Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2431
Revised: July 29, 2004
Accepted: September 4, 2004
Published online: April 28, 2005
AIM: To investigate the effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells in vitro and its anticancer mechanism.
METHODS: Human gallbladder carcinoma GBC-SD cells were cultured by cell culture technique. The growth and the invasiveness of GBC-SD cells in vitro were evaluated by the tetrazolium-based colorimetric assay and by the Matrigel experiment and the crossing-river test. Expression of PCNA, Ki-67, MMP2 and TIMP2 proteins of GBC-SD cells was determined by streptavidin–biotin complex method.
RESULTS: In vitro norcantharidin inhibited the growth and proliferation of GBC-SD cells in a dose- and time-dependent manner, with the IC50 value of 56.18 μg/mL at 48 h. Norcantharidin began to inhibit the invasion of GBC-SD cells at the concentration of 5 μg/mL, and the invasive action of GBC-SD cells was inhibited completely and their crossing-river time was prolonged significantly at 40 μg/mL. After treatment with norcantharidin, the expression of PCNA, Ki-67, and MMP2 was significantly decreased. With the increase in TIMP2 expression, the MMP2 to TIMP2 ratio was decreased significantly (P<0.05).
CONCLUSION: Norcantharidin inhibits the proliferation and growth of human gallbladder carcinoma cells in vitro at relatively low concentrations by inhibiting PCNA and Ki-67 expression. Its anti-invasive activity may be the result of decrease in MMP2 to TIMP2 ratio and reduced cell motility.
- Citation: Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol 2005; 11(16): 2431-2437
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2431.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2431
Primary carcinoma of the gallbladder represents a highly lethal and aggressive malignant tumor because of its dormancy course, difficult diagnosis, early metastasis, strong invasion and poor prognosis[1,4]. The only potentially curative therapy for gallbladder carcinoma is surgical resection. Unfortunately, most patients with this type of cancer present with advanced and unresectable disease-only 10-30% of patients can be considered for surgery on presentation[1,3,5,7] and should be considered for palliative treatment such as chemotherapy[1,3,6,8,9] and radiotherapy[1,3,6,10,11]. However, reports of chemotherapy and radiotherapy in gallbladder carcinoma are disappointing, results are conflicting and most series have a small number of patients[1,3,6,8-11]. Obviously, there is an urgent need to identify new therapeutic agents for the treatment of gallbladder carcinoma in vivo. Many lines of evidence have shown that Chinese medicine contains many chemical compounds with anticancer effects. We reported the influence of norcantharidin (NCTD, a demethylated form of cantharidin, which is an active ingredient of Chinese medicine-Mylabris) on growth and apoptosis of GBC-SD cell lines of human gallbladder carcinoma[12,13]. In the present study, human gallbladder carcinoma GBC-SD cell lines were used to study the in vitro effect of NCTD on proliferation and invasion of human gallbladder carcinoma GBC-SD cells and its mechanism.
GBC-SD cell lines of human gallbladder carcinoma were purchased from Shanghai Cell Institute Country Cell Bank. NCTD was purchased from Beijing Fourth Pharmaceutical Works, China. Matrigel invasion chamber, which is composed of a layer of artificial basement membrane matrix above PET membrane with 8.0-μm hole, were purchased from Becton Dickinson, USA. Rat monoclonal antibody proliferating cell nuclear antigen (PCNA) and Ki-67 were respectively purchased from Calbiochem Co. and Antibody Diagnostica Co.; Monoclonal MMP2 antibody was purchased from Neomarker and TIMP2 antibody from Boster. Bovine calf serum, RPMI-1640 medium, trypsin and D-Hanks’ solution were all purchased from Gibco; MTT solution and DMSO were purchased from Sigma; SABC kit and DAB are all purchased from Boster.
Cell cultures[12,13] GBC-SD cells of human gallbladder carcinoma were cultured in RPMI-1640 medium supplemented with 10% bovine calf serum in an incubator with 50 mL/L at 37 °C. The medium was changed every 2 d. When the cells became confluent, namely a 95% plating efficiency, they were trypsinized with 0.25% trypsin. Then the cells were returned to culture at 37 °C in 50 mL/L CO2 for 24 h, and they were washed twice with Hanks’ balanced salt solution, and used in this experiment.
Inhibitory effect of NCTD on growth of GBC-SD cells The tetrazolium-based colorimetric assay (MTT) was used to evaluate the inhibitory effect of NCTD on growth of GBC-SD cells in vitro, namely the tumor cytotoxicity test[12,13]. After GBC-SD cells were cultured in a 96-well plate (3×105 cells·100 μL/well) in culture medium overnight, they were treated with various concentrations of NCTD in fresh culture medium at 37 °C for 24 h. The tumor cell cytotoxicity was determined by MTT. The optical densities (A values) at 540 nm were measured using an ELISA reader (DG3032, Shanghai). The A540 value of the experimental groups was divided by the A540 value of untreated controls and presented as a percentage of the cells. The inhibitory percent of various concentrations of NCTD on GBC-SD cells (%) = (1-A540 value in the experimental group/A540 value of control group) ×100%. Three separate experiments were performed. The concentration of drug giving 50% growth inhibition (IC50) was calculated from the formula LC50 = lg-1[Xm-I(Σp-0.5)].
Matrigel invasion experiment of GBC-SD cells (1) Living GBC-SD cells were trypsinized with 0.25% trypsin and washed with fresh culture medium, suspended in the culture medium with 10% bovine calf serum (1×106 cells/mL). The tumor cell suspension was transferred to the above layer of the Matrigel invasion chamber (0.3 mL/every chamber), while 0.8 mL of RPMI-1640 medium with 10% bovine calf serum was only added to the bottom layer of the Matrigel invasion chamber. Then the cells were cultured in 50 mL/L CO2 at 37 °C for 24 h; (2) The cells were treated without (untreated control group) or with various concentrations of NCTD (the six-concentration groups, every concentration ×3) in fresh culture medium (0.3 mL/every chamber), were cultured in an incubator with 50 mL/L at 37 °C for 72 h; (3) The passing-membrane cells were collected from the above layer of the Matrigel invasion chamber, centrifuged (200 r/min, 10 min), dyed by Trypan blue dye, and counted in a hemocytometer. Each experiment was performed thrice.
Crossing-river experiment of GBC-SD cells The suspension of GBC-SD cells (3×105 cells/mL) according to the above-mentioned confection method were fed (3 mL/well) into six-well culture plates and incubated in 50 mL/L at 37 °C for 24 h; (2) The cells were treated without (untreated control group) or with various concentrations of NCTD in fresh culture medium, and were again cultured in 50 mL/L at 37 °C for 84 h; (3) After being washed with PBS and one beeline as a river was then laid out on the well with 10 μL pipette tip, the cells of culture plate well were washed twice with PBS and cultured in the medium supplemented with 10% bovine calf serum in 50 mL/L at 37 °C. Beeline mark on the well was observed every 2 h till the mark was filled with the cells. Three separate expe-riments were performed for each concentration/exposure time combination.
Immunohistochemistry assay of PCNA, Ki-67, MMP2, TIMP2 Expression of PCNA, Ki-67, MMP2 and TIMP2 proteins of GBC-SD cells was determined by streptavidin-biotin complex method (SABC). PCNA, Ki-67, MMP2 and TIMP2 antibodies were respectively used at a concentration of 1:100. Goat serum, biotinylated secondary antibody (goat anti-mouse IgG) and DAB are all purchased from Boster. For negative control, the slides were treated with PBS in place of primary antibody. Ten slides were made up per experimental group. More than 10 visual fields were observed or more than 500 cells were counted per slide. The positive index of PCNA represented expression of PCNA protein. The positive index of PCNA = number of PCNA-positive cells/1000 cells. The positive index of Ki-67 represented expression of Ki-67 protein. The positive percentage of MMP2 or TIMP2 showed expression of MMP2 or TIMP2 protein.
Data statistical analysis All the statistical analyses were performed using SPSS 10.0 for Windows. P<0.05 or F<0.05 was considered to be of statistical significance.
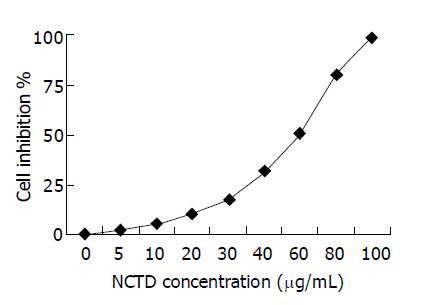
Inhibitory effect of various concentrations of NCTD on GBC-SD cells The effect of NCTD on cell growth was examined at doses between 0 and 100 μg/mL. As shown in Figure 1, inhibitory effect of NCTD at low concentrations (5 μg/mL) on GBC-SD cells was not obvious; but as concentration increased, proliferation of GBC-SD cells was markedly inhibited by NCTD with 98.59% growth inhibition at 100 μg/mL concentration, in a dose-dependent manner. IC50 is 56.18 μg/mL.
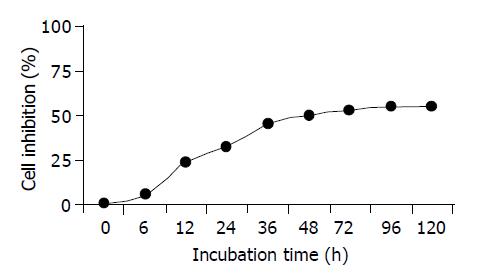
Inhibitory effect of IC50 NCTD on GBC-SD cells at different time The effect of NCTD on cell growth was examined at times between 0 and 120 h. As shown in Figure 2, anti-proliferation effect of IC50 NCTD on GBC-SD cells began to show after being cultured for 6 h; moreover, the inhibitory effect was markedly intensified as time prolonged with 52.18% growth inhibition for 48 h, in a time-dependent manner. So it was most obvious after 48 h.
Influence of NCTD on Matrigel invasion of GBC-SD cells in vitro As shown in Table 1, GBC-SD cells in untreated control group passed more of the membrane and had more invasive capability in vitro; NCTD began to inhibit the invasion of GBC-SD cells at the concentration of 5 μg/mL and as its concentration increased, their passing-membrane cells markedly decreased, the Trypan blue dyed cells, namely the dead passing-membrane cells obviously increased (P<0.01). At 40 μg/mL of NCTD, the invasive action of GBC-SD cells was inhibited completely. Its effect was also in a dose-dependent manner. The experiment showed that NCTD could inhibit obviously the in vitro invasive capability simulating human basement membrane of GBC-SD cells.
Influence of NCTD on the crossing-river test of GBC-SD cells As shown in Table 2, the crossing-river time of GBC-SD cells in various experiment groups was prolonged significantly after treatment with NCTD. When compared with control group, the crossing-river time was prolonged by 25% in 5 μg/mL NCTD group; 160% in 30 μg/mL NCTD group; GBC-SD cells did not still cross-river completely after 72 h in more than 40 μg/mL NCTD group. The crossing-river test indicated that NCTD could inhibit movement capability of GBC-SD cells in vitro markedly.
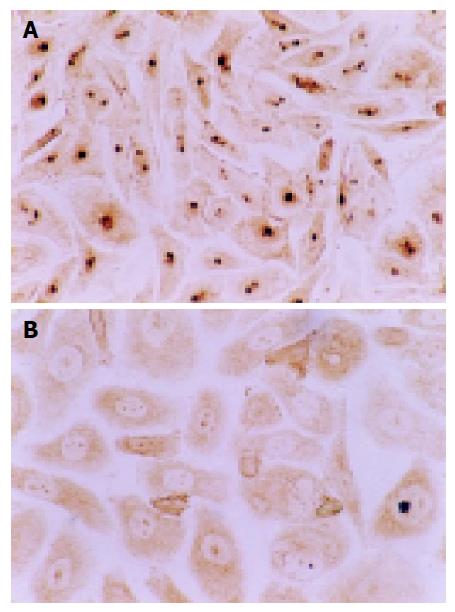
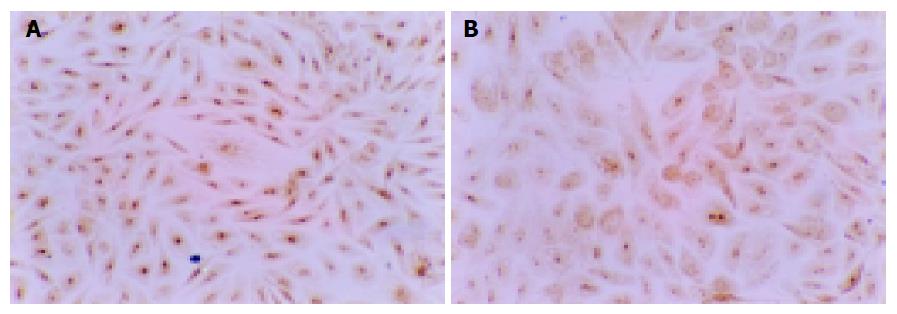
Influence of NCTD on expression of PCNA, Ki-67 proteins of GBC-SD cells The positive expression, with brown or yellow dye, of PCNA or Ki-67 occurred in cell nucleoli. GBC-SD cells in control group showed mostly the positive dye of PCNA and Ki-67 (Figures 3A and 4A), the positive index of PCNA and Ki-67 reached 0.932±0.031 and 0.964±0.092, respectively. After treatment with IC50 NCTD for 48 h, the positive cells of expression of PCNA and Ki-67 proteins decreased significantly, the dye of cell nucleoli became light and shallow (Figures 3B and 4B), the positive index of PCNA and Ki-67 came down 0.318±0.023 and 0.297±0.018 respectively, when compared with the control group (Table 3, P<0.05).
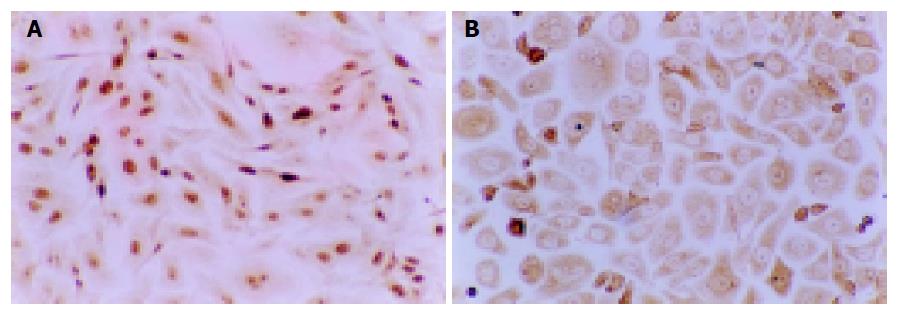
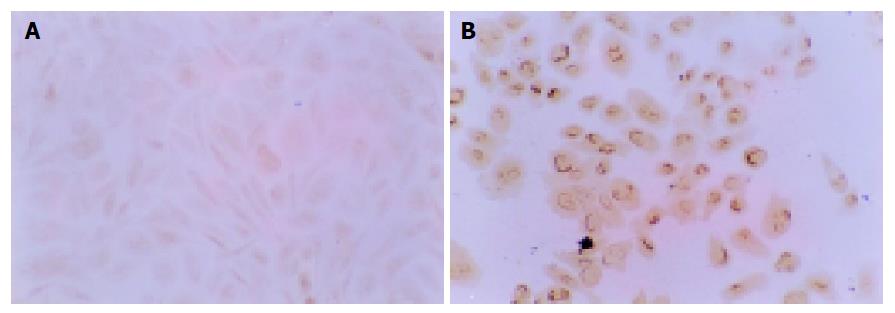
Influence of NCTD on expression of MMP2, TIMP2 proteins and MMP2/TIMP2 of GBC-SD cells The positive expression, with brown or yellow dye, of MMP2 occurred in cytoplast, TIMP2 in cytoplast or on nucleoli membrane. The positive expression of MMP2, the negative expression of TIMP2 was observed in most GBC-SD cells of control group (Figures 5A and 6A). After treatment with various concentrations of NCTD for 48 h and as its concentration increased, the positive cells of MMP2 expression was decreased and the dye became light and race, the positive percentage of MMP2 expression was reduced significantly; the positive cells and the positive percentage of TIMP2 expression were all increased significantly; the MMP2 to TIMP2 ratio was decreased significantly (Figures 5B and 6B; Table 4, P<0.05).
Primary carcinoma of the gallbladder is one of the malignant neoplasms with increasing incidence recently. As the special site of anatomy, the feature of biology and epidemiology of the disease, it is hitherto a poor prognostic disease with difficult diagnosis, early metastasis and dismal treatment result[1,4]. The only potentially curative therapy for the disease is still surgical resection. Unfortunately, most patients with this type of cancer present with advanced and unresectable disease-only 10-30% of patients can be considered for surgery on presentation, furthermore, only about 5% of patients were alive after 5 years since operation[1,3,5,7]. So, it is important that these patients should be considered for palliative, integrated treatment such as chemotherapy[1,3,6,8,9] and radiotherapy[1,3,6,10,11]. However, no specific chemoradio-therapy program for carcinoma of the gallbladder has emerged as the definitive acceptable standard of care, most series have small number of patients and there is much room for improvement[1,3,6,8-11]. With the deep research on the etiology, molecular biology of tumor and tumorigenesis, there is obviously an urgent need to identify new therapeutic agents for the treatment of gallbladder carcinoma.
Many lines of evidence have shown that Chinese medicine contains many chemical compounds with anticancer effects. We reported the effect of norcantharidin (NCTD) on growth and apoptosis of GBC-SD cell lines of human gallbladder carcinoma[12,13]. NCTD, the demethylated analog and the low-cytotoxic derivative of cantharidin, a 7-oxabicyclo[2.2.1]heptane-2, 3-dicarboxylic acid derivative, a natural toxin and the active ingredient extracted from Chinese medicine Mylabris. NCTD is synthesized from furan and maleic anhydride via the Diels-Alder reaction[14-17]. It has been reported that NCTD inhibit the proliferation and growth of a variety of human tumor cell lines in vitro, and are used to treat human cancers with stimulation of the bone marrow and increase of the peripheral leukocyte count[14,18-21]. There were, however, very few reports describing the effect of NCTD on human gallbladder carcinoma. In the present study, we investigated the in vitro effect of NCTD on proliferation and invasion of human gallbladder carcinoma GBC-SD cells and its mechanism.
Modern oncology research has shown that genesis and development of tumor are obviously related with proliferation and apoptosis of the cells. Proliferation and apoptosis of normal cells comparatively maintain their balanceable condition. If cell apoptosis is arrested or cell proliferation exceeds its apoptosis, the cells grow predominately. This is a significant basis of genesis and development of tumor. One of the most important mechanisms, on which many anti-cancer agents inhibit the growth of tumor is inhibition of the proliferation of tumor cells or inducement of their apoptosis[21,22].
In this study, we investigated the effect of NCTD on proliferation of human gallbladder carcinoma GBC-SD cells and its anti-cancer mechanism. NCTD was shown obviously effective in inhibiting the proliferation of GBC-SD cells in a dose- and time-dependent manner. This is consistent with the foreign reports about the effect of NCTD on other tumor cells[22,23]. The reports demonstrated that NCTD is the inhibitor of protein phosphatase type 2A[24-26] and can inhibit the growth of human colon cancer HT29 cells, inhibit cell growth and arrest the cell cycle at G2/M phase in K562 human myeloid leukemia cells, inhibit DNA synthesis in H L-60 cells and induce apoptosis in human tumor cells[23,27-29]. We reported that after treatment with NCTD, percentage of the G2/M phase in human gallbladder carcinoma GBC-SD cells was increased, percentage of the S phase was decreased with the increased rate of cell apoptosis by flow cytometry; cell nuclear shrinkage, membrane budding and karyorrhexis in some GBC-SD cells were shown by light microscope; microvillus decreasing, cell apparatus including Golgi and mitochondria atrophy, and typical apoptosis cells were observed by electron microscope, and there was the morphological change of apoptosis of GBC-SD cells[12]. It was shown that the mechanisms, on which NCTD inhibit the proliferation and the growth of human gallbladder carcinoma GBC-SD cells might be correlated with the inhibition of cell proliferation, arresting of cell cycle, blockage of DNA synthesis, influence of cell metabolism and inducement of cell apoptosis[12].
PCNA and Ki-67, gene proteins of cell proliferation-related, are the markers for reflection of cell proliferation[30-33]. In this study, after treatment with IC50 NCTD for 48 h, the positive GBC-SD cells of expression of PCNA and Ki-67 proteins were decreased significantly; their positive indexes were also decreased significantly (P<0.05). It was shown that NCTD could influence the expression of the proliferation-related gene proteins, PCNA and Ki-67, of GBC-SD cells. This may be one of the mechanisms by which NCTD inhibit proliferation and growth of human gallbladder carcinoma GBC-SD cells.
Tumor invasion, one of the essential characters of malignant neoplasm, is considered to be a dynamic, complex and multi-step process[34], but the essential step is the degradation of extracellular matrix (ECM) and basement membrane (BM)[34-37]. It was reported that matrix metalloproteinases (MMPs) are important for the degradation of ECM. MMPs hydrolyze specifically type IV, V, VII, X collagens and fibronectin, elastin, etc., which are all important components of ECM and BM, and are closely associated with the invasiveness and metastasis of tumor[34-37]. Tissue inhibitors of metalloproteinases (TIMPs), as the specific inhibitors of MMPs, have such ability to form tight-binding, non-covalent inhibitory complexes with multiple members of the MMP family that they inhibit MMP activity of ECM degradation and have anti-metastasis function[34-37]. The organic balance or homeostasis of MMPs to TIMPs ratio is the decisive factor for the maintenance of ECM steadiness and integrity[38-40]. So, inhibition of cancer cell invasion, maintenance of ECM integrity and homeostasis of expression of MMPS and TIMPS became one of the basic mechanisms of anti-cancer treatment for invasion and metastasis of tumor[41-43].
In the present study, The Matrigel experiment, the crossing-river test and immuno-histochemistry assay of MMP2, TIMP2 in human gallbladder carcinoma GBC-SD cells were shown that NCTD began to inhibit the in vitro invasion and movement of GBC-SD cells at the concentration of 5 μg/mL; and that after treatment with norcantharidin, the expression of MMP2 was significantly decreased, with the increase in TIMP2 expression the MMP2 to TIMP2 ratio were decreased significantly (P<0.05). Also, these changes were obviously related with the decrease of passing-membrane GBC-SD cells in the Matrigel experiment. NCTD could affect the expression of matrix dissolution-related gene proteins- MMP2 and TIMP2, and the ratio of MMP2/TIMP2, consequently, exert the inhibiting effect of invasion of GBC-SD cells. So, NCTD in vitro inhibits not only proliferation of human gallbladder carcinoma GBC-SD cells but also invasion and metastasis of the cells at relatively low concentrations. Its anti-invasive activity may be the result of decrease in MMP2 toTIMP2 ratio and reduced motility of the cells.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 465] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Bartlett DL. Gallbladder cancer. Semin Surg Oncol. 2000;19:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg. 2004;8:83-89; discussion 89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Ito H, Matros E, Brooks DC, Osteen RT, Zinner MJ, Swanson RS, Ashley SW, Whang EE. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg. 2004;8:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Muratore A, Polastri R, Capussotti L. Radical surgery for gallbladder cancer: current options. Eur J Surg Oncol. 2000;26:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Todoroki T. Chemotherapy for gallbladder carcinoma--a surgeon's perspective. Hepatogastroenterology. 2000;47:948-955. [PubMed] |
| 9. | Ishii H, Furuse J, Yonemoto N, Nagase M, Yoshino M, Sato T. Chemotherapy in the treatment of advanced gallbladder cancer. Oncology. 2004;66:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Houry S, Barrier A, Huguier M. Irradiation therapy for gallbladder carcinoma: recent advances. J Hepatobiliary Pancreat Surg. 2001;8:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kresl JJ, Schild SE, Henning GT, Gunderson LL, Donohue J, Pitot H, Haddock MG, Nagorney D. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Fan YZ, Fu JY, Zhao ZM, Chun CQ. Influence of norcantharidin on proliferation and apoptosis of GBC-SD cell lines of human gallbladder carcinoma. Shanghai Yixue. 2003;26:1-4. |
| 13. | Fan YZ, Fu JY, Zhao ZM, Chen CQ. The in vitro effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells and its mechanism. Zhonghua ZhongLiu ZaZhi. 2004;26:271-274. [PubMed] |
| 14. | Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26:147-162. [PubMed] [DOI] [Full Text] |
| 15. | Liu J, Gao J, Liu X. Advances in the study of Cantharidin and its derivatives. ZhongYaoCai. 2003;26:453-455. [PubMed] |
| 16. | Williams LA, Möller W, Merisor E, Kraus W, Rösner H. In vitro anti-proliferation/cytotoxic activity of cantharidin (Spanish Fly) and related derivatives. West Indian Med J. 2003;52:10-13. [PubMed] |
| 17. | Ho YP, To KK, Au-Yeung SC, Wang X, Lin G, Han X. Potential new antitumor agents from an innovative combination of demethylcantharidin, a modified traditional Chinese medicine, with a platinum moiety. J Med Chem. 2001;44:2065-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | McCluskey A, Ackland SP, Bowyer MC, Baldwin ML, Garner J, Walkom CC, Sakoff JA. Cantharidin analogues: synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg Chem. 2003;31:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Mack P, Ha XF, Cheng LY. Efficacy of intra-arterial norcantharidin in suppressing tumour 14C-labelled glucose oxidative metabolism in rat Morris hepatoma. HPB Surg. 1996;10:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Yang EB, Tang WY, Zhang K, Cheng LY, Mack PO. Norcantharidin inhibits growth of human HepG2 cell-transplanted tumor in nude mice and prolongs host survival. Cancer Lett. 1997;117:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Yi SN, Wass J, Vincent P, Iland H. Inhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitro. Leuk Res. 1991;15:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, Lee MS, Hsiao M, Lin SK. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol. 2003;39:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Chen YN, Cheng CC, Chen JC, Tsauer W, Hsu SL. Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells. Br J Pharmacol. 2003;140:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Liu XH, Blazsek I, Comisso M, Legras S, Marion S, Quittet P, Anjo A, Wang GS, Misset JL. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer. 1995;31A:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Hart ME, Chamberlin AR, Walkom C, Sakoff JA, McCluskey A. Modified norcantharidins; synthesis, protein phosphatases 1 and 2A inhibition, and anticancer activity. Bioorg Med Chem Lett. 2004;14:1969-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Baba Y, Hirukawa N, Tanohira N, Sodeoka M. Structure-based design of a highly selective catalytic site-directed inhibitor of Ser/Thr protein phosphatase 2B (calcineurin). J Am Chem Soc. 2003;125:9740-9749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Hong CY, Huang SC, Lin SK, Lee JJ, Chueh LL, Lee CH, Lin JH, Hsiao M. Norcantharidin-induced post-G(2)/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun. 2000;276:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, Hsu SL. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer. 2002;100:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, Mao YQ, Kan B, Lei S, Wang GS. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002;128:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1103] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 31. | Takasaki Y, Kogure T, Takeuchi K, Kaneda K, Yano T, Hirokawa K, Hirose S, Shirai T, Hashimoto H. Reactivity of anti-proliferating cell nuclear antigen (PCNA) murine monoclonal antibodies and human autoantibodies to the PCNA multiprotein complexes involved in cell proliferation. J Immunol. 2001;166:4780-4787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710-1715. [PubMed] |
| 33. | Aoki T, Tsukinoki K, Karakida K, Ota Y, Otsuru M, Kaneko A. Expression of cyclooxygenase-2, Bcl-2 and Ki-67 in pleomorphic adenoma with special reference to tumor proliferation and apoptosis. Oral Oncol. 2004;40:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43 Suppl:S42-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 509] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 37. | Zhang JT, Fan YZ. Matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs) and their roles in invasion and metastasis of tumor. Zhongliu. 2002;22:161-163. |
| 38. | Gohji K, Fujimoto N, Ohkawa J, Fujii A, Nakajima M. Imbalance between serum matrix metalloproteinase-2 and its inhibitor as a predictor of recurrence of urothelial cancer. Br J Cancer. 1998;77:650-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Gohji K, Fujimoto N, Fujii A, Komiyama T, Okawa J, Nakajima M. Prognostic significance of circulating matrix metalloproteinase-2 to tissue inhibitor of metalloproteinases-2 ratio in recurrence of urothelial cancer after complete resection. Cancer Res. 1996;56:3196-3198. [PubMed] |
| 40. | Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642-6650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 397] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 41. | Fan YZ, Zhang JT. Significance of MMP-2/TIMP-2 in gallbladder carcinoma. J Tumor Marker Oncol. 2001;16:339. |
| 42. | Fan YZ, Zhang JT. Study on the ratio of MMP-2/TIMP-2 in patients with carcinomas of the gallbladder and their clinical value. Zhonghua Gandan Waike Zazhi. 2002;8:630-631. |
| 43. | Zhang JT, Fan YZ, Yang HC, Yang YQ. Expression and its significance of MMP-2 and TIMP-2 proteins in gallbladder carcinomas. US Chin J Lymphol Oncol. 2003;2:36-41. |