Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1764
Revised: September 14, 2004
Accepted: December 3, 2004
Published online: March 28, 2005
AIM: To investigate the pharmacological effects of rice flavone (5,4’-dihydroxy-3’,5’-dimethoxy-7-O-β-D-glucopyranosyloxy-flavone, RF) separated from panicle-differentiating to flowing rice on rat experimental hepatic injury.
METHODS: Models of rat acute hepatic injury induced by carbon tetrachloride (CCl4) administration, rat hepatic fibrosis induced by thioacetamide, injury of primary cultured rat hepatocytes induced by CCl4, respectively, were established. After treated with RF, content of serum alanine transaminase (ALT), aspartate aminotransferase (AST) and albumin (Alb), hyaluronic acid (HA), the activity of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and hydroxyproline (Hyp) were measured and liver tissue was observed pathologically by hematoxylin-eosin (HE) staining. Effects of RF on pathological changes, function index, enzyme of scavenging free radicals and blood rheology were evaluated.
RESULTS: In model of rat acute hepatic injury induced by CCl4, RF can significantly decrease the contents of serum ALT, AST, increase the content of Alb, improve the dropsy and fat denaturalization of hepatocytes. In model of rat hepatic fibrosis induced by thioacetamide, RF can inhibit the increase of HA, Hyp and whole blood viscosity, and improve the activities of GSH-Px and SOD, and inauricular microcirculation.
CONCLUSION: RF has apparent protective effects on hepatic injury by increasing activity of GSH-Px and SOD, scavenging free radicals produced by CCl4, reducing blood viscosity, and improving microcirculation and blood supply.
-
Citation: Xu DH, Mei XT, Chen Y, Li YM, Lv JY, Xu SB. Protective effects of 5,4’-dihydroxy-3’,5’-dimethoxy-7-O-
β -D-glucopyranosyloxy-flavone on experimental hepatic injury. World J Gastroenterol 2005; 11(12): 1764-1768 - URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1764
Oxidation-reduction, blood viscosity, and microcirculation state are important indexes to evaluate the pathological state of liver. Free radicals’ injury can induce hepatic fibrosis when it comes to a certain degree. In tradition hepatic fibrosis is thought to be reversed while liver cirrhosis is not, so interdicting and reversing hepatic fibrosis are crucial in curing liver cirrhosis and a lot of intractable liver diseases.
In recent years, lots of in vitro experiments show that activation of phenotypic antigens on hepatic stellate cells (HSCs) is very important in the process of hepatic fibrosis. And oxidative stress can induce the activity of HSCs, especially in the early stage. Nowadays antioxidants have become a hotspot of drugs of anti-hepatic fibrosis.
From the past, people use Chinese herbal medicine containing high content of polyphenols to cure inflammatory, edema and hepatic injury. Many substances of flavones have effects of anti-oxidation; many flavones are chosen for studying anti-hepatic injury and anti-hepatic fibrosis, such as Silymarin, Scutellarin, Baicalin and Ginkgo biloba extract[1]. Experiments demonstrate that they really have effects of anti-hepatic fibrosis. We have separated 5,4’-dihydroxy-3’,5’-dimethoxy-7-O-β-D-glucopyranosyloxy-flavone from panicle-differentiating to flowering rice (Oryza sativa L. IR.72 T2)[2]. The protective effects and mechanisms on hepatic injury and hepatic fibrosis will be tested by establishing models of rat acute hepatic injury induced by CCl4 administration, rat hepatic fibrosis induced by thioacetamide, injury of primary cultured rat hepatocytes induced by CCl4.
Male SD rats (230±25 g in body mass) were supplied by Guangdong Experimental Animal Center. Matrine was purchased from Guangzhou Mingxing Pharmacy Company. N-(2-hydroxyethyl)piperazine-N’-2-ethanesulfonic acid (HEPES) and collagenase were purchased from Sigma Corporation. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Gibco BRL Company. Trypan blue was purchased from Huamei Company. Newborn calf serum was purchased from Guangzhou Stock Farm. Ninety-five percent of rice flavone (RF), provided by Laboratory of Drugs Pharmacology, School of Pharmaceutical Sciences, Sun Yat-Sen University, was dissolved to needed concentrations by 0.5% carboxymethyl cellulose sodium (CMC-Na) solution.
Protective effects on acute hepatic injury in rats induced by CCl4 Sixty healthy male SD rats (weighing 230±25 g) were divided randomly into six groups: normal control group, model control group, a matrine administration group, low-, medium-, high-dose groups of RF administration, 10 animals in each. Masculine group was given the injection of matrine at a dose of 13.5 mg/(kg·d). Three groups received RF injections at the doses of 4.5, 13.5 and 40.5 mg/(kg·d), respectively. Rats of normal control group and model control group were injected with 0.5% sterilized CMC-Na per day. On the seventh day, after the administration for 2 h, normal group rats were injected intraperitoneally peanut oil at 0.5 mL/100 g body mass, while rats from other groups were injected intraperitoneally with peanut oil containing 25% CCl4 at 0.5 mL/100 g body mass. The fasted rats had free access to water for 24 h. After this, the animals were exsanguinated, one blood sample was taken, centrifugated (3000 r/min for 10 min), and the plasma stored until analysis. Alb and the activities of ALT and AST in serum were determined by routine laboratory methods. Rat livers were resected for histological examination.
Protective effects on rat hepatic fibrosis induced by thioacetamide Sixty healthy male SD rats (weighing 230±25 g) were divided randomly into six groups: normal control group, model control group, a matrine administration group, low-, medium-, high-dose groups of RF administration, 10 animals in each. All groups except normal group were injected intraperitoneally with thioacetamide at first dose of 20 mg/kg body mass, at later dose of 20 mg/kg body mass, twice a week for 8 wk. From the 5th wk, masculine group was given matrine injection at a dose of 13.5 mg/(kg·d) for 4 wk. Three groups received RF injections at the doses of 4.5, 13.5 and 40.5 mg/(kg·d) for 4 wk, respectively. Normal control group and model control group rats were injected with 0.5% sterilized CMC-Na per day for 4 wk. On the 56th d, fasted rats had free access to water for 24 h. After this, the animals were exsanguinated, whole blood viscosity was measured, and HA in serum was determined by routine laboratory methods. Rat livers were resected for Hyp examination.
Injury of primary cultured rat hepatocytes induced by CCl4 Hepatocytes were harvested from SD rat (weighing 250 g) anesthetized with phenobarbitol sodium at a dose of 50 mg/kg body mass; abdomens of the rat was opened and shaved, the portal vein was exposed and cannulated. It was perfused at 37 °C in situ first with calcium-free phosphate-buffered saline solution (PBS) for 5 min. The liver was removed and put into the salver. The perfusion changed to 0.5 g/L collagenase in PBS buffer for 15 min. Then the liver was removed and the cells were combed gently in tissue culture medium, hepatocytes were pelleted, washed by centrifugations at 400 r/min for 3 min at 4 °C. The supernate was abandoned and the remainder continued to be cleaned and centrifuged with the solution (142 mmol/L NaCl, 67 mmol/L KCl, 12 mmol/L CaCl2·2H2O, 10 mmol/L HEPES, 55 mmol/L NaOH, pH7.4) for three times. Finally, viability of cells were stained by 0.6% trypan blue exclusion to observe their survival rate.
The cell concentration was adjusted to 7.5×105 /mL with culture medium (10% NBS, 10 mmol/L HEPES, 100 U/mL penicillin and streptomycin, 10 nmol/L insulin, 1×10 nmol/L dexamethasone). The hepatocytes were plated onto 24-well plastic tissue-culture plates (2 mL in each well) and 96-well plastic tissue-culture plates (0.2 mL in each well) culture medium; primary cultures were maintained for 24 h at 37 °C in 50 mL/L CO2. Then RF was added to make its concentrations to 5×10-4, 5×10-5, 5×10-6 and 5×10-7 mg/mL respectively, and CCl4 was added to make its concentrations to 10 mmol/L. After 24 h, cell suspension in 24-well plates was tested for the concentrations of ALT, AST, Alb, MDA, SOD and GSH-Px. After 48 h, 5 g/L MTT was added to 96-well plates (20 μL in each well). After maintaining for 4 h, dimethyl sulfoxide was added to dissolve the black crystal. Measurement was read at 570 nm of enzyme-labeled instrument.
Data are presented as mean±SE of the means (mean±SD). Comparisons among different groups were performed by t-test with Primer of Biostatistics.
Effects of RF on rat liver function RF at the dose of 40.5 mg/kg can significantly decrease the contents of ALT and AST in serum (P<0.001), and significantly increase the content of Alb (P<0.001). RF at the dose of 13.5 mg/kg can also significantly improve the contents of ALT (P<0.001), Alb (P<0.01) and AST (P<0.05) (Table 1).
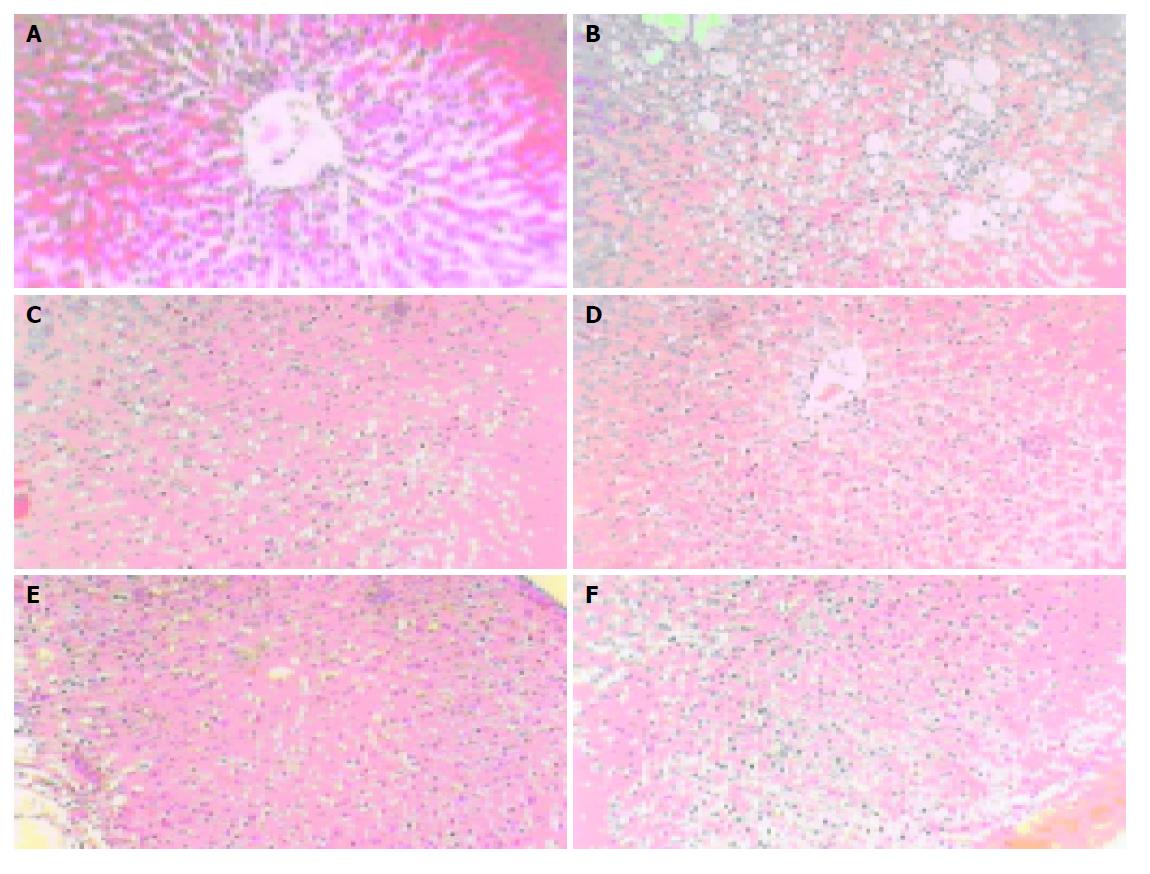
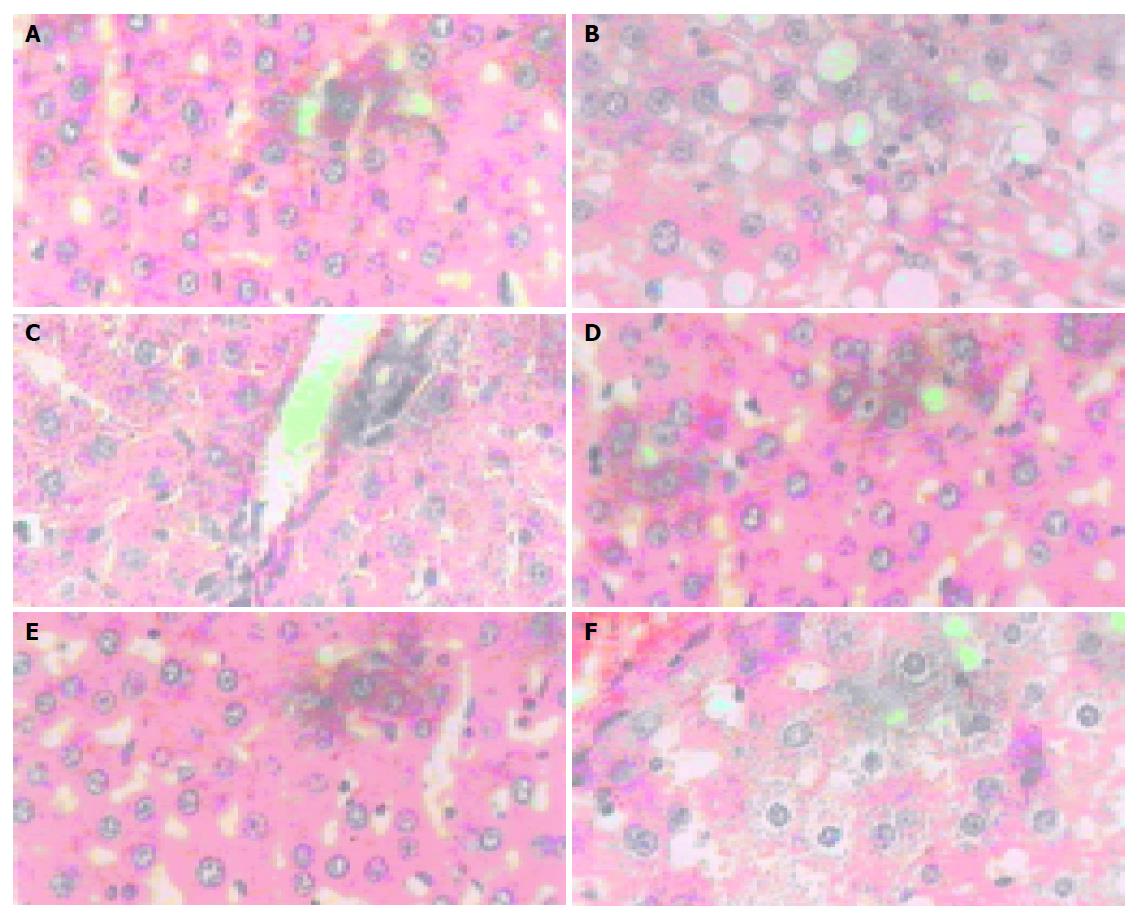
Histological analysis The hepatocytes in normal group were funicular around the central vein. The boundaries of the polygonal cells were blurry, with one to two nucleoli in the central (Figures 1A and 2A). The hepatocytes in model control group had apparent dropsy and much metamorphic fat (Figure 1B and 2B). The hepatocytes treated with RF 40.5 mg/kg were in normal shape except for a few metamorphic ones, without metamorphic fat (Figures 1D and 2D). A little dropsy, much less severe than that in model control group, appeared in the groups treated with RF 13.5 mg/kg (Figures 1E and 2E) and 4.5 mg/kg (Figures 1F and 2F), as well as in the matrine group (Figures 1C and 2C).
Effects of RF on rat in hepatic fibrosis model induced by thioacetamide Compared with normal group, lower, medium and high sheer rates of whole-blood viscosity, HA and Hyp of rat in hepatic fibrosis model induced by thioacetamide significantly increase the content of Alb (P<0.001). RF at the dose of 13.5, 40.5 mg/kg can significantly reduce the lower, medium and high sheer rates of whole-blood viscosity (P<0.01), inhibit the increase of HA in serum and Hyp in liver (P<0.001, Tables 2, 3).
| Group | Dose (mg/kg) | Whole-blood viscosity (P, mPa/s) | ||
| Lower sheer rate 10 s | Medium sheer rate 60 s | High sheer rate 120 s | ||
| Normal | – | 10.03±2.36d | 5.12±0.95d | 4.04±1.03d |
| Model | – | 22.60±4.64 | 10.73±1.82 | 7.83±1.35 |
| Matrine | 13.5 | 12.28±2.72b | 6.67±0.88d | 5.28±0.71d |
| RF | 4.5 | 14.44±1.74a | 9.60±1.39 | 7.18±0.60 |
| 13.5 | 16.34±1.76+ | 8.33±0.711b | 6.55±0.40d | |
| 40.5 | 14.44±1.17d | 7.76±1.23d | 5.96±0.78d | |
Effect of RF on ALT, AST and Alb in the supernatants of cultured rat hepatocytes injured by CCl4 The contents of ALT and AST in all RF groups decreased significantly (P<0.05-0.001), and the contents of Alb in the RF groups of 5×10-4 and 5×10-5 mg/mL increased significantly (P<0.01, P<0.001). RF at the dose of 5×10-5 mg/mL was the most effective among the four doses. These data indicate that RF can improve the injury induced by CCl4 (Table 4).
Effect of RF on SOD, GSH-Px and MDA in the medium of cultured rat hepatocytes injured by CCl4 As showed in Table 5, RF can markedly prevent the increase of MDA and the inactivation of GSH-Px and SOD in liver (P<0.001). Moreover, RF at the dose of 5×10-5 mg/mL was the most effective among all doses. The result demonstrates that RF can enhance the activity of SOD and GSH-Px enzyme to scavenge the free radicals and inhibit the production of MDA.
Effect of RF on the survival rate of primary cultured hepatocytes Observed under light microscopy, hepatocytes after being treated with CCl4 for 48 h obviously changed as cytomembrane rough, organelle swollen, chromatin condensed or ruptured. Most of the cells appeared to be necrotic or degenerative. Although hepatocytes in all RF doses appear with different percentages of necrosis, the number was much lower than that in CCl4 control. The result determined by MTT showed that the survival rate in normal control was 91.64%, compared to that of 49.65% in CCl4 control (P<0.001). The survival rates at all doses of RF increased significantly with dose dependence, which was 55.9%, 66.48%, 81.37% and 84.81%, respectively (Table 6).
The mechanism of hepatic injury induced by CCl4 is that P450 in liver activate CCl4 and produced CCl3. And the latter attacked the lipid molecules on the cell and intracellular membrane by hydrogen adsorption to produce LPO. Whole-blood viscosity and HA of hepatic injury of hepatic fibrosis patients are higher than those of normal people, having direct effects on blood microcirculation and tissue perfusion. It will result in slowing blood flow and ischemia among pathological section in liver, and aggravate pathological changes.
Theory of “blood flow-activation and stasis-removal to nourish the liver” is the theoretical basis in the treatment of hepatic fibrosis in traditional Chinese medicine. Pathological studies also show that serious microcirculation barrier occurs in chronic liver diseases. Studies show that RF has extensive pharmacological effects. RF could significantly improve the learning and memory ability in animal dementia models, such as the impairment of acquisition of memory in mice induced by M-anticholinergic agents like anisodine, the impairment of retrieval of memory in mice induced with central neuron system depressants like ethanol, mice induced with cerebral ischemia-reperfusion[3]. RF has obvious suppression effect on hepatic fibrosis by improving liver function of scavenging the free radicals and MDA to reduce their injury to liver tissue[4]. The mechanism works at least in two ways: Firstly, by preventing LPO from being reproduced. Secondly, by improving the activities of the enzymes of endogenous free radicals in liver. In the present study, RF significantly improved the liver function in rats with hepatic injury induced by CCl4 and decreased the contents of ALT and AST. All of these results indicate that RF can enhance the liver function and reduce the hepatic injury and fibrosis by reducing blood viscosity, improving microcirculation and blood supply, providing the hepatocytes with sufficient oxygen and nutrition, enhancing the activity of anti-oxidation enzymes and scavenging free radicals.
In general, RF is effective in anti-hepatic injury and anti-oxidation, improving microcirculation. As for its rich resource, RF is promising to be produced in industry and developed into a new drug of anti-hepatic fibrosis.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Luo YJ, Yu JP, Shi ZH, Wang L. Ginkgo biloba extract reverses CCl4-induced liver fibrosis in rats. World J Gastroenterol. 2004;10:1037-1042. [PubMed] |
| 2. | Xu DH, Li BJ, Liu Y, Huang ZS, Gu LQ. Identification of rice (Oryza sativa L.) signal factors of inducing Agrobacterium vir Gene expression. Sci China Ser C Life Science. 1996;39:8-16. |
| 3. | He R, Mei X, Xu D, Xu S. [Improvement of learning and memory functions by rice flavoids in mice]. Zhong Yao Cai. 2002;25:108-111. [PubMed] |
| 4. | Xu DH, Chen Y, Mei XT, Xu SB. Effect of the RF on experimental rathepaic firbrosis. Zhongguo Yaoke Daxue Xuebao. 2002;33:234-237. |