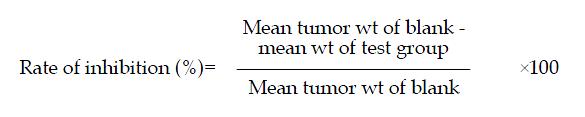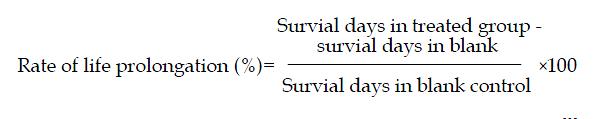Published online Feb 15, 2004. doi: 10.3748/wjg.v10.i4.484
Revised: May 28, 2003
Accepted: June 7, 2003
Published online: February 15, 2004
AIM: To study the anti-neoplastic effect of Haimiding and its mechanisms of action.
METHODS: Experiments using MTT and colony formation were carried out to study the in vitro anti-neoplastic action of Haimiding, its in vivo anti-neoplastic action was studied by observing its effect on the weight of tumors in FC mice and S180, H22 tumor bearing mice, as well as their life spans. The effect of Haimiding on cell apoptosis and different stages of cell cycles in human gastric carcinoma cells were studied by flow cytometry. Its effect on [Ca2+]i of human gastric carcinoma cells and the source of Ca2+ during the change of [Ca2+]i were observed by confocal laser scanning technique.
RESULTS: Haimiding showed a definite cytotoxicity to 8 human tumor cell lines, which was most prominent against BGC-823, Eca-109 and HCT-8 tumor cells. It also exhibited an obvious inhibition on colony formation of the above tumor cell lines, which was most prominent in Eca-109 tumor cells. It showed obvious inhibition on the growth of tumor in FC mice and S180 bearing mice as well as prolonged the life span of H22 bearing mice. It was able to induce apoptosis and elevate intracellular [Ca2+]i concentration of tumor cells. The source of Ca2+ came from both extracellular Ca2+ influx and intracellular Ca2+ release.
CONCLUSION: Haimiding is composed of a TCM preparation and 5-flurouracil. Its anti-neoplastic potency is highly enhanced by synergism as compared with either one of its components. Its mechanisms of anti-neoplastic action can be attributed to its action to initiate apoptosis of tumor cells by opening the membrane calcium channel and inducing intracellular Ca2+ release to elevate [Ca2+]i of the tumor cells.
- Citation: Ji YB, Gao SY, Ji HR, Kong Q, Zhang XJ, Yang BF. Anti-neoplastic efficacy of Haimiding on gastric carcinoma and its mechanisms. World J Gastroenterol 2004; 10(4): 484-490
- URL: https://www.wjgnet.com/1007-9327/full/v10/i4/484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i4.484
Haimiding (HMD) is an anti-neoplastic preparation made of a traditional Chinese medicine (TCM) preparation and 5-fluorouracil (5-Fu). Its TCM preparation consists of active ingredients from the extracts of Sargassum fusiforme (Harv.) Setch, Ecklonia kurome okam, Astragalus chrysopterus Buge, and Sophora flavescens Aits. The present study was to observe the in vivo and in vitro anti-neoplastic activities of HMD, and the effect of HMD on apoptosis of human gastric carcinoma SGC-7901 and intracellular [Ca2+]i, so to better understand the mechanism of its anti-neoplastic action.
Drug samples Sterile HMD powder with or without 5-Fu for parenteral use (Lot No.010606) was prepared by the Pharmaceutical Laboratory, Institute of Materia Medica, Harbin Commercial University. 5-Fu (Lot No. 990304) was provided by Haipo Pharmaceutical Factory, Shanghai, China.
Cancer cell lines Human oral epithelial KB, human esophageal carcinoma cell Eca-109, human proventriculas BGC-823, human pulmonary adenocarcinoma A-549, human colon HCT-8, human breast MCF-7, human ovary A2780 and human liver Bel-7402 were provided by the Department of Pharmacology, Institute of Materia Medica, Chinese Academy of Medical Sciences. SGC-7901 was from College of Public Health, Harbin Medical University.
Test animals Kunming strain mice were provided by Veterinary Department of Harbin Medical University, pure 615 strain mice were provided by Institute of Materia Medica, Chinese Academy of Medical Sciences.
Tumor bearing mice strain FC mice, S180 A and H22 mice of same sex weighing 20 ± 2 g were supplied by Institute of Materia Medica, Chinese Academy of Medical Sciences.
Reagents Bovine serum and RPM1-1640 culture medium were products of Gibo. MTT was provided by Sigma. Fluo-3/AM fluorescence probe was from Molecular Probe Co., USA. Verapamil was a product of Heng Rui Pharmaceutical Factory, Jiangsu Province, China.
Anti-neoplastic study in vitro
MTT test[1,2] About 1200 cells were added to each well of a 96 multi-well culture plate. After 24 h each dose of the drug was added to 3 different wells in the test group. Vehicle was added as control in another group. The plate was placed into a humidified incubator (CAN-111, Japan) containing 5% CO2 and incubated at 37 °C for 4 d. Ten μl MTT stock solution (5 mg/ml) was added to each well and shaken well, the incubation was continued for a further 4 h. One hundred μl acidified 10% sodium dodecanoate sulfonate (SDS) (pH = 4.7) was added to each well, which was then left overnight. The absorbance was determined by an El-311 enzyme labeling apparatus (El-311, Bio-Tek, USA) at a wave length of 570 nm and a reference wave length of 630 nm. The drug concentration inhibiting 50% of cell growth (IC50) was measured.
Experiments on formation of colonies[3,4] About 100 test cells were inoculated onto a 35 mm plastic petridish for 24 h and the assigned dose of test drugs was added to each dish. The culture liquid was discarded on the 9th day and the residue was washed 3 times in PBS. The cells were fixed in 10% formaldehyde solution and stained with 0.25% gentian violet. The number of colonies containing more than 50 cells in each dish was counted under a microscope. Vehicles were used instead of test drugs as blank control.
Anti-neoplastic studies in vivo
Effect of HMD on tumor weight of FC mice[5] Tumor cells were transplanted to 615 FC mice. Nine days after transplantation, FC mice bearing the well growing tumor were placed in an ice basin inside an enclosed aseptic working table. Tumor tissues, were separated and then cut into small pieces and homogenized with a glass homogenizer. The homogenate was made into a suspension containing about 5.6 × 106/ml of tumor cells with sterile normal saline in the conventional way[6], and 0.2 ml of the suspension was transplanted to the right axilla of each 615 strain mouse. After 24 h, the mice were weighed and randomized into 3 HMD groups (treated respectively ip with high, medium and low doses of HMD, equivalent to 27.05 g/kg, 13.53 g/kg and 6.76 g/kg of crude drugs in 0.4 ml injectable solution, once daily), a 5-Fu group treated ip with 25 mg/kg 5-Fu in 0.4 ml solution once daily, and a compound TCM group treated ip with HMD (without 5-Fu) equivalent to 13.50 g/kg of crude drug in 0.4 ml solution daily. A blank control group was treated ip with 0.4 ml normal saline once daily. All drugs were withdrawn on the 11th day. The animals were sacrificed on the next day and their tumors were resected and weighed. Results of 3 repeated experiments were collected and the data were statistically analyzed. The rate of tumor inhibition was calculated according to the following equation:
Math 1
Effect of HMD on tumor weight of S180 bearing mice[5] S180 tumor cells were transplanted into Kunming strain mice. After 7 days, ascites was drawn from the mice bearing well growing tumor under aseptic condition. The tumor cells were diluted with sterile normal saline in ice-bath to 4:1 to produce a suspension containing about 5.8 × 106/ml of tumor cells. This suspension should have a semi-transparent creamy appearance. Any ascitic fluid with blood streaks should not be used. The mice were transplanted by sc injection of 0.2 ml of the suspension to the right axilla of each Kunming mouse according to the procedure described in “National Requirements for in vivo Screening of Anti-neoplastic Drugs”[7]. After 24 h, the transplanted mice were weighed and randomized into groups to be treated with different doses of test drugs. Their tumors were resected, weighed and the rate of tumor inhibition was calculated as above (results of 3 repeated trials).
Effect of HMD on life span of H22 bearing mice[8] Kunming mice were transplanted with H22 tumor cells. The mice bearing well growing transplanted tumor were selected after 7 d, and their ascites were drawn under aseptic condition and diluted in ice-cold sterile normal saline to a concentration of 4:1. The tumor cells were mixed well to generate a suspension containing about 6 × 106 tumor cells/ml, and 0.2 ml of the suspension was transplanted ip to each Kunming mouse. After 24 h, the mice were weighed and randomized to different dosage groups as described above. The number of dead mice was recorded every day until no survival left. The rate of life prolongation was calculated according to the following equation:
Math 2
Effect of HMD on apoptosis of gastric carcinoma cells[9] Culturing of tumor cells: Human gastric carcinoma SGC-7901 cells were resuscitated conventionally and cultured in RPMI 1640 culture medium (Gibo) containing 0.1 mass fraction of bovine serum and incubated at 37 °C with 95% relative humidity in an incubator containing a volume fraction of 0.05 CO2. The cells were generated every 2-3 days.
Experimental groups: Culture flasks containing human gastric carcinoma SGC-7901 cells were divided into 5 groups, 10 flasks each. HMD was added to each flask in the 3 dosage groups to a final concentration of 50 µl/ml, 100 µl/ml and 200 µl/ml, respectively. 5-Fu was added to another group of 10 flasks to a final concentration of 100 µl/ml. A similar amount of culture media was added to the remaining 10 flasks as blank control. They were incubated in an incubator containing 5% CO2 for 24 h.
Determination of cell apoptosis by flow cytometry[7]: Nine volumes of 70% ethanol were added to 1 volume (about 106 cells) of cell suspension and fixed at -20 °C for 12 h. The cells were kept in 70% ethanol at -20 °C for 2-3 weeks, centrifuged and washed in PBS solution. The resulting cells were again suspended in 1 ml of PI staining solution, and stained at room temperature for 30 min. The red fluorescence FCM was detected with a laser (488 nm) or blue light (BG 12 light filter).
Effect of HMD on [Ca2+]i of gastric cancer cells Culturing of cells Human gastric carcinoma SGC-7901 Cells, after resuscitated conventionally, were cultured in RPMI-1640 medium containing 0.1 mass fraction of bovine serum and incubated at 37 °C with 95% relative humidity in an incubator containing 0.05 volume fraction of CO2. Every 2-3 days were taken for a generation.
Experimental grouping and treatment with test drugs Trypan blue was used to show the number of cells, which was adjusted to about 2 × 105/ml with culture medium, and stored in different culture bottles.
The experiments consisted of 6 groups (A, B, C1, C2, C3 and D) and 4 different periods of time (12, 24, 36 and 48 h). Groups A and B were negative and positive controls respectively. Groups C1, C2 and C3 were low, medium and high HMD dose groups, and group D was medium dose of HMD plus verapamil.
When the cells attached to the wall after culturing, the culture medium of each group was adjusted to different concentrations. Group B was adjusted to a final concentration of 50 µg/mL of 5-Fu, groups C1, C2 and C3 to a final concentration of 200 µg/ml, 100 µg/ml and 50 µg/ml of HMD, respectively and group D to a final concentration of 100 µg/mL of HMD plus 20 µg/mL of verapamil. Group A, as a negative control was cultured in RPMI-1640 medium containing 10% bovine serum. These media were cultured for 12, 24, 36 and 48 h respectively, and the resulting cells were collected.
Determination of free Ca2+ concentration in cells[38] SGC-7901 human gastric carcinoma cells, digested after addition of test drugs at different times, were collected and washed twice with 0.1 mass fraction RPMI-1640 and then stained with 0.25 mass fraction of trypan blue. The viable cell count was over 95%. The cell suspension was preheated at 37 °C for 5 min and then washed 2-3 times in PBS and calcium free Dakins solution, stained with 100 µl Fluo-3/AM solution at 37 °C for 45 min. A cell suspension was prepared with an appropriate amount of calcium free Dakins solution. The change of intracellular Ca2+ concentration was examined under a confocal laser scanning microscope (Insught Plus-type IQ, meridian, USA), using the intensity of fluorescence light as the criteria of Ca2+ concentration, and was observed with a 40 × objective lens at an exciting wavelength of 488 nm.
t test was used for statistical analysis and results were expressed as mean ± SD.
Cytocidal activities of HMD against 8 human cancer cell lines are shown in Table 1. It demonstrated that the actions were more prominent in BGC-823, Eca-109 and HCT-8 cell lines in a dose dependent manner. Results from regression calculation showed that IC50 of HMD against BGC-823, Eca-109 and HCT-8 was 25.28 mg/ml, 12.78 mg/ml and 28.29 mg/ml (equivalent to the crude drug) respectively.
| Drug | IC50(mg/ml) | |||||||
| KB | BGC-823 | A549 | MCF-7 | A2780 | Bel-7420 | HCT-8 | Eca-109 | |
| HMD | 83.89 | 25.28 | 292.41 | 34.14 | 16.84 | 36.57 | 28.29 | 12.78 |
| 5-Fu | 0.12 | 0.06 | 0.19 | 0.10 | 0.08 | 0.09 | 0.08 | 0.04 |
| HMD without 5-Fu | 428.58 | 274.90 | 685.70 | 365.00 | 124.65 | 295.60 | 187.58 | 100.42 |
HMD had an obvious inhibitory effect on colony formation of BGC-823, Eca-109 and HCT-8 tumor cell lines, being most prominent in Eca-109 in a positive dose-effect manner. The data are shown in Table 2.
| Drug | Percentage of colony formation (mean ± SD) | |||
| Dose (mg/ml) | BGC-823 | HCT-8 | Eca-109 | |
| HMD | 10.00 | 72.18 ± 7.29c | 83.18 ± 9.01 | 61.58 ± 4.12a |
| HMD | 20.00 | 50.21 ± 5.18d | 59.45 ± 6.78c | 22.43 ± 3.65d |
| HMD | 40.00 | 28.43 ± 3.42d | 30.15 ± 4.20d | 0 |
| 5-Fu | 0.04 | 61.18 ± 4.27c | 68.70 ± 5.15a | 69.27 ± 5.48a |
| HMD without 5-Fu | 19.63 | 90.51 ± 7.42 | 82.15 ± 7.92 | 78.29 ± 6.25a |
| Blank control | 98.27 ± 6.58 | 95.68 ± 9.12 | 97.14 ± 8.26c | |
Effect of HMD on tumor weight of FC mice HMD at doses of 27.05 g/kg·d, 13.53 g/kg·d and 6.76 g/kg·d demonstrated an obvious inhibitory effect on the growth of tumor in FC mice in a positive dose- effect manner. The inhibitory effect of 13.53 g/kg HMD (containing 25 mg 5-Fu) was more potent than 25 mg/kg of 5-Fu alone with statistical significance (P < 0.05). Results are shown in Table 3.
Effect of HMD on tumor weight of S180 bearing mice HMD at doses of 27.05 g/kg·d, 13.53 g/kg·d and 6.76 g/kg·d significantly inhibited the growth of S180 sarcoma in tumor-bearing mice in a positive dose-effect manner (P < 0.01, P < 0.05). Results are shown in Table 4.
Effect of HMD on life span of H22 bearing mice HMD at doses of 27.05 g/kg·d, 13.53 g/kg·d, and 6.76 g/kg·d obviously prolonged the life span of H22 bearing mice in a positive dose-effect manner. HMD at 13.53 g/kg (containing 25mg 5-Fu) showed a more potent effect than that of 5-Fu alone (P < 0.05), and HMD without 5-Fu (P < 0.05). Results are shown in Table 5.
Studies on the effects of HMD on cell apoptosis and cell cycles of tumor cell lines showed that HMD was able to induce cell apoptosis to a certain degree (Table 6 and Table 7), which was statistically significant (P < 0.01) as compared with the blank control. 5-Fu, however, was devoid of such an effect. HMD caused a decrease of cells at stage S, resulting in cells differentiation at G0-G1 and G2-M stages. But most cells remained at stage G2-M with an increase of HMD doses.
Effect of HMD on intracellular [Ca2+]i of tumor cells
Changes of intracellular [Ca2+]i in human gastric carcinoma cells after treated with HMD for 12 h. It showed that HMD significantly increased the intracellular free calcium ion concentration (P < 0.001) in a dose dependent manner (Table 8, Table 9, Table 10, 11). Calcium ion concentration of the verapamil group(group D) was between that of the negative control and medium dose HMD group, both of which were statistically significant (P < 0.01).
The experimental results indicated that when HMD was used for treatment of tumor cells, an increase of intracellular free calcium ion concentration was observed regardless of the duration of treatment. But comparatively, at the early stage of treatment (12 h), the range of increase was most evident (Table 12).
| Different durations of treatment (h) | No. of cells | [Ca2+]i (FI) |
| 12 | 28 | 916.78 ± 151.46 |
| 24 | 23 | 672.65 ± 165.16 |
| 36 | 26 | 684.31 ± 148.41 |
| 48 | 28 | 787.00 ± 178.19 |
Gastric carcinoma, as one of the most common human malignant tumors, ranks the first leading cause of gastrointestinal cancer-related mortality worldwide. In China, it now ranks the second. It has been shown that tumor apoptosis played an important role in its growth, invasion, metastasis and recurrence[10-29].
HMD is a compound TCM preparation for injection in combination with modern synthetic drugs. It has an obvious anti-neoplastic activity shown by extensive pharmacological studies. In the present study in vitro experiments with MTT and cell colony formation were carried out to assess its inhibition on 8 human cancer cell lines. It showed that HMD could inhibit the growth of cancer cell lines. The anti-neoplastic activity of HMD was most prominent against human gastric carcinoma BGC-823, human esophagus carcinoma Eca-109, and human colon carcinoma HCT-8 cell lines in a dose dependent manner. These results were consistent with the clinical effects of HMD on dysphagia and regurgitation, suggesting that HMD was extremely sensitive to carcinomas of the digestive tract. In vivo anti-neoplastic activity of HMD was studied by transplanting various cancer cell lines (proventricular FC, S180 sarcoma and H22 liver cancer) into experimental animals to observe its inhibition on tumor growth. It showed that HMD could inhibit the growth of FC and S180 tumors in a positive dose-effect manner. It also significantly prolonged the life span of H22 bearing mice, though its potency was not comparable to those obtained in FC and S180-bearing mice. This indicates that HMD is more potent for solid tumors than ascitic carcinoma, which also conforms with the clinical obserbations.
HMD injection is composed of 2 parts, a compound TCM formula and a synthetic drug 5-fluorouracil (5-Fu). In the above in vivo and in vitro anti-neoplastic studies, controls with TCM formula or 5-Fu alone were included to compare their individual and combined efficacy. Results showed that the anti- neoplastic action of HMD injection exceeded that of TCM formula or 5-Fu alone (at the same dosage level). Thus, it seemed that TCM formula and 5-Fu had an excellent synergism when used in combination.
To obtain a deeper understanding of the mechanism of the anti-neoplastic action of HMD on human gastric carcinoma, we carried out further studies on the induction of cell apoptosis of human gastric carcinoma SGC-7901 by HMD.
Cell apoptosis, as an autonomic process of organisms, is intrinsically different from necrosis in pathological conditions. Cell apoptosis is a “suicidal” process on its own accord under physiological condition, though it may also involve some pathological events. But, undoubtedly it plays an important role in the recovery and maintenance of normal physiological function. Carcinogenesis is not merely due to abnormal cell proliferation and differentiation. but the mechanisms of cell apoptosis, which leads to neoplastic occurrence, development, evolution, metastasis and eventually death of tumor-bearing hosts should also be duly considered. The mechanism of cell apoptosis in the etiology of carcinogenesis might be induced by many factors, such as inactivation and mutagenesis of apoptotic gene or inhibition of the process of apoptotic gene expression, apoptotic mechanism caused by chemical carcinogens or virus, apoptosis insufficiency of precancerous cell, apoptosis insufficiency of cancer cells due to immunological response of the organism, inhibition of tumor cell apoptosis by adhesion factor and growth factor of the host, attempts of tumor cells to avoid apoptosis by lowering their dependence on survival signal while enhancing their survival competence thereby aggravating the disease with metastasis[30].
The relationship between cell apoptosis and carcinogenesis provides us a new pharmacological mechanism and target for the study of anti-neoplastic drugs. As an anti-neoplastic drug, if it is very potent to induce tumor cell apoptosis and to activate the programed cell death process, then it can avoid a great number of untoward side effects due to the release of waste material resulted from cell necrosis, and alleviate the damage of normal cells caused by chemotherapy and prolong the life of patients.
Experimental studies of the effect of HMD on apoptosis of human gastric cancer cell line showed that there was a definite trend to promote cell apoptosis, but with the increase of dosage no further increase of cell apoptosis could be observed. This might be due to the fact that the increased dose caused a sufficient amount of tissue necrosis, which overshadowed cell apoptosis. The positive control 5-Fu was found unable to induce cell apoptosis which coincided with the finding that 5-Fu could enhance the tolerability of cells toward apoptosis as reported in the literature[30]. Therefore it seems to be possible that the effective ingredient responsible for the induction of apoptosis is the polysaccharides present in HMD formula. Sea weed polysaccharides is known to possess cytotoxicity that can inhibit and kill cancer cells directly, and when excited by low doses of a cytotoxic agent it can induce cell apoptosis, while polysaccharides from astragalus chrysopterur can enhance the cytotoxicity of NK cells of mice. Such NK cell induced death of the target cell is known as apoptosis.
It has been found that the majority of active cells were proliferating at the S stage of DNA synthesis, while the differentiated cells were stagnant at the G1/G0 stage[31], when DNA synthesis was not in progress. Anti-neoplastic agents can markedly change the kinetic features of cell cycle such as that occurred in tumor cells from S180 mice by the action of HMD. Moreover, different doses of HMD had different effects on cell cycle kinetics. At small (50 µg/ml) and medium (100 µg/ml) doses, HMD could block cell growth at G1/G0 stage and S stage. Larger doses of HMD could cause the cells to remain at G2 and M stage resulting in an obvious increase of G2 and M cells, indicating that different doses of HMD showed different anti-neoplastic mechanisms. Regarding its induction of cell apoptosis, HMD also showed several different mechanisms.
HMD exerts its anti-neoplastic action by inducing cell apoptosis of the tumor cells. Ca2+ has been found to play an important role in the transmission system of this pathway[37]. To clarify the relationship between the mechanisms of the anti-neoplastic activity of HMD and the intracellular Ca2+ concentration, we tried to observe the effect of HMD on the Ca2+ concentration in cancer cells by confocal laser scanning microscopic technique.
Calcium presents in the body in two different forms: the combined form and the ionic form (Ca2+). Only the ionic form shows physiological activity. Such calcium ions are divided into intracellular and extracellular types. It was shown that Ca2+ concentration in extracellular fluid was about 10-3 mol/L, while the intracellular concentration was only 10-7 mol/L. They were always maintained at this definite level with intracellular concentration being only 1/10 000 of that of the extracellular one. It has become increasingly evident nowadays that this seemingly negligible change of intracellular Ca2+ concentration interferes with many physiological and metabolic functions, especially transmission of cellular signals. At present, Ca2+ is regarded as a rather important second messenger and at the same time, participates in or coordinates with the metabolism of other second messengers and the regulation of cell functions.
In the transmission of cell signals, either intrinsic or extrinsic signals pass through the cell membrane to get into cytoplasms and enter into cell nuclei to induce changes of gene expression. Three pathways for a signal to cross over cell membrane have been found[38]: through the mediation of tyrosine protein kinase, G-protein or the ionic channel[32]. The signal transmission in cytoplasm was mainly accomplished by the messenger adenylate cyclase, lipositol and calcium calmodulin[33]. Five corresponding transmission pathways which can induce apoptosis may be derived from the above 3 messengers. They are the Ca2+ signal system in cells, the cAMP/PKA signal system, the DG/PKC signal system, the tyrosine protein kinase system, and the acylsphingosine pathway. The main signal molecules in these messengers include cAMP, IP3, PKC and Ca2+, in which Ca2+ could play a pivotal role in the transmission[7,38]. Therefore, in our study on the mechanism of transmission of apoptosis signal to tumor cells, Ca2+ was first selected.
In the present study, Fluo-3/AM was used as the fluorescence indicator, and confocal laser scanning microscopy technique was used to determine the dynamic change of [Ca2+]i in human gastric carcinoma SGC-7901 cell line after different durations of drug administration. Therefore, the study was performed at 4 different stages of 12, 24, 36 and 48 h. In each stage, the dose-dependent manner of the effect of HMD on intracellular [Ca2+]i was observed. Experimental results showed that the Ca2+ concentration of all the HMD treated groups, regardless of the stage, was higher than that of the negative blank control (P < 0.001), and the extent of elevation by high, medium and low dosage of HMD was dose-dependent. As to groups dosed for different length of time, the group dosed for 12 h showed a most drastic elevation of Ca2+ concentration. All the other time groups showed a larger Ca2+ concentration change than the negative control though smaller than that of the 12 h group. This result conformed with literature reports that the elevation of Ca2+ was closely related to the early apoptosis.
Cohen and Duke[34] found a continual increase of [Ca2+]i when apoptosis of thymocytes was induced by glucocorticoid. Further studies found that the intrinsic endonuclease which caused DNA fragmentation was Ca2+ and Mg2+ dependent and the activity of this enzyme was obviously enhanced as the concentration in cytoplasms was elevated. Similarly, when VP-16 or TNF was used to induce apoptosis of breast cancer cells, [Ca2+]i was also elevated to activate the Ca2+ dependent endonuclease, causing DNA fragmentation and cell apoptosis[35]. As shown in the present study HMD could induce apoptosis of cancer cells, and cause an increase of intracellular [Ca2+]i similar to the changes of [Ca2+]i when apoptosis of other cancer cell lines occurred[38], which could explain why Ca2+ could activate Ca2+ dependent endonuclease leading to apoptosis.
While Ca2+, with its pivotal role in those signal transmission systems may induce cell apoptosis through several different approaches: (1) to activate the Ca2+ dependent endonuclease causing apoptosis; (2) the elevation of Ca2+ activates PKC directly and at the same time helps DG to activate PKC, which is a bifunctional kinase that not only regulates cell differentiation but also induces cell apoptosis; (3) after the elevation of Ca2+, it combines with CaM to form a complex Ca·CaM, which activates adenylate cyclase (AC) resulting in the activation of cAMP/PKA signal system, thereby activating PKA to induce tumor cell apoptosis; (4) Ca2+ could control the activity of glutamyl transferase resulting in cell apoptosis, though its exact mechanism is still unclear. In brief, Ca2+ plays a specific role in transmission of cell signals of apoptosis. HMD is capable of inducing tumor cell apoptosis by elevating intracellular [Ca2+]i.
Ca2+ comes from two sources when cellular [Ca2+]i increases. One is the inflow of Ca2+ from extracellular sources, mainly by opening calcium channels, The other is the release of Ca2+ from intracellular calcium storage. Ca2+ in cells is mainly stored in endoplasmic reticulum/seroplasmic reticulum and is mainly regulated by IP3. To trace the source of Ca2+ when intracellular [Ca2+]i in gastric carcinoma cells was elevated by HMD, we tried on an additional “verapamil group”, with the use of a calcium channel blocker added to the medium dosage group of HMD. We deduced that one of the following results might occur. (1) If Ca2+ only came from extracellular calcium inflow, the [Ca2+]i value should be similar to that of the negative control. (2) If Ca2+ came only from the release of intracellular storage, the [Ca2+]i value should be similar to that of the medium dose of HMD group. (3) If the Ca2+ came from both extracellular Ca2+ inflow and intracellular Ca2+ release, the [Ca2+]i value should be between those of negative control and medium dose HMD with statistical significance. Results of the experiment showed that the [Ca2+]i value was higher than that of the negative control, but lower than that of the medium dose HMD group with statistical significance (P < 0.001). It indicated that HMD elevatd intracellular [Ca2+]i to induce tumor cell apoptosis and caused the opening of calcium channels, which facilitated Ca2+ inflow and interacted with receptors on the membrane to produce IP3 for the release of Ca2+ from its storage.
From this study we conclude that HMD shows a remarkable cytotoxicity against tumor cells. Its mechanism of action is to increase intracellular [Ca2+]i by opening the calcium channels of cell membrane, and initiate the release of Ca2+ from its storage to induce tumor cell apoptosis.
Edited by Wang XL and Zhu LH
| 1. | Chen W, Zhou X, Xu Q, Guo W, Lin L. [Chemosensitivity testing of oral and maxillofacial cancer with biopsy specimens]. Zhonghua Kouqiang Yixue Zazhi. 2002;37:404-407. [PubMed] |
| 2. | Gui LR, Zhou Y, Zhang BL, Li WB. [Pituitary adenylate cyclase activating polypeptide protects neuro-2a cells from beta amyloid protein cytotoxicity by modulating intracellular calcium]. Shengli Xuebao. 2003;55:42-46. [PubMed] |
| 3. | Han R, Xu CX, Li ZR. The Research and Laboratory Technique of Anti-tumor Drug. Beijing: The United Press of Beijing Medical University and Peking Union Medical College 1997; 283. |
| 4. | Wang Q, Wu LM, Li AY, Zhao Y, Wang NP. [Experimental studies of antitumor effect of artesunate on liver cancer]. Zhongguo Zhongyao Zazhi. 2001;26:707-78, 720. [PubMed] |
| 5. | Wang LJ, Wang Y, Chen SW, Ma JS, Fu Q, Wang BX. [The antitumor activity of Diosgenin in vivo and in vitro]. Zhongguo Zhongyao Zazhi. 2002;27:777-779. [PubMed] |
| 6. | Gao J. Basic and Experiment of Oncolog. Beijing: The United Press of Beijing Medical University and Peking Union Medical College 1992; 61-67. |
| 7. | Han R. The Research and Laboratory. Technique of Anti-tumor Drug. Beijing: The United Press of Beijing Medical University and Peking Union Medical College 1997; 400. |
| 8. | Wang Z, Wang Y, Huang Z, Zhong S, Wu Y, Yu L. [Study on antitumor effect and mechanism of aloe polysaccharides]. Zhongyaocai. 2001;24:350-353. [PubMed] |
| 9. | Huang YH, Zhen YS. [Rhein induces apoptosis in cancer cells and shows synergy with mitomycin]. Yaoxue Xuebao. 2001;36:334-338. [PubMed] |
| 10. | Zhang T, Cao EH, Li JF. A laser scanning confocal microscopy method. Simultaneous detection of intracellular Ca2+ and apoptosis using Fluo-3 and Hoechst 33342. Anal Quant Cytol Histol. 2000;22:93-97. [PubMed] |
| 11. | Miao ZH, Tang T, Zhang YX, Zhang JS, Ding J. Cytotoxicity, apoptosis induction and downregulation of MDR-1 expression by the anti-topoisomerase II agent, salvicine, in multidrug-resistant tumor cells. Int J Cancer. 2003;106:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144-1155. [PubMed] |
| 13. | Kaledin VI, Nikolin VP, Baimak TY, Galyamova MR, Popova NA, Andreeva EM. Phenobarbital modifies antitumor effect of cyclophosphamide depending on the type of tumor cell death caused by it. Bull Exp Biol Med. 2003;135:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment. Mol Cancer Ther. 2003;2:573-580. [PubMed] |
| 15. | Guzman E, Langowski JL, Owen-Schaub L. Mad dogs, Englishmen and apoptosis: the role of cell death in UV-induced skin cancer. Apoptosis. 2003;8:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Steinbach JP, Wolburg H, Klumpp A, Probst H, Weller M. Hypoxia-induced cell death in human malignant glioma cells: energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell Death Differ. 2003;10:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kim WH, Hong F, Radaeva S, Jaruga B, Fan S, Gao B. STAT1 plays an essential role in LPS/D-galactosamine-induced liver apoptosis and injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G761-G768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Kountouras J, Zavos C, Chatzopoulos D. Apoptosis in hepatocellular carcinoma. Hepatogastroenterology. 2003;50:242-249. [PubMed] |
| 20. | Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1282] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 21. | Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 836] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003;88:31-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Schmitt CA. Senescence, apoptosis and therapy--cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Nijhawan R, Hemachandran M, Joshi K. Apoptosis in breast cancer. Acta Cytol. 2003;47:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Tsuruo T. Molecular cancer therapeutics: recent progress and targets in drug resistance. Intern Med. 2003;42:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 371] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Hernandez MO, Neves I, Sales JS, Carvalho DS, Sarno EN, Sampaio EP. Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: a possible role for tumour necrosis factor-alpha. Immunology. 2003;109:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Liu JD, Wang YJ, Chen CH, Yu CF, Chen LC, Lin JK, Liang YC, Lin SY, Ho YS. Molecular mechanisms of G0/G1 cell-cycle arrest and apoptosis induced by terfenadine in human cancer cells. Mol Carcinog. 2003;37:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Han R. The Research and Laboratory Technique of Anti-tumor Drug Beijing: The Union Press of Beijing Medical University and Peking Union Medical College 1997: 346. . |
| 33. | Lee YS, Jin DQ, Kwon EJ, Park SH, Lee ES, Jeong TC, Nam DH, Huh K, Kim JA. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002;186:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Xing C, Chen J, Xu H. [Changes in [Ca2+]i and IP3 levels in the process of cisplatin-induced apoptosis of gastric carcinoma]. Zhonghua Zhongliu Zazhi. 1999;21:256-258. [PubMed] |
| 35. | Sun DY, Guo YL, Ma LG. Cell Signal Transduction(the second edition). Beijing: Science and Technology Press 1998; 28,42. |
| 36. | Cao ZY. Hormone Acceptor and Clinical Application. Beijing: The Union Press of Beijing Medical University and Peking Union Medical College 1993; 48-70. |
| 37. | Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38-42. [PubMed] |
| 38. | Robinson JM, Smith DF, Davis EM, Gilliam EB, Capetillo SC, Walborg EF Jr. Partial characterization of rat hepatoma cell-surface glycopeptides possessing concanavalin A receptor activity. Biochem Biophys Res Commun. 1976;72:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |