Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.945
Revised: July 7, 2003
Accepted: July 30, 2003
Published online: April 1, 2004
AIM: To investigate the effects of vitamin E succinate (VES) on the expression of Fas and PCNA proteins as well as its clinical significance in human gastric carcinoma, and to explore the mechanism of VES-induced inhibition of gastric carcinoma cell growth.
METHODS: Immunohistochemical methods were used to detect Fas and PCNA expression both in human gastric cancer SGC-7901 cells treated with VES at different doses and in human gastric carcinoma tissues.
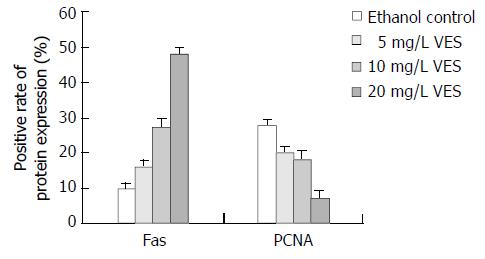
RESULTS: After the SGC-7901 cells were treated with VES at 5, 10, 20 mg/L for 48 h, the positive rates of Fas expression were 16%, 27% and 48%, respectively, significantly increased compared to that of control group (P < 0.05); while the positive rates of PCNA expression in groups treated with different doses of VES were 20%, 18% and 7%, respectively, which were significantly decreased compared to that of the control group (P < 0.05). In human gastric carcinoma tissues, the Fas positive expression rate was 42.4%(25/59), which declined with the decrease in the degree of tumor differentiation (P < 0.05) and with the existence of lymph node metastasis (P < 0.001). While the PCNA positive expression rate was 91.5%(54 / 59), no relationship was observed between PCNA expression and clinico pathologic parameters.
CONCLUSION: VES inhibited the growth of gastric cancer cells by inducing Fas expression and inhibiting PCNA expression. It is, therefore, considered that the expression of Fas and PCNA genes, through tumor cell apoptosis and proliferation, respectively, may be useful as a clinical predictive index in the application of VES to gastric carcinoma therapy, where as Fas may be of more value than PCNA.
- Citation: Wu K, Zhao L, Li Y, Shan YJ, Wu LJ. Effects of vitamin E succinate on the expression of Fas and PCNA proteins in human gastric carcinoma cells and its clinical significance. World J Gastroenterol 2004; 10(7): 945-949
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.945
RRR-α-tocopheryl succinate (vitamin E succinate, VES), a derivative of natural vitamin E, has been shown to be a potent growth inhibitor of many kinds of cancer cell types such as human monocytic leukemia cell[1], murine Bl6 melanoma cell[2,3], human prostate cancer cell[4], human breast cancer cell[5,6] and murine EL-4 thymic lymphoma cell[7,8]. Characterization of cellular processes involved in VES antiproliferative effects have shown that VES inhibits tumor cell growth by a variety of mechanisms, including cell cycle blockage[9,10], DNA synthesis arrest[11], induction of differentiation[12-14], and triggering of apoptosis[15-19]. Meanwhile, VES is also noteworthy because of its non-toxic and non-inhibitory effects on normal cell types[20], indicating that VES can be used as a chemopreventive/ chemotherapeutic agent against tumors.
Gastric cancer is one of the most common malignant tumors in China[21-29]. Our previous studies found that VES could inhibit human gastric cancer SGC-7901 cell growth by blocking cell cycle, arresting DNA synthesis and triggering apoptosis[30-32]. In addition, our in vivo research demonstrated that VES inhibited benzo (a) pyrene (B (a) P)-induced forestomach carcinogenesis in female mice[33]. In this study, the expression of Fas and PCNA both in SGC-7901 cells treated with VES and in human gastric carcinoma tissues was investigated further for its clinical significance. It may provide reference indices for VES application in clinical therapy.
Human gastric cancer cell lines SGC-7901 were maintained in RPMI 1640 medium supplemented with 100 mL/L fetal calf serum (FCS), 100 kU/L penicillin, 100 mg/L streptomycin and 2 mmol/L L-glutamine, incubated in a humidified atmosphere containing 50 mL/L CO2 at 37 °C. The SGC-7901 cells were collected after incubation for 48 h in the presence of VES at 5, 10 and 20 mg/L and of ethanol at 1 mL/L as the control group (VES was dissolved in absolute ethanol and diluted in RPMI 1640 complete condition media correspondingly to a final concentration of VES and 1 mL/L ethanol). The cells were then fixed in 40 g/L formaldehyde and embedded in paraffin.
Fifty-nine paraffin-embedded specimens of gastric cancer were obtained from the pathological laboratory of the First Affiliated Hospital of Harbin Medical University. These specimens were from resections of gastric cancer in this hospital from 1991 to 2001. Of these, 35 were from males, and 24 from females and the age of the patients varied from 31 to 77 years. These specimens had been histologically classified in which 7 were highly to moderately differentiated adenocarcinomas, 52 were poorly differentiated. Thirty-three tumors had invaded the serosa by histology, and 22 cases had local lymph node metastasis.
VES was purchased from Sigma Co. Ltd. RPMI 1640 medium was obtained from Gibco BRL. Antibodies against either PCNA or Fas and SP immunohistochemical reagent kit were purchased from Beijing Zhongshan Biotechnology Co. Ltd.
Serial sections of 4 μm thick were sliced from each of the cell and clinical tissue blocks. These were deparaffinized and rehydrated, and immersed in 30 mL/L H2O2 for 10 min to remove the endogenous peroxidase activity. The sections were further incubated with 100 mL/L normal goat serum for 1 h to reduce nonspecific binding. They were, then, incubated with either primary Fas or PCNA antibody at 4 °C for 12-16 h. All sections were washed in phosphate buffer solution (PBS) (0.01 mol/L, pH7.2). Afterwards, they were sequentially incubated with biotin conjugated secondary antibody, and avidin biotin enzyme reagent. Staining was done by first immersing the slides in 3, 3’-diaminobenzidine tetrahydrochloride (DAB). All slides were counterstained with haematoxylin/methyl-green, dehydrated and mounted. PBS substituted for the primary antibody was used as the negative control. For the slides initially incubated with primary Fas antibody, if the proportion of positively stained cells was more than 10%, it was considered as a Fas positive sample. For the slides initially incubated with primary PCNA antibody, if the proportion of positively stained cells as more than 50%, it was considered as a PCNA positive sample.
The data of Fas and PCNA expression were analyzed by t-test. Correlations between Fas and PCNA expression and clinicopathologic parameters were examined using c2 test. P < 0.05 was considered to be statistically significant.
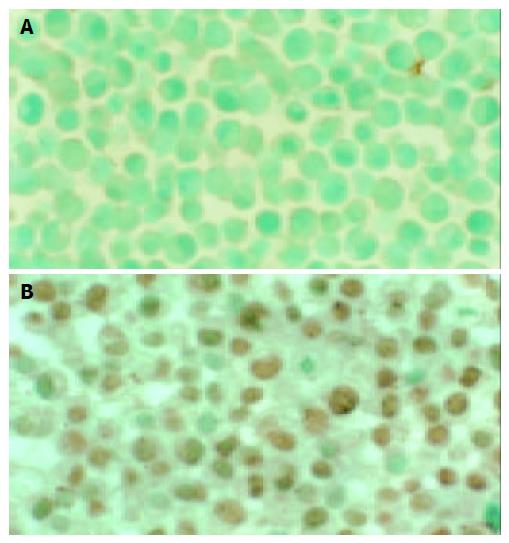
The Fas positive expression appeared as brown granules located in the membrane and cytoplasm, and the cell nucleus appeared green after counterstaining with methyl green (Figure 1). After the SGC-7901 cells were treated with VES at 5, 10, 20 mg/L and ethanol at 1 mL/L for 48 h, the Fas positive rates were 16%, 27%, 48% and 10%, respectively (Figure 2). The positive rates for the VES treated groups at different doses were significantly increased compared to that of the control group (P < 0.05), with an evident dose-effect relationship.
In contrast to Fas positive expression, the positive PCNA brown stained granules were in the nucleus, while the negative cell nucleus appeared blue after counterstaining with hematoxylin (Figure 3). PCNA positive expression rates, when the cells were treated with VES at 5, 10, 20 mg/L for 48 h were 20%,18% and 7%, respectively (Figure 2), and in the control group 28%. The positive rates of the cells treated with VES at different doses were significantly decreased compared to that of the control group (P < 0.05), with an evident dose-effect relationship.
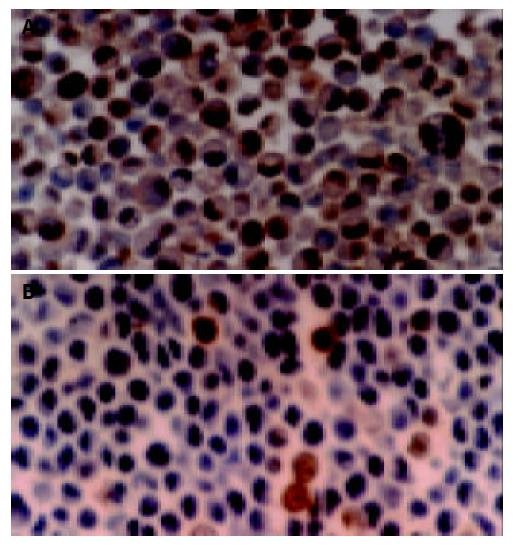
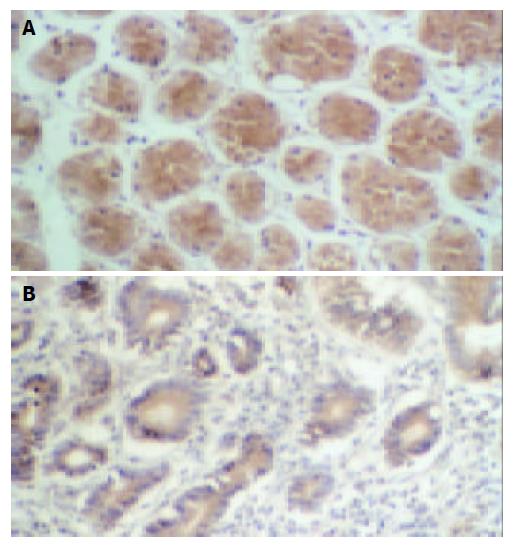
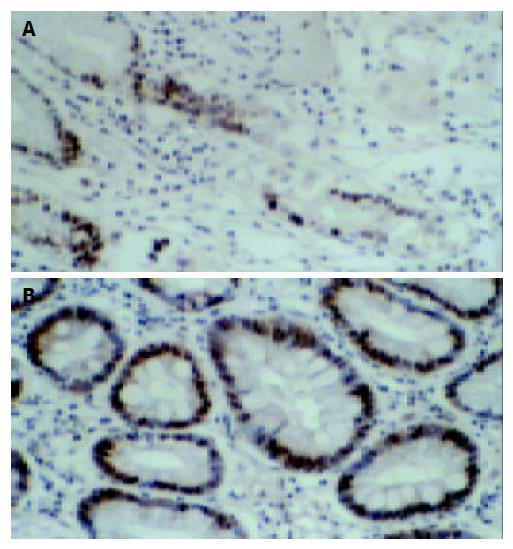
As shown in Figure 4A, the expression of Fas protein in normal gastric tissues was stronger, for there were many brown stained granules in the membrane and cytoplasm of both glandular and interstitial cells. In the gastric carcinoma tissues, the Fas expression was decreased (Figure 4B) or even negative, the Fas positive rate was only 42.4%(25 / 59).
There were brown stained granules located in the nuclei of PCNA positive cells, while the negative cell nucleus appeared blue after counterstaining with hematoxylin (Figure 5). The positive expression rate of PCNA in normal gastric tissues was not high; the stained cell rate was 49.6%. The stained cell rate in gastric carcinoma tissues was between 33-99%, and the PCNA positive rate was 91.5%(54 / 59).
As shown in Table 1, the rates of Fas and PCNA protein expression were not correlated with either the patient’s age or sex (P > 0.05). The immunoreactivity of Fas was significantly associated with tumor differentiation and lymph node metastasis. Among the 59 cases of gastric carcinoma, Fas positive expression rate in the 7 well-differentiated tissues (85.7%) was obviously higher than that in the 52 cases that were poorly differentiated tissues (36.5%)(P < 0.05). The Fas positive expression rate in the group with lymph node metastasis was 13.6%(3/11), lower than in that without lymph node metastasis (P < 0.01). PCNA positive expression was not associated with clinicopathologic parameters (P > 0.05).
| Pathologic parameters | n | Fas expression | PCNA expression | ||
| + | (%) | + | (%) | ||
| Age (yr) | |||||
| ≤ 45 | 11 | 4 | (36.3) | 11 | (100) |
| > 45 | 48 | 21 | (43.7) | 43 | (89.5) |
| Sex | |||||
| Male | 35 | 17 | (48.5) | 30 | (85.7) |
| Female | 24 | 8 | (33.3) | 24 | (100) |
| Tumor differentiation | |||||
| Well-moderate | 7 | 6 | (85.7) | 5 | (71.4) |
| Poor | 52 | 19 | (36.5) | 49 | (94.2) |
| Tumor invasion | |||||
| Muscularis | 26 | 14 | (53.8) | 24 | (92.3) |
| Serosa | 33 | 11 | (33.3) | 30 | (90.9) |
| Node metastasis | |||||
| ( + ) | 22 | 3 | (13.6) | 22 | (100) |
| ( - ) | 37 | 22 | (59.6)b | 32 | (99.8) |
The mechanisms of VES-induced inhibition of tumor cell growth have been a topic of great interest. Turley et al[34] observed that the expression of Fas, a cell surface receptor, was increased after treatment of breast cancer cells with VES. They also observed that VES-induced apoptosis in breast cancer cells was inhibited when using Fas neutralizing antibody or transfecting Fas antisense oligonucleotides to cancer cells. Since then, other studies have shown that Fas-mediated apoptosis may be another important pathway by which VES inhibits tumor cell growth[35-37].
Fas/APO-1, a 45 ku type I transmembrane protein, belongs to the nerve growth factor (NGF)/tumor necrosis factor (TNF) receptor superfamily. As a member of the five death domain-containing receptors, Fas initiates a signal-transduction cascade leading to programmed cell death[38-46]. In recent studies, Fas protein expression has been identified in various organs. It was reported that Fas antigen expression could be related to lymph node status and clinical stages in cancer patients. The rate of Fas antigen expression was significantly higher in gastric carcinoma tissues without lymph node metastasis than in those with lymph node metastasis, and also higher in clinical stages I and II than in clinical stages III and IV of gastric carcinoma. Moreover, the prognosis of patients with negative expression of Fas was worse[47,48]. In this study, we determined the Fas expression both in SGC-7901 cells treated with VES and in human gastric carcinoma tissues. The data showed that after 48 h of VES treatment, the expression of Fas protein was evidently increased with a marked dose-dependent relationship compared to that of the control group, indicating that Fas signal pathway was involved in VES-triggered apoptosis. In addition, the expression of Fas protein in gastric carcinoma tissue was depressed with the decrease of tumor differentiation degree and with the existence of lymph node metastasis. These results are in accordance with the reports mentioned earlier. It suggested that Fas protein expression may be one of indices to predict prognosis of patients with gastric carcinoma, thus establishing a theoretical foundation for using Fas protein expression to assess the effect and prognosis of VES application in clinic therapy.
PCNA functions as a cofactor for DNA polymerase δ. It is associated with DNA repair in both S phase and DNA synthesis phase. As an index of cellular proliferative status, PCNA was determined in various lesions. The overexpression of PCNA with high frequency was usually used as a reliable marker for assessment of tumor progression, premalignant evolution and clinical prognosis of patients with various malignancies[49-59]. Our in vitro results showed that VES at different doses could decrease the PCNA expression in SGC-7901 cells to different degrees, indicating that the inhibition of the growth of human gastric carcinoma by VES might be associated with its depression of PCNA protein, decrease in DNA-polymerase δ activity and interference of DNA synthesis. Additionally, the expression of PCNA in clinical gastric carcinoma tissue was higher than that in normal gastric tissues, but we failed to demonstrate the correlation between PCNA expression and clinicopathological parameters in gastric carcinoma in this study, which was conflicted with the reports about PCNA expression in gastric carcinoma[60,61] and the reports that PCNA could be an effective indicator of prognosis for tumor. Based on the in vitro results that VES has effects on PCNA expression in gastric carcinoma cells in our study and the reports we have mentioned, we presumed that the conflicting results in our study might be related to fewer cases we have collected, such as only seven cases of highly-moderately differentiated carcinoma.
With the progress of tumor research, it has been realized that the tumorigenesis is a result of imbalance between cell proliferation and apoptosis. On the one hand, cells proliferate massively; on the other hand, cellular apoptosis is inhibited. Fas and PCNA are the two genes closely related to apoptosis and proliferation. Our study indicates that both of them play important roles in the inhibitory effects of VES on gastric carcinoma cells in vitro. But for evaluating the effect of VES on gastric carcinoma tissue, Fas, as an effective predicting index for VES application to gastric carcinoma therapy may be of more value than PCNA.
We thank Dr. Love (University of Calgary, Canada) for his comments on the manuscript.
Edited by Zhu LH and Xu FM
| 1. | Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346-3351. [PubMed] |
| 2. | Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Prasad KN, Edwards-Prasad J. Effects of tocopherol (vitamin E) acid succinate on morphological alterations and growth inhibition in melanoma cells in culture. Cancer Res. 1982;42:550-555. [PubMed] |
| 4. | Zhang Y, Ni J, Messing EM, Chang E, Yang CR, Yeh S. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc Natl Acad Sci U S A. 2002;99:7408-7413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668-2675. [PubMed] |
| 6. | Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Kline K, Sanders BG. RRR-alpha-tocopheryl succinate inhibition of lectin-induced T cell proliferation. Nutr Cancer. 1993;19:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Kline K, Yu WP, Zhao BH, Israel K, Charpentier A, Simmons-Menchaca M, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Nutrients in Cancer Pre-vention and Treatment. Prasad KN. Santamaria L, Williams RM (eds). Totowa.NY:. Humana Press Inc. 1995;39-56. [DOI] [Full Text] |
| 10. | Kline K, Yu WP, Sanders BG. Vitamin E: Mechanisms of Action as Tumor Cell Growth Inhibitors. Cancer and Nutrition. Prasad KN, Cole WC (Eds). IOS Press. 1998;37-53. |
| 11. | Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111:435-441. [PubMed] |
| 13. | You H, Yu W, Munoz-Medellin D, Brown PH, Sanders BG, Kline K. Role of extracellular signal-regulated kinase pathway in RRR-alpha-tocopheryl succinate-induced differentiation of human MDA-MB-435 breast cancer cells. Mol Carcinog. 2002;33:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Israel K, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits the proliferation of human prostatic tumor cells with defective cell cycle/differentiation pathways. Nutr Cancer. 1995;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569-6576. [PubMed] |
| 16. | Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Stícha M. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Weber T, Lu M, Andera L, Lahm H, Gellert N, Fariss MW, Korinek V, Sattler W, Ucker DS, Terman A. Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (Apo2 ligand) in vivo. Clin Cancer Res. 2002;8:863-869. [PubMed] |
| 18. | Yu W, Simmons-Menchaca M, You H, Brown P, Birrer MJ, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induction of prolonged activation of c-jun amino-terminal kinase and c-jun during induction of apoptosis in human MDA-MB-435 breast cancer cells. Mol Carcinog. 1998;22:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Hua JS. Effect of Hp: cell proliferation and apoptosis on stomach cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:647-648. |
| 21. | Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5-Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669-673. |
| 22. | Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistochemically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113-1116. |
| 23. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 24. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374-376. [PubMed] |
| 25. | Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. hTERT ex-pression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508-512. |
| 26. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |
| 27. | Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500-505. [PubMed] |
| 28. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] |
| 29. | Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230-232. [PubMed] |
| 30. | Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83-87. [PubMed] |
| 31. | Wu K, Zhao Y, Liu BH, Li Y, Liu F, Guo J, Yu WP. RRR-alpha-tocopheryl succinate inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26-30. [PubMed] |
| 32. | Zhao Y, Wu K, Xia W, Shan YJ, Wu LJ, Yu WP. The effects of vitamin E succinate on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:782-786. [PubMed] |
| 33. | Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60-65. [PubMed] |
| 34. | Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881-890. [PubMed] |
| 35. | Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953-961. [PubMed] |
| 36. | Israel K, Yu W, Sanders BG, Kline K. Vitamin E succinate induces apoptosis in human prostate cancer cells: role for Fas in vitamin E succinate-triggered apoptosis. Nutr Cancer. 2000;36:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:982-986. [PubMed] |
| 38. | Salih HR, Starling GC, Knauff M, Llewellyn MB, Davis PM, Pitts WJ, Aruffo A, Kiener PA. Retinoic acid and vitamin E modulate expression and release of CD178 in carcinoma cells: consequences for induction of apoptosis in CD95-sensitive cells. Exp Cell Res. 2001;270:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Rubio CA, Jacobsson B. The Fas-FasL system and colorectal tumours. J Clin Pathol. 2002;55:559; author reply 559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 40. | Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88-92. [PubMed] |
| 41. | Geng YJ. Molecular signal transduction in vascular cell apoptosis. Cell Res. 2001;11:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Li ZY, Zou SQ. Fas counterattack in cholangiocarcinoma: a mechanism for immune evasion in human hilar cholangiocarcinomas. World J Gastroenterol. 2001;7:860-863. [PubMed] |
| 43. | Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 44. | Hou L, Li Y, Jia YH, Wang B, Xin Y, Ling MY, Lü S. Molecular mechanism about lymphogenous metastasis of hepatocarcinoma cells in mice. World J Gastroenterol. 2001;7:532-536. [PubMed] |
| 45. | Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664-670. [PubMed] |
| 46. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] |
| 47. | Reimer T, Koczan D, Müller H, Friese K, Thiesen HJ, Gerber B. Tumour Fas ligand: Fas ratio greater than 1 is an independent marker of relative resistance to tamoxifen therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2002;4:R9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Liu HF, Liu WW, Fang DC, Liu FX, He GY. Clinical significance of Fas antigen expression in gastric carcinoma. World J Gastroenterol. 1999;5:90-91. [PubMed] |
| 49. | Zhang W, Meng R, Fu C, Yu D. [Gene expression significance of beta-catenin, p53 and PCNA in PJS polyposis]. Zhonghua Waike Zazhi. 2002;40:104-106. [PubMed] |
| 50. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] |
| 51. | Li JQ, Zhang CQ, Feng KT. PCNA, P53 protein and prognosis in primary liver cancer. China Nati J New Gastroenterol. 1996;2:220-222. |
| 52. | Wang YK, Ji XL, Gu YG, Zhang SC, Xiao JH. P53 and PCNA expression in glandular dilatation of gastric mucosa. China Natl J New Gastroenterol. 1996;2:106-108. |
| 53. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 54. | Shen LJ, Zhang HX, Zhang ZJ, Li JY, Chen MQ, Yang WB, Huang R. Detection of HBV, PCNA and GST-pi in hepatocellular carcinoma and chronic liver diseases. World J Gastroenterol. 2003;9:459-462. [PubMed] |
| 55. | Yue H, Na YL, Feng XL, Ma SR, Song FL, Yang B. Expression of p57kip2, Rb protein and PCNA and their relationships with clinicopathology in human pancreatic cancer. World J Gastroenterol. 2003;9:377-380. [PubMed] |
| 56. | Huang ZH, Fan YF, Xia H, Feng HM, Tang FX. Effects of TNP-470 on proliferation and apoptosis in human colon cancer xenografts in nude mice. World J Gastroenterol. 2003;9:281-283. [PubMed] |
| 57. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. [PubMed] |
| 58. | Ouyang GL, Li QF, Peng XX, Liu QR, Hong SG. Effects of tachyplesin on proliferation and differentiation of human hepatocellular carcinoma SMMC-7721 cells. World J Gastroenterol. 2002;8:1053-1058. [PubMed] |
| 59. | Jia XD, Han C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in Wistar rats. World J Gastroenterol. 2000;6:699-703. [PubMed] |
| 60. | Liu WZ, Zheng X, Shi Y, Dong QJ, Xiao SD. Effect of Helicobacter pylori infection on gastric epithelial proliferation in progression from normal mucosa to gastriccarcinoma. World J Gastroenterol. 1998;4:246-248. [PubMed] |
| 61. | Elpek GO, Gelen T, Aksoy NH, Karpuzoglu T, Keles N. Microvessel count, proliferating cell nuclear antigen and Ki-67 indices in gastric adenocarcinoma. Pathol Oncol Res. 2000;6:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |