Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4566
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: August 7, 2005
AIM: In the inflammatory state, intercellular adhesion molecule-1 (ICAM-1) and vascular cellular adhesion molecule-1 (VCAM-1) play a key role in promoting migration of immunological cells from the circulation to target site. Aim of our study was to investigate soluble forms of these molecules in patients with virus-related chronic liver diseases, to assess their behavior in different pathologies and correlation with severity of liver damage.
METHODS: Circulating ICAM-1 and VCAM-1 were assayed by EIA commercial kits (R&D System Co., Abington, UK) in 23 patients with chronic active hepatitis (CH), 50 subjects affected by liver cirrhosis (LC) and 15 healthy controls comparable for sex and age. In patients, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were also detected by autoanalyzer.
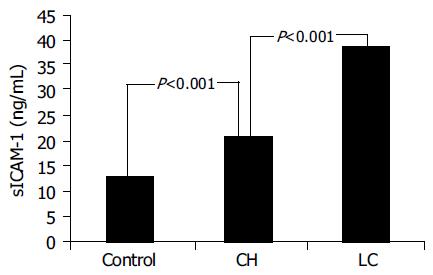
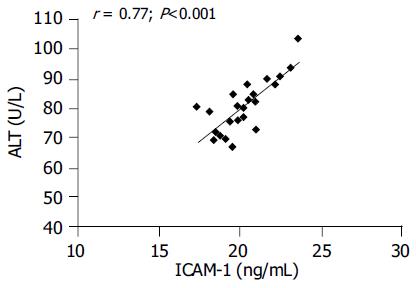
RESULTS: LC patients had significantly higher ICAM-1 values than CH patients (38.567.4 ng/mL vs 20.896.42 ng/mL; P<0.001) and these ones had significantly higher values than controls (12.921.08 ng/mL; P<0.001). In CH group, ICAM-1 levels were significantly related to inflammatory activity (P = 0.041) and ALT values (r = 0.77; P<0.05). VCAM-1 values were significantly increased only in LC patients (P<0.001) and related to severity of liver impairment.
CONCLUSION: These findings suggest that the determination of serum ICAM-1 can be considered as an additional useful marker of hepatocellular necrosis and inflammatory activity in chronic hepatitis, while serum VCAM-1 is an indicator of liver fibrogenesis and severity of disease in cirrhosis.
- Citation: Bruno CM, Sciacca C, Cilio D, Bertino G, Marchese AE, Politi G, Chinnici L. Circulating adhesion molecules in patients with virus-related chronic diseases of the liver. World J Gastroenterol 2005; 11(29): 4566-4569
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4566
During inflammation, cytokines enhance the presentation on cell endothelial surface of adhesion molecules, which interact with corresponding leukocyte receptors and promote their adhesion and migration from the circulation to target site[1].
Intercellular adhesion molecule-1 (ICAM-1) and vascular cellular adhesion molecule-1 (VCAM-1), are single-chain membrane-bound glycoproteins, belonging to the immunoglobulin supergene family. They are expressed on endothelial and other cells and play a crucial role in the trans-migration of inflammatory cells[2,3].
Cellular expression is associated to the release of soluble forms (sICAM-1 and sVCAM-1) which are detectable in the peripheral blood[4,5].
Although the biological function of these soluble forms are not clearly established yet, their increased serum concentration reflects cellular overexpression in inflammatory state[6].
In patients with inflammatory diseases of the liver, increased sICAM-1 and sVCAM-1 values have been reported but their clinical usefulness is still controversial[7-11].
Aim of the study was to investigate circulating ICAM-1 and VCAM-1 in patients affected by virus-related liver diseases, namely chronic active hepatitis (CH) and liver cirrhosis (LC), compared to healthy control subjects, to verify whether there are differences between these two molecules and to evaluate a possible correlation with severity of liver damage as clinically and histologically assessed.
We examined three groups of subjects: 23 neodiagnosed and untreated patients with CH, 50 patients with LC and 15 control subjects.
All groups were comparable for sex and age.
Diagnosis, in patients, was based on clinical (medical history, physical examination), instrumental (ultrasonography, endoscopy) and laboratory (liver function tests) data. In all patients with chronic hepatitis and in 24 cirrhotic subjects, diagnosis was confirmed by liver biopsy (in the remaining cirrhotic subjects the procedure was not necessary, as diagnosis was clinically evident).
Patients with evidence of other chronic or acute infective processes (altered white blood cells count, temperature, urinary tract infection, spontaneous bacterial peritonitis, airway infections) were excluded as well as those with suspected hepatocellular carcinoma (on the basis of ultrasonography, alpha-fetoprotein and carcinoembryonic antigen levels performed during the screening).
In 6 out of 23 CH patients, the etiological agent was HBV while in the remaining 17 hepatitis C virus (HCV). Of the 50 LC subjects, 19 were infected by HBV, and 31 by HCV.
All sections from liver biopsies were examined by a histopathologist who was unaware of the clinical details. With regard to histological findings, all patients with chronic hepatitis had none or mild fibrosis (staging score 0-1); according to grading score, they were classified into two groups: (a) 12 subjects with minimal or mild inflammatory activity (score 1-8); (b) 11 subjects with moderate or severe inflammatory activity (score 9-18).
As liver biopsy was not performed in all cirrhotics, we could not grade severity of histological picture in this group. For this reason we chose the Child-Pugh’s classification[12] to state the seriousness of liver impairment.
Twenty-three cirrhotic patients were in class B and 27 belonged to class C.
Informed consent was obtained for the whole study series and the study confirmed to Helsinki Declaration.
A blood sample was withdrawn, fasting in the morning, from all subjects. Sample was centrifugated and plasma was stored at -20 °C until determination.
Circulating ICAM-1 and VCAM-1 were assayed by EIA commercial kit (R&D System Co., Abington, UK), according to procedures described by the manufacturer and concentrations expressed as nanogram per milliliter. The sensitivity of sVCAM-1 assay was less than 2.0 ng/mL. Intra- and inter-assay variability averaged 4.3% and 8.5%, respectively. For sICAM-1 assay, the sensitivity was <0.35 ng/mL. Intra- and inter-assay variability averaged 4.0% and 7.0%, respectively.
In patients, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were also detected by autoanalyzer.
Analysis of variance and Kruskall-Wallis test were used to compare mean±SD between various groups. Relationship between continuous variables was investigated by correlation test. Statistical significance was set at P<0.05.
Mean±SD. ICAM-1 was 12.92±1.08 ng/mL in controls, 20.89±6.42 ng/mL in CAH patients and 38.56±7.4 ng/mL in LC patients.
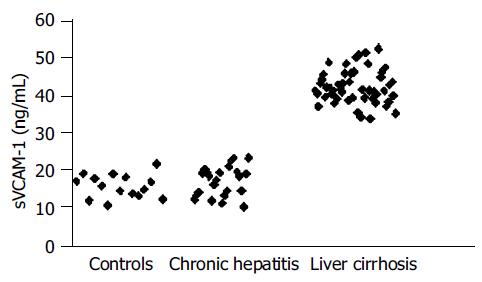
Mean±SD. VCAM-1 was 15.96±4.02 ng/mL in controls, 17.96±8.43 ng/mL in CH patients and 42.29±5.76 ng/mL in LC patients, respectively.
Mean±SD. AST was 73.9±9 U/L in CH patients and 62.7±8.5 U/L in LC subjects.
ALT values averaged 84±12 U/L in CH subjects and 58±7.2 U/L in cirrhotic patients.
Statistical analysis showed that LC patients had significantly higher ICAM-1 values than CH patients (P<0.001) and these ones had significantly higher values (P<0.001) than controls (Figure 1). Among CH patients, a significant difference in serum was found between ICAM-1 with score 1-8 and those with score 9-18 (18.1±4.9 ng/mL vs 23.5±6.9 ng/mL, respectively; P = 0.041).
VCAM-1 values were significantly higher in LC patients than in CH subjects (P<0.001) and controls (P = 0.000). There was no significant difference (P>0.05) in VCAM-1 values between controls and CH patients and among these ones with regard to grading score (17.41±8.3 ng/mL vs 18.13±8.2 ng/mL; P>0.05).
With regard to Child-Pugh classification, in cirrhotic patients there was no difference in ICAM-1 levels (P>0.05) between class B (37.7±6.1 ng/mL) and class C (40.2±4.3 ng/mL) subjects; circulating VCAM-1 was significantly higher (P = 0.005) in class C (43.6±4.1 ng/mL) than in class B (40.53.1 ng/mL) patients.
Correlation test showed a significant relationship (r = 0.77; P<0.001) between ICAM-1 and ALT values in CH patients (Figure 2). No significant correlation was found between ICAM-1 and AST values in CH patients, as well as between VCAM-1 concentration and ALT and AST values both in CH and in LC patients.
Main characteristics and findings of our investigated patients are summarized in Table 1.
| Controls, n = 15 | CH patients, n = 23 | LC patients, n = 50 | |
| Mean age±SD | 57.4± 4.1 yr | 58.2±5.1 yr | 61.1±8.3 yr |
| Males | 8 | 13 | 29 |
| Females | 7 | 10 | 21 |
| ALT (mean±SD) | 84±12 U/L | 58±7.2 U/L | |
| AST (mean±SD) | 73.9±9 U/L | 67.2±8.5 U/L | |
| ICAM-1 | 12.92±1.08 ng/mL | 20.89±6.42 ng/mL | 38.56±7.4 ng/mL |
| (mean±SD) | (score 1–8) 18.1±4.9 | Child B 37.7±6.1 | |
| (score 9–18) 23.5±6.9 | Child C 40.2±4.3 | ||
| VCAM-1 | 15.96±4.02 ng/mL | 17.96±8.43 ng/mL | 42.29±5.76 ng/mL |
| (mean±SD) | (score 1–8) 17.41±8.3 | Child B 40.5±3.1 | |
| (score 9–18) 18.13±8.2 Child C 43.6±4.1 |
Increased serum ICAM-1 and VCAM-1 have been previously described in patients with liver diseases, but such increase does not seem to be able to distinguish among various etiologies and its clinical significance is still controversial.
Some authors reported that circulating levels of these molecules are related to degree of inflammatory activity and to histological score, suggesting a putative role in monitoring the follow-up[13,14]. Others have declared that their measurement adds little to the information provided by traditional biochemistry[15].
Recently, it has been reported that treatment with alpha-interferon, in chronic hepatitis, is capable to decrease circulating ICAM-1 in responder but not in non-responder patients, while VCAM-1 does not differentiate between these two groups[11,16,17].
Our results show that, in patients affected by chronic hepatitis, serum ICAM-1 is increased and mean values in subjects with moderate or severe inflammation (score 9-18) are significantly higher (P<0.05) than in those with minimal or mild inflammation (score 1-8).
Conversely, in this group, serum VCAM-1 was similar to controls and no difference was observed with regard to inflammatory activity.
Moreover, we found a significant positive correlation between ICAM-1 and ALT values.
Then, our findings appear in agreement with literature data and suggest that ICAM-1, but not VCAM-1, can be considered, in subjects with chronic hepatitis, a useful marker to assess severity of inflammation and hepatocellular necrosis.
Nevertheless, the relationship between ICAM-1 and ALT values does not implicate a direct involvement of this adhesion molecule in physiopathology of hepatocellular damage but it could only reflect the activation of undergoing immunological mechanisms.
Elevated serum VCAM-1 in chronic hepatitis have been reported by some authors[16,18], but we found enhanced levels only in cirrhosis, when fibrosis progresses to an irreversible state.
This discordance is likely due to the selection of patients.
Notably, our CH patients had none or mild fibrosis (staging score 0-1). Therefore, we cannot exclude that subjects with chronic hepatitis and more severe fibrosis have increased sVCAM-1.
On the other hand, in cirrhotic patients, both circulating ICAM-1 and VCAM-1 were higher than in CH patients and controls. However, in this group, neither ICAM-1 nor VCAM-1 values correlated to AST and ALT levels.
In advanced phases of evolving liver diseases, the formation of fibrous tissue, stimulated by chronic inflammation, can be predominant on cellular necrosis, leading to structural alterations of hepatic architecture and development of cirrhosis.
In fact, our cirrhotic patients had lower transaminases values than subjects affected by chronic hepatitis.
In these conditions, increased ICAM-1 likely reflects the persistence of inflammation rather than severity of cellular necrosis. This could explain the lack of correlation between serum ICAM-1 and transaminases values in cirrhotic patients.
Interestingly, in our experience, no overlapping resulted in VCAM-1 values between LC and CH patients because the lowest value in LC group was greater than highest value in CH group (Figure 3). Furthermore, mean serum ICAM-1 was similar in classes B and C cirrhotic subjects while in the last ones mean VCAM-1 value was higher than in class B patients (43.64.1 ng/mL vs 40.53.1 ng/mL; P = 0.005).
Therefore, cirrhotic subjects with disease in a more advanced phase, belonging to Child-Pugh class C, had a greater VCAM-1 increase.
These findings, altogether considered, suggest the association of VCAM-1 levels to amount of liver fibrosis rather than to severity of inflammation and cellular necrosis.
However, the pathogenic link between VCAM-1 and liver fibrosis remains unclear.
In the liver, the formation of fibrous tissue depends upon the balance between matrix synthesis and its removal. Extra-cellular matrix production and degradation is a complex process involving cells, cytokines and proteinases. The interaction between membrane receptors of stellate cells (also called Ito cells) and proteins, included in the extra-cellular matrix, is modulated by adhesion molecules. Such interaction determines its effects through cytoplasmic signaling pathways which can influence collagen synthesis and metalloprotease activity, resulting in a raised production and/or a reduced removal of hepatic connective tissue.
Thus, a role in this process can be supposed even though, to date, the expression of VCAM-1 on stellate cell surface has not been demonstrated.
Alternatively, enhanced VCAM-1 values could be the consequence of persistent activation of vascular endothelial cells which are able to produce connective tissue growth factor, a highly profibrogenic molecule involved in several fibrotic disorders, including those of the liver[19].
Activated endothelial cells may also contribute, by expressing de novo integrins in the space of Disse, to the formation of a pathological basement membrane in the capillarized sinusoids of cirrhotic liver[20].
Even though further investigations are required, from a clinical overview, the serial determination of serum VCAM-1 could be useful to weigh intensity of fibrogenesis and to identify the switch from prevalence of necro-inflammation to prevalence of sclerofibrotic processes, marked by an accentuated increase of values.
In conclusion, our results confirm that, in patients with chronic virus hepatitis, serum ICAM-1 is increased and related to severity of necro-inflammation as assessed by biochemistry and histological score. In cirrhotic patients, ICAM-1 is also augmented but it is not related to transaminase values. Circulating VCAM-1 is increased in cirrhosis and appears related to seriousness of liver impairment.
These findings suggest that serum ICAM-1 is an additional useful marker of cytonecrotic activity in chronic hepatitis. Serum VCAM-1 could be instead considered as a noninvasive indicator of liver fibrosis and severity of disease in cirrhosis.
Co-first-author: Cosimo Marcello Bruno
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4705] [Cited by in RCA: 4727] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 2. | Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990;12:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | García-Monzón C, Sánchez-Madrid F, García-Buey L, García-Arroyo A, García-Sánchez A, Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995;108:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147:3788-3793. [PubMed] |
| 5. | Pigott R, Dillon LP, Hemingway IH, Gearing AJ. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 463] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 876] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 7. | Girón-González JA, Rodríguez-Ramos C, Elvira J, Galán F, Del Alamo CF, Díaz F, Martín-Herrera L. Serial analysis of serum and ascitic fluid levels of soluble adhesion molecules and chemokines in patients with spontaneous bacterial peritonitis. Clin Exp Immunol. 2001;123:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Thomson AW, Satoh S, Nüssler AK, Tamura K, Woo J, Gavaler J, van Thiel DH. Circulating intercellular adhesion molecule-1 (ICAM-1) in autoimmune liver disease and evidence for the production of ICAM-1 by cytokine-stimulated human hepatocytes. Clin Exp Immunol. 1994;95:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tortorella C, Sacco R, Orlando P, Salerno MT, Schiraldi O, Antonaci S. sICAM-1, sCD95 and sCD95L levels in chronic liver diseases of different etiology. Immunopharmacol Immunotoxicol. 2000;22:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | de Caestecker JS. Bile acid therapy and markers of immune-mediated damage in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1997;9:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Granot E, Shouval D, Ashur Y. Cell adhesion molecules and hyaluronic acid as markers of inflammation, fibrosis and response to antiviral therapy in chronic hepatitis C patients. Mediators Inflamm. 2001;10:253-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5729] [Article Influence: 110.2] [Reference Citation Analysis (2)] |
| 13. | Douds AC, Lim AG, Jazrawi RP, Finlayson C, Maxwell JD. Serum intercellular adhesion molecule-1 in alcoholic liver disease and its relationship with histological disease severity. J Hepatol. 1997;26:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Lo Iacono O, García-Monzón C, Almasio P, García-Buey L, Craxí A, Moreno-Otero R. Soluble adhesion molecules correlate with liver inflammation and fibrosis in chronic hepatitis C treated with interferon-alpha. Aliment Pharmacol Ther. 1998;12:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Falleti E, Pirisi M, Fabris C, Bortolotti N, Soardo G, Gonano F, Bartoli E. Circulating standard CD44 isoform in patients with liver disease: relationship with other soluble adhesion molecules and evaluation of diagnostic usefulness. Clin Biochem. 1997;30:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Dejica D, Grigorescu M, Dejica V, Radu C, Neculoiu D. Serum levels of soluble intercellular-1 and vascular cell-1 adhesion molecules in chronic hepatitis C and the influence of interferon-alpha + ribavirin therapy. Rom J Gastroenterol. 2002;11:277-283. [PubMed] |
| 17. | Radu C, Dejica D, Grigorescu M, Zaharie T, Neculoiu D. Correlation of sICAM-1 and sVCAM-1 level with biochemical, histological and viral findings in chronic hepatitis C after interferon-alpha + ribavirin therapy. Rom J Gastroenterol. 2003;12:91-95. [PubMed] |
| 18. | Kaplanski G, Farnarier C, Payan MJ, Bongrand P, Durand JM. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Quondamatteo F, Kempkensteffen C, Miosge N, Sonnenberg A, Herken R. Ultrastructural localization of integrin subunits alpha3 and alpha6 in capillarized sinusoids of the human cirrhotic liver. Histol Histopathol. 2004;19:799-806. [PubMed] |