A). Portal venous vessels were enhanced by microbubbles in about 28-39 s, and then hepatic venous vessels were stained in about 35-71 s. In the vascular phase, intrahepatic portal and hepatic veins were visualized on contrast-enhanced images, peripheral hepatic vessels were regular in caliber, course and ramification, tapering smoothly as they run to the periphery (Figures 3B and 3C). When the vessel images on contrast-enhanced US were evaluated by another two authors blindly, all the normal subjects were regarded to having no abnormal morphologic changes.
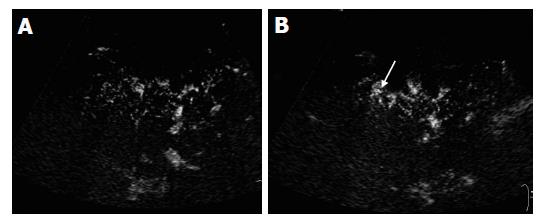
The microbubble arrival times at peripheral artery, portal and hepatic vein were shorter in cirrhotic patients than in chronic hepatitis patients and normal subjects. However, it showed statistical significant differences only in hepatic venous arrival time (P<0.05) (Table 1). Whereas, there were no significant differences in arrival times at different vessels between chronic hepatitis patients and normal controls (Table 1). Markedly morphologic changes of peripheral hepatic vessels including poor delineation of vessels, irregularity in caliber, course, and ramification without tapering of vessels (Figure 4A), or visualization of abnormal structures such as ring-like vessels (Figure 4B), were found in 5 of the 6 cirrhotic patients (5/6, 83.3%) Slight morphologic changes were identified in one cirrhotic patient (1/6, 16.7%). Marked, slight and no morphologic changes were found in 1 (1/10, 10%), 6 (6/10, 60%), and 3 (3/10, 30%) chronic hepatitis patients, respectively (P<0.001, Table 2). If marked morphologic changes of peripheral hepatic vessels were taken as a criterion for the diagnosis of liver cirrhosis, the diagnostic sensitivity and specificity were 83.3% and 93.3%, respectively.
| Artery (n) | Portal vein (n) | Hepatic vein (n) | |
| Cirrhosis | 16.4±5.3 (5) | 21.3±6.7 (3) | 21.3±7.7 (3) |
| Chronic hepatitis | 23.5±9.1 (8) | 35.1±19.4 (8) | 44.3±20.8 (7) |
| Normal control | 20.3±5.4 (4) | 31.3±6.7 (3) | 45.5±17.1 (4) |
| P1 | 0.537 | 0.077 | 0.046 |
| P2 | 0.185 | 0.26 | 0.03 |
| Marked (%) | Slight (%) | None (%) | Total | |
| Cirrhosis | 5 (83.3) | 1 (16.7) | 0 | 6 |
| Chronic hepatitis | 1 (10) | 6 (60) | 3 (30) | 10 |
| Normal control | 0 | 0 | 5 (100) | 5 |
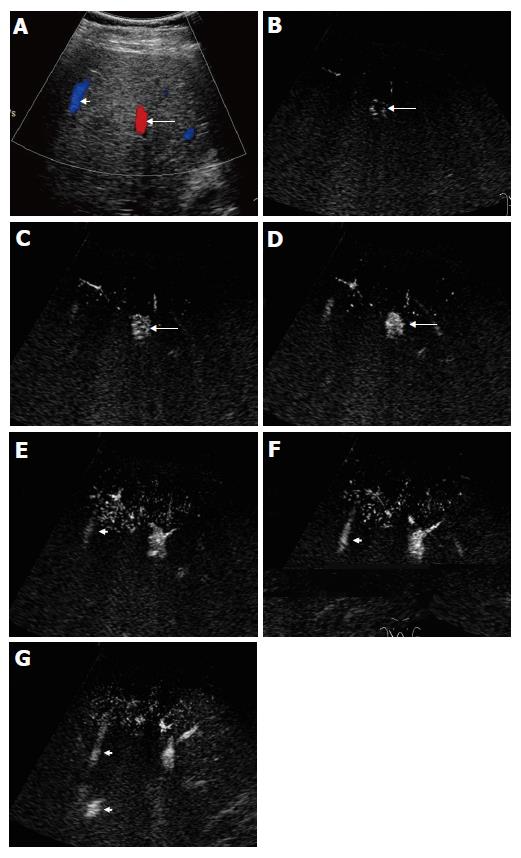
The microbubble arrival time at hepatic vein was shorter in cirrhotic patients who had marked morphologic changes than in those who had slight morphologic changes of peripheral hepatic vasculature (16-25 s vs 37 s). In one of the cirrhotic patients without HCC, the arterial arrival time was short (11 s). During the early arterial phase, one distal portal vein branch was enhanced by microbubbles (14 s) prior to the enhancement of proximal portal vein and other branches at the same level (Figure 5A). An adjacent peripheral hepatic vein was also stained (16 s) (Figures 5B and 5C). A small intrahepatic arterioportal shunt was suspected. Unfortunately, the patient did not undergo hepatic angiography.
In liver cirrhosis, circulatory changes play an important role in the progression of regenerative nodular formation and extensive stromal fibrosis and vice versa[8]. Microscopically, these circulatory changes in the liver, such as capillarization of sinusoids, arterialization, and intrahepatic shunts[9-11], can be observed by microcirculation evaluation methods such as microvascular casts[8], color gelatin injection postmortem[11]. Macroscopically, circulatory morphological changes in liver cirrhosis can be demonstrated by different angiographic techniques, such as hepatic angiography, arterial portography or transhepatic portography and hepatic venography[7]. However, angiographic techniques are invasive and cannot be used routinely. Color and power Doppler ultrasonography can also be used in the evaluation of hepatic vasculature. However, they can not achieve satisfactory results due to their limitations in detecting slow blood flow signals in fine vessels.
Contrast-enhanced code harmonic angio is an advanced microbubble-specific technique that combines PIHI with coded technology, which amplifies the wide-bind signals from microbubbles and suppresses signals from the tissue[2,4]. It provides the possibility to assess the morphology of hepatic vessels in real time under physiological conditions. In this study, the morphology of peripheral hepatic vasculature was clearly demonstrated by a high frequency probe, which has high resolution in showing superficial structures. Compared to normal subject, marked morphologic changes including poor delineation of vessels, irregularity in caliber, course, and ramification without tapering of the vessels, or visualization of abnormal structures, were portrayed in most cirrhotic patients (83.3%), while few of chronic hepatitis patients (10%) and none of normal subjects had marked morphologic changes of peripheral hepatic vasculature, suggesting that it has a relatively high sensitivity and specificity for the diagnosis of liver cirrhosis. The results indicate that contrast-enhanced code harmonic angio can recognize the differences in peripheral vasculature between cirrhotic and non-cirrhotic subjects, and might provide useful information for the diagnosis of cirrhosis.
In addition, evaluation of peripheral hepatic vasculature might facilitate the differential diagnosis of pseudotumors caused by intrahepatic arterioportal shunt, which is not rare in liver cirrhosis especially in peripheral area of the liver[7,12,13]. It was reported that these pseudolesions mimic hypervascular tumors on hepatic imaging and bring about problems of differential diagnosis in cirrhotic patients especially when they are round or nodular in shape[12,13]. Usually, intrahepatic arterioportal shunts are confirmed by hepatic angiography. However, when the arterioportal shunt-induced pseudolesion is small (less than 1 cm in diameter), it can hardly be visualized on hepatic angiogram because of the limited spatial resolution and overlapping of liver parenchyma[13]. In this situation, contrast-enhanced US may show the small intrahepatic arterioportal shunt in peripheral area of the liver. Theoretically, when distal portal venous branches are enhanced earlier in the early arterial phase before microbubbles arrive at the proximal branches, an intrahepatic arterioportal shunt can be diagnosed by hepatic angiography. In addition, the abnormal ring-like structures on contrast-enhanced US images in some cirrhotic patients might also indicate small intrahepatic shunts or abnormal anastomoses in artery, portal or hepatic vein. Unfortunately, this assumption was not confirmed in this study because we did not perform any prospective angiography. Further investigations are needed to validate the possibility.
Compared to angiography, contrast-enhanced coded harmonic angio imaging has several advantages in showing morphologic changes of peripheral vessels. First, it is non-invasive. Second, peripheral vessels including hepatic artery, portal and hepatic vein, can be seen. Third, the kinetics of microbubble contrast agent in peripheral hepatic vessels can be evaluated simultaneously. However, it has some disadvantages. The morphologies of different hepatic vessels displayed on contrast-enhanced US are not so clear compared to angiography because of overlapping of different vessels. In addition, the visual fields on contrast-enhanced US cannot display the whole vascular tree.
We could also assessed the microbubble arrival time at different peripheral vessels in some subjects using contrast-enhanced coded harmonic angio mode. The results showed that hepatic venous arrival time was shorter in cirrhotic patients than in other subjects, which is in accordance with those observed by Albrecht et al[3] Furthermore, the venous arrival time was shorter in cirrhotic patients who had marked morphological changes of peripheral hepatic vasculature, indicating that hemodynamic changes in liver cirrhosis are due to the morphologic changes of hepatic circulation. We also noticed that not only the hepatic venous arrival time, but also the arterial and portal venous arrival time were shorter in cirrhotic patients than in chronic hepatitis patients and normal controls, though there was no statistically significant difference, suggesting that both intrahepatic and extrahepatic hemodynamic changes such as pulmonary arteriovenous shunt and systemic hyperdynamic circulatory state may occur in liver cirrhosis[3], which might contribute to the early arrival of microbubbles in hepatic artery and portal vein.
Except for the evaluation of hemodynamics changes in chronic liver disease, assessing microbubble arrival time at different hepatic vessels might help to study the time phases on contrast-enhanced US images, which is important in the diagnosis and differential diagnosis of liver tumors.
There are several limitations in this preliminary study. First, the number of patients with liver cirrhosis was not large enough. Second, angiography examinations were not prospectively studied, lacking of data to validate the findings on contrast-enhanced the United States. Third, the microbubble arrival time was not available in some subjects because of several interfering factors. Furthermore, we did not use the time-intensity analyzing software to measure the microbubble arrival time, the accuracy was certainly not very high. However, the results obtained in our study and other studies[5] are not significantly different from those obtained using time-intensity analyzing software[3].
In conclusion, contrast-enhanced coded pulse inversion harmonic imaging can evaluate the hemodynamics and morphology of peripheral hepatic vasculature and provide useful information for the diagnosis of liver cirrhosis.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology. 1985;89:186-191. [PubMed] |
| 2. | Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Contrast-enhanced subtraction harmonic sonography for evaluating treatment response in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2001;176:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Kudo M. Imaging blood flow characteristics of hepatocellular carcinoma. Oncology. 2002;62 Suppl 1:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Bang N, Nielsen MB, Rasmussen AN, Osterhammel PA, Pedersen JF. Hepatic vein transit time of an ultrasound contrast agent: simplified procedure using pulse inversion imaging. Br J Radiol. 2001;74:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Futagawa S, Fukazawa M, Musha H, Isomatsu T, Koyama K, Ito T, Horisawa M, Nakayama S, Sugiura M, Kameda H. Hepatic venography in noncirrhotic idiopathic portal hypertension. Comparison with cirrhosis of the liver. Radiology. 1981;141:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Okuda K, Takayasu K, Matsutani S. Angiography in portal hypertension. Gastroenterol Clin North Am. 1992;21:61-83. [PubMed] |
| 8. | Haratake J, Hisaoka M, Yamamoto O, Horie A. Morphological changes of hepatic microcirculation in experimental rat cirrhosis: a scanning electron microscopic study. Hepatology. 1991;13:952-956. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Villeneuve JP, Dagenais M, Huet PM, Roy A, Lapointe R, Marleau D. The hepatic microcirculation in the isolated perfused human liver. Hepatology. 1996;23:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zoli M, Magalotti D, Bianchi G, Ghigi G, Orlandini C, Grimaldi M, Marchesini G, Pisi E. Functional hepatic flow and Doppler-assessed total hepatic flow in control subjects and in patients with cirrhosis. J Hepatol. 1995;23:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Popper H, Elias H, Petty DE. Vascular pattern of the cirrhotic liver. Am J Clin Pathol. 1952;22:717-729. [PubMed] |
| 12. | Yu JS, Kim KW, Sung KB, Lee JT, Yoo HS. Small arterial-portal venous shunts: a cause of pseudolesions at hepatic imaging. Radiology. 1997;203:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Kim TK, Choi BI, Han JK, Chung JW, Park JH, Han MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |