Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.69
Revised: August 28, 2003
Accepted: October 27, 2003
Published online: January 7, 2005
AIM: To study the blocking effects of genistein on cell proliferation cycle in human gastric carcinoma cells (SGC-7901) and the possible mechanism.
METHODS: MTT assay was applied in the detection of the inhibitory effects of genistein on cell proliferation. Flow cytometry was used to analyze the cell cycle distribution. Immunocytochemical technique and Western blotting were performed to detect the protein expression of cyclin D1, cyclin B1 and p21waf1/cip1.
RESULTS: Genistein significantly inhibited the growth and proliferation of human gastric carcinoma cells (SGC-7901). Seven days after treatment with different concentrations of genistein (2.5, 5.0, 10.0, 20.0 μg /mL), the growth inhibitory rates were 11.2%, 28.8%, 55.3%, 84.7% respectively and cell cycles were arrested at the G(2)/ M phase. Genistein decreased cyclin D1 protein expression and enhanced cyclin B1 and p21waf/cip1 protein expression in a concentration-dependent manner.
CONCLUSION: The growth and proliferation of SGC-7901 cells can be inhibited by genistein via blocking the cell cycle, with reduced expression of cyclin D1 and enhanced expression of cyclin B1 and p21waf/cip1 protein in the concentration range of 0-20 μg /mL.
- Citation: Cui HB, Na XL, Song DF, Liu Y. Blocking effects of genistein on cell proliferation and possible mechanism in human gastric carcinoma. World J Gastroenterol 2005; 11(1): 69-72
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/69.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.69
Genistein is a natural ingredient in soybean. Recently, it has attracted more and more attention in the field of cancer prevention[1-3]. A number of epidemiological and laboratory studies have shown that genistein is a potential cancer chemopreventive agent for sex hormone-dependent cancers, such as breast cancer and prostate cancer[4-9]. However, there are few reports about the effect of genistein on non-sex hormone-dependent cancers, such as gastric cancer[10-12]. Gastric cancer is common in China and supposed to be caused by environmental factors, in which diet is an important modifying agent[13,14].
In this study, human gastric carcinoma cells (SGC-7901) were used as the model in vitro to investigate the effect of genistein on cell proliferation and its possible mechanism.
Genistein (purity >98%) and trypsin were purchased from Sigma. 3H-TdR was purchased from China Atomic Energy Research Academy. SP-9000 kit was the product of Zyme. Monoclonal antibodies to cyclin D1, cyclin B1 and p21waf1/cip1 were the products of Santa Cruz and purchased from Zhongshan Co., China.
Human gastric carcinoma cells (SGC-7901), provided by the Cancer Research Institute of Beijing, were cultured in RPMI1640 (Gibco) medium supplemented with 10% fetal calf serum, penicillin (100×103U/L) and streptomycin (100 mg/L) at 37 °C in a 50 mL/L CO2 atmosphere. Genistein was dissolved in DMSO at the concentration of 20 mg/mL and then diluted to the required concentration with culture medium.
MTT assay was conducted to detect the cell proliferation. SGC-7901 cells were seeded in 96- well plates, each well containing 5×103 cells. After 24 h, the culture medium was replaced by media in which genistein concentrations were 0, 2.5, 5.0, 10.0 and 20.0 μg /mL respectively. There were four wells for each concentration. From 1 to 7 d, one of the plates was taken out and 20 μL fresh 3-[4,5-dimethhylthiaoly]-2,5-diphenyl-tetrazolium bromide (MTT, 5g/L PBS) was added to each well. After 4 h incubation, the culture media were discarded, 150 μL of DMSO was added to each well and vibrated to dissolve the depositor. The optical density (A value) was measured at 570 nm with a microplate reader. The inhibitory rate (IR) of genistein on SGC-7901 cells on the 7th d was calculated as follows: IR (%) = (1- treated group A/control group A)×100%.
After an exponential growth phase, SGC-7901 cells were treated with different concentrations of genistein (0, 5.0, 10.0 and 20.0 μg/mL) for 24 or 48 h. The cells were collected and stained with propidium iodide (PI), then the DNA content of cells was measured using flow cytometry to monitor the cell cycle changes.
Cultured cells treated with genistein for 24 or 48 h were harvested and fixed in 4% citromint solution, and then embedded in paraffin. Four micrometer-thick sections were cut and deparaffinized in xylene and dehydrated with graded alcohol. Sections were treated with microwave to retrieve antigens, then incubated overnight at 4 °C with cyclin B1 and cyclin D1 antibodies (1:50 dilution) respectively. Other steps were according to the description of SP kit. Chromogenic reaction was developed with diaminobenzidine (DAB), and restained with methylgreen. All sections were observed under microscope and the number of positive cells per 1000 cells was counted.
Cultured cells treated with genistein for 48 h were harvested and washed with PBS. The cells were lysed in protein extract solution. Protein concentration was determined by Coomassie light blue methods. One hundred micrograms of cell protein was degenerated by heat, separated on 10% polyacrylamide gel electrophoresis and transferred to nitrocellulose filter membrane at 30 V. The membranes were incubated with blocking solution (containing antibodies against p21waf1/cip1) for 2 h at 37 °C and washed twice with PBS, then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Chromogenic reaction was developed with DAB and the bands were recorded and the peak areas of protein were scanned by the digital image instrument (ChemiImager 4000).
Data analysis was performed using Student’s t test. P<0.05 was considered statistically significant.
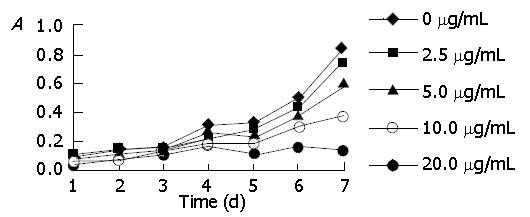
MTT assay was conducted to detect the inhibitory effect of genistein on SGC-7901 cells. As shown in Figure 1, cell proliferation slowed down with the increase of genistein concentration and elongation of action time in a dose- and time-dependent manner. On d 7, the inhibitory rates of genistein on SGC-7901 cell growth at concentrations of 2.5, 5.0, 10.0 and 20.0 μg/mL were 11.2%, 28.8%, 55.3% and 84.7%, respectively.
As shown in Table 1, the cell cycle of SGC-7901 cells was changed obviously. The number of cells in G0/G1 phase of cell cycle was decreased gradually. The progression of cell cycle was partly arrested at G2/M phase, but the change of S phase was insignificant.
| Genistein(mg/mL) | 24 h | 48 h | ||||
| G0/G1 | S | G2/M | G0/G1 | S | G2/M | |
| 0 | 57.9 | 32.52 | 9.57 | 64.13 | 29.75 | 6.12 |
| 5 | 50.64b | 30.05 | 19.31b | 56.16b | 29.41 | 14.43b |
| 10 | 43.01b,d | 30.18a | 27.80b,d | 49.85b,d | 30.01 | 20.14b,d |
| 20 | 36.96b,d,f | 30.66a | 32.38b,d,f | 39.26b,d,f | 36.88b,d,f | 23.86b,d,f |
After SGC-7901 cells were incubated with different concentrations of genistein for 24 and 48 h, the expression of cyclin B1 was significantly increased while that of cyclin D1 was significantly decreased. There were significant differences between each dosage group and control group. The results are shown in Table 2.
| Genistein (mg/mL) | Positive rate (%, 24 h) | Positive rate (%, 48 h) | ||
| cyclin B1 | cylinD1 | cyclin B1 | cylinD1 | |
| 0 | 36.8 | 91.9 | 48.2 | 88.1 |
| 5 | 46.5b | 70.5b | 54.8b | 54.3b |
| 10 | 53.4b,d | 49.3b,d | 62.8b,d | 43.9b,d |
| 20 | 72.3b,d,f | 25.4b,d,f | 85.2b,d,f | 22.1b,d,f |
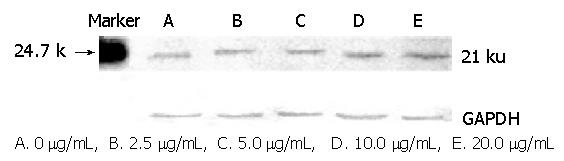
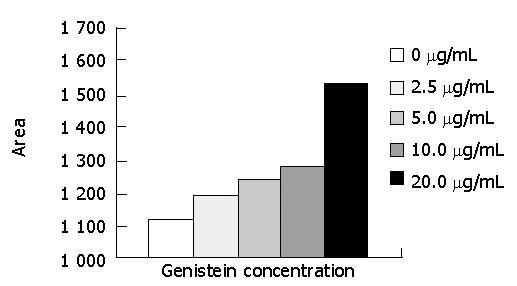
The expression of p21waf1/cip1 protein is shown in Figure 2 and the peak areas of bands were analyzed with gel digit image instrument (Figure 3). Genistein at concentrations of 2.5, 5.0, 10.0 and 20.0 μg/mL increased the expression of p21waf1/cip1 in a concentration-dependent manner.
MTT chromatometry is a common method to detect cell stock and growth. Ectogenesis of MTT can be reduced by succinic acid dehydrogenase existing in mitochondria of live cells and forms indissoluble blue-purple crystal mass (formazan) and deposits in cells. The crystal mass is dissolved by DMSO. By detecting the A value with a microplate reader, the quantity of live cells can be gained indirectly. The findings from our research group suggest that genistein could significantly inhibit the proliferation of SGC-7901 cells in a dose- and time-dependent manner. As shown in Figure 1, the inhibitory rates of different genistein concentrations (2.5, 5.0, 10.0 and 20.0 μg/mL) on d 7 are 11.2%, 28.8%, 55.3% and 84.7%, respectively. Genistein is a growth inhibitor of gastric carcinoma cells, the mechanism is unknown. However, we discovered that supplemented with genistein, the number of SGC-7901 cells after incubation in culture media was decreased and the cell cycle was arrested at G2/M phase.
Cyclins are a group of proteins with cell cycle specificity. Up to the present, cyclins A, B (B1-2), C, D (D1-3), E, F, G and H have been found. Cyclin D1 is synthesized in pre-DNA-synthetic gap (early G1 phase), and plays an important role in G1 to S phase and induces cells into S phase. In general, cyclin D1 is the key regulator of cell cycle progression and the key protein of the signal transduction in G1 phase cell proliferation. If cyclin D1 is over-expressed, the checkpoint of G1/S will be out of control and lose its role in the signaling of proliferation. This further promotes cell cycle progression and cell proliferation, and causes carcinomatous change of cells. Thus cyclin D1 is called the shirking protein of G1/S checkpoint. It has been proved that cyclin D1 is overexpressed in several neoplasms, such as esophageal carcinoma, mammary cancer, pulmonary and gastric carcinoma[15]. Suppressed expression of cyclin D1 in cancer cells would help recover normal cell cycle and control proliferation speed of tumor cells. In this study, we found that genistein showed significant inhibition on the expression of cyclin D1 in SGC-7901 cells, suggesting that genistein might inhibit cell proliferation of gastric carcinoma by decreasing the over-expression of cyclin D1.
Cyclin B1 and cyclin-dependent kinase 1 (CDK1) are two proteins required for cells to traverse from G(2) into M. G(2) arrest occurs in response to DNA damage caused by a variety of agents and treatments. Cyclin B1 is synthesized in late S and G2 phase. It binds to CDK1 and is activated to form maturation promoting factor (MPF). Cyclin B1 is degraded in M phase. We investigated the expression of cyclin B1 in SGC-7901 cells treated with various concentrations of genistein for 24 and 48 h. The results showed that the expression of cyclin B1 did not decrease with increased concentrations of genistein as cyclin D1, instead it increased. Some researches indicate that sustained increase of cyclin B1 causes cell cycle blockage in cell cleavage phase. However, other results show that when cell cycle blockage occurs in G2/M phase, cyclin B1 is not degraded, but accumulated in cells[16-19]. Cappelletti et al[16] demonstrated that genistein could block mammary cancer cells in G2/M phase, but the expression of cyclin B increased 2.8, 8, and 103 times respectively in BT20, MDA-MB-231 and ZR75.1 cells. It is stated that G2/M blockage does not always follow the decrease of cyclin B1 expression. In this experiment, genistein blocked SGC-7901 cell proliferation and increased the number of cells in G2/M phase more than three times, as well as the expression of cyclin B1. The increased cyclin B1 expression did not make cancer cells escape the regulation of checkpoint from G2 to M phase. Maybe it is because cyclin B1 protein accumulates during interphase, while cell cycle progression is arrested at G2/M phase. The molecular mechanism underlying G2/M phase blockage requires clarification in further studies.
To find out the effect of genistein on cell proliferation cycle, we detected the expression of CKI-p21waf1/cip1 protein by Western blotting. Researchers previously believed that p21waf1/cip1 protein was a regulatory factor of cell cycle in G1 phase. But now, more and more evidence indicates the expression of p21waf/cip1 protein relates with G2/M phase arrest[6,20-23]. While p21waf1/cip1 binds to a variety of CDKs and cyclins, and exerts inhibitory activity on cyclin/CDK complexes, including cyclinA-CDK1 and cyclinB1-CDK1. Therefore p21waf/cip1 protein has an intimate relationship with G2 and M phases of cell cycle. When SGC-7901 cells are incubated with genistein for 48 h, the expression of p21waf1/cip1 is reduced in a dose- dependent manner. All these demonstrate that the inhibitory effect of genistein on human gastric carcinoma cells relates with genistein-induced expression of p21waf/cip1 and genistein arrests tumor cells in G2/M phase.
Cell cycle regulation involves many factors and is very complicated[23]. The data from our studies indicate that genistein could arrest cell cycle progression of SGC-7901 cells at G2/M phase. The possible mechanism is that genistein promotes the expression of p21waf1/cip1 and reduces the degradation of cyclin B1 protein in tumor cells. Therefore tumor cells are unable to pass the checkpoint pathway of G2/M and can not proceed to mitosis. Genistein could also inhibit the expression of cyclin D1 in tumor cells. In a word, neoplasm is a disease of cell over-proliferation and correlates with cell cycle regulation disorder. Genistein inhibits tumor cell growth and proliferation by increasing the expression of cyclin B1 and p21waf/cip1 and decreasing the expression of cyclin D1 in SGC-7901 cells. This result suggests that the inhibitory effect of genistein on SGC-7901 cell proliferation relates to cell cycle.
Edited by Wang XL, Zhang JZ and Zhu LH
| 1. | Myoung H, Hong SP, Yun PY, Lee JH, Kim MJ. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer Sci. 2003;94:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Arliss RM, Biermann CA. Do soy isoflavones lower cholesterol, inhibit atherosclerosis, and play a role in cancer prevention? Holist Nurs Pract. 2002;16:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Jones JL, Daley BJ, Enderson BL, Zhou JR, Karlstad MD. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am Surg. 2002;68:575-577; discussion 577-578. [PubMed] |
| 5. | Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S-558S. [PubMed] |
| 6. | Frey RS, Li J, Singletary KW. Effects of genistein on cell proliferation and cell cycle arrest in nonneoplastic human mammary epithelial cells: involvement of Cdc2, p21(waf/cip1), p27(kip1), and Cdc25C expression. Biochem Pharmacol. 2001;61:979-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Castle EP, Thrasher JB. The role of soy phytoestrogens in prostate cancer. Urol Clin North Am. 2002;29:71-81, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett. 2002;186:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132:3623-3631. [PubMed] |
| 10. | Song D, Liu Y, Wang X, Yang Y. Inhibitory effects of genistein on the synthesis of DNA and the protein expression of cyclin D1 in human gastric carcinoma cell-line. Wei Sheng Yan Jiu. 2002;31:106-108. [PubMed] |
| 11. | Song D, Na X, Liu Y, Chi X. Study on mechanisms of human gastric carcinoma cells apoptosis induced by genistein. Wei Sheng Yan Jiu. 2003;32:128-130. [PubMed] |
| 12. | Piontek M, Hengels KJ, Porschen R, Strohmeyer G. Antiproliferative effect of tyrosine kinase inhibitors in epidermal growth factor-stimulated growth of human gastric cancer cells. Anticancer Res. 1993;13:2119-2123. [PubMed] |
| 13. | Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901). World J Gastroenterol. 2002;8:224-229. [PubMed] |
| 14. | Wang DX, Fang DC, Liu WW. Study on alteration of multiple genes in intestinal metaplasia, atypical hyperplasia and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:855-859. |
| 15. | Barnes DM, Gillett CE. Cyclin D1 in breast cancer. Breast Cancer Res Treat. 1998;52:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 188] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Cappelletti V, Fioravanti L, Miodini P, Di Fronzo G. Genistein blocks breast cancer cells in the G(2)M phase of the cell cycle. J Cell Biochem. 2000;79:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by arsenic trioxide on gastric cancer cell SGC-7901. Shijie Huaren Xiaohua Zazhi. 1999;7. |
| 18. | Kasahara T, Kuwayama C, Hashiba M, Harada T, Kakinuma C, Miyauchi M, Degawa M. The gene expression of hepatic proteins responsible for DNA repair and cell proliferation in tamoxifen-induced hepatocarcinogenesis. Cancer Sci. 2003;94:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Palazón LS, Davies TJ, Gardner RL. Translational inhibition of cyclin B1 and appearance of cyclin D1 very early in the differentiation of mouse trophoblast giant cells. Mol Hum Reprod. 1998;4:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by p21waf1/cip1 induction, G2/M arrest, and apoptosis. J Cell Biochem. 1998;69:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Stewart ZA, Leach SD, Pietenpol JA. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205-215. [PubMed] |
| 22. | Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Kim MH, Gutierrez AM, Goldfarb RH. Different mechanisms of soy isoflavones in cell cycle regulation and inhibition of invasion. Anticancer Res. 2002;22:3811-3817. [PubMed] |