Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.4
Revised: August 20, 1995
Accepted: September 15, 1995
Published online: October 1, 1995
- Citation: Pan BR, Yang SF, Ma LS. Acute liver failure: A progress report. World J Gastroenterol 1995; 1(1): 4-8
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/4.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.4
Acute liver failure (ALF) includes many conditions in which severe injury of hepatocytes or massive necrosis occurs. Loss of hepatocyte function sets in motion a multiorgan response, and death may occur even when the liver has begun to recover. This article reviews recent advances in our understanding and treatment of ALF. Altered mental status (hepatic encephalopathy) and coagulopathy in the setting of an acute hepatic disease help define ALF. In 1946, Lucke et al paid attention to two fatal types of acute hepatitis: a fulminant form with an extremely rapid outcome and a subacute form with a slower course. ALF encompasses all these clinical presentations.
Existing definitions of clinical syndromes in ALF do not accurately reflect important differences in the clinical features and prognoses. Based on a large series of patients with ALF treated at King’s College Hospital, London between 1972 and 1985, O’Grady et al proposed a new terminology. Hyperacute liver failure was suggested for cases in which encephalopathy occurs within 7 d of the onset of jaundice; this group includes the sizable cohort likely to survive with medical management despite a high incidence of cerebral edema. ALF was suggested for cases with an interval of 8-28 d from jaundice to encephalopathy; these patients also have a high incidence of cerebral edema, but have a poor prognosis without liver transplantation. Subacute liver failure was suggested for cases with encephalopathy that occurs 5-12 wk after the onset of jaundice; these patients are characterized by a low incidence of cerebral edema, but still have a poor prognosis. Adoption of this terminology should help in the management of these patients, in addition to standardizing the structure and interpretation of controlled trials of therapies (Figure 1, Table 1).
| Characteristics | Hyperacute liver failure | Acute liver failure | Subacute liver failure |
| Encephalopathy | Yes | Yes | Yes |
| Duration of jaundice | 0-7 d | 8-28 d | 29-72 d |
| Cerebral edema | Common | Common | Seldom |
| Prothrombin time | Prolonged | Prolonged | Least prolonged |
| Bilirubin | Slightly elevated | Elevated | Elevated |
| Prognosis | Moderate | Poor | Poor |
Viral hepatitis and drug-induced liver injury account for most cases of ALF, but there are great differences in incidence among countries. It is important to determine the cause as carefully as possible, since the prognosis and use of antidotes for certain forms of ALF depend on the identification of the causative agent (Table 2).
| Causes | Agents responsible |
| Viral hepatitis | Hepatitis A, B, C, D, E, or F (?) virus |
| Herpes simplex virus | |
| Drug related liver injury | Epstein-Barr virus, Cytomegalovirus |
| Toxins | Adenoviruses, Paramyxovirus |
| Vascular events | Acetaminophen |
| Miscellaneous | Idiosyncratic reactions |
| Drug-induced steatosis | |
| Carbon tetrachloride | |
| Amanita phalloides | |
| Phosphorus | |
| Ischemia or shock | |
| Veno-occlusive disease | |
| Heat stroke and Hypothermia | |
| Malignant infiltration | |
| Wilson's disease | |
| Acute fatty liver of pregnancy | |
| Reye's syndrome, Cryptogenic |
The foremost cause of ALF is acute viral hepatitis, accounting for up to 72% of all cases. Viral hepatitis leads to hepatic failure in only a small proportion of cases (< 1%), and each of the five primary hepatotropic viruses (A, B, C, D, E) has been implicated in ALF. Hepatitis A rarely leads to ALF (0.35% of cases), but when it does, the patients have a good prognosis (> 60% survival) and seldom need liver transplantation. Acute hepatitis B may lead to ALF in 1% of patients, accounting for more than 70% of the patients with virus-induced disease in most countries. Hepatitis C virus has rarely been implicated in ALF, but may contribute to the massive necrosis that occurs in patients co-infected with hepatitis B virus. Hepatitis D virus in former carriers of hepatitis B virus accounts for fewer than 10% of all cases of acute hepatitis related to hepatitis B virus, and more than half the cases of ALF in patients positive for hepatitis B are due to hepatitis D virus rather than to hepatitis B alone. Hepatitis E occurs in epidemics exhibiting a high incidence of ALF, with the case fatality rate approaching 40% in pregnant women. Cytomegalovirus, Epstein-Barr virus, and Herpes viruses 1, 2, and 6 have occasionally been implicated as a cause of ALF. ALF related to herpes virus is usually responsive to immunosuppressive therapy, and can be treated with acyclovir.
Drugs that cause liver injury can be divided into two categories: predictable and idiosyncratic. Acetaminophen is representative of the predictable toxic group; the toxicity of acetaminophen is dose-dependent and exaggerated by starvation or drugs (particularly alcohol) that induce the cytochrome P 450 isoenzyme. Many other drugs produce rare catastrophic insults to hepatocytes that are considered to be idiosyncratic reactions (Table 3).
| Infrequent causes | Rare causes | Synergistic causes |
| Isoniazid | Carbamazepime | Alcohol and acetaminophen |
| Valproate | Ofloxacin | Trimethoprim and sulfamethoxazole |
| Halothane | Ketoconazole | Rifampin and isoniazid |
| Sulfonamides | Niacin | Amoxicillin and clavulanic acid |
| Propylthiouracil | Labetalol | |
| Aiodarone | Etoposide (VP-16) | |
| Disulfiram | Imipramine | |
| Dapsone | Interferon alfa | |
| Flutamide |
The fluorinated hydrocarbons trichoroethylene and tetrachloroethane produce hepatic injury in people exposed to industrial cleaning solvents. Amanita phalloides, the death-cap mushroom, causes numerous deaths among amateur mushroom gatherers in China’s mountainous areas. ALF is preceded by muscarinic effects, such as profuse sweating, vomiting, and diarrhea. Early identification is helpful to use antidotes (penicillin and silybin). Successful liver transplantation has been reported.
ALF is one of the presentations of Wilson’s disease. Cardiac-related hepatic ischemia produces centrilobular necrosis and ALF. Causes include myocardial infarction, cardiac arrest, cardiomyopathy, and pulmonary embolism. Hepatic sinusoidal obstruction with subsequent ischemia or interruption of sinusoidal flow has been reported in metastatic gastric carcinoma, carcinoid syndrome, breast cancer, oat-cell carcinoma, amyloidosis, and blastic infiltration with leukemic cells. Occlusion of hepatic venous outflow may occasionally produce a similar picture, either as Budd-Chiari syndrome or as veno-occlusive disease, in the setting of intensive cancer chemotherapy or bone marrow transplantation.
Acute fatty liver can occur in the third trimester of pregnancy and is characterized by the sudden onset of jaundice and altered mental status, accompanied by hypoglycemia and preeclampsia. ALT levels may be very high in late pregnancy and may be accompanied by hemolysis, thrombocytopenia, and preeclampsia in what is termed the HELLP syndrome (Hemolysis, Elevated Liver enzyme levels, Low Platelet counts). Other rare causes of ALF include amebic abscesses, disseminated tuberculosis, recrudescence of hepatitis B virus after the withdrawal of cancer chemotherapy, and bone marrow transplantation.
Although the causative agent is frequently known, a full understanding of the pathogenesis of ALF is elusive. A shock-like state and cerebral edema, shared by all forms of ALF, suggest a unified pathological mechanism. Endotoxemia is common and the levels of tumor necrosis factor α are increased. Prostaglandin metabolism is perturbed and may be important in producing or protecting against tissue hypoxia. Levels of prostaglandin E2, thromboxane A2, and prostacyclin are increased.
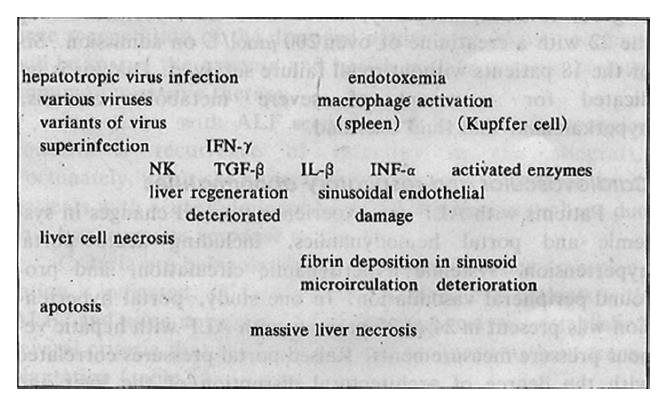
A group-specific component protein (Gc) is markedly diminished in ALF. The survival of patients with ALF after acetaminophen poisoning can be predicted by the level of Gc and the quantity of actin-Gc complexes. Depletion of Gc protein may contribute to the disease, since exhaustion of this actin scavenging mechanism would enhance the precipitation of actin filaments (and platelets) in the microcirculation. Plasma thrombomodulin levels are elevated in ALF. Endothelin-1 levels are elevated in patients with ALF and are highest in association with renal failure. It seems unlikely that a single pathological mechanism can explain all the abnormal events. Two aspects are involved – the host and the virus – as summarized schematically in Table 4 and Figure 2.
| Etiologic agents | |
| Viruses | various hepatotropic viruses |
| superinfection of hepatotropic viruses | |
| variants of a hepatotropic virus (mutants) | |
| Chemicals | |
| Miscellaneous | |
| Host factors | |
| Hyperfunction of cellular immunity | |
| Hyperfunction of antibody production → immune complexes | |
| Endogenous endotoxemia | |
| Deficient phagocytosis of reticuloendothetial system | |
| Activation of macrophages | overproduction of TNF-α and IL-1 |
| release of leukotrienes | |
| release of superoxides | |
| Liver regeneration failure | overproduction of regeneration-suppressing factors |
| disorders in cell receptors and signal transduction | |
| Apoptosis | |
The onset of encephalopathy is often abrupt. Agitation, delusional ideas, and hyperkinesis are common, and coma rapidly ensues. Benzodiazepine-like substances have been implicated in the pathogenesis of the encephalopathy. Elevated concentrations of 1,4-benzodiazepines have been detected in brain tissue.
Cerebral edema occurs in 75%-80% of ALF patients who progress to grade IV encephalopathy, and is the leading cause of death in these patients. Intracranial blood flow is markedly reduced in the patients. Cerebral edema in the confinement of the cranial vault raises intracranial pressure and decreases intracerebral perfusion. Transferring comatose patients to transplantation facilities by airplane or helicopter is hazardous, since the decreased pressure in flight exacerbates the condition. A CT scan to rule out an intracranial hemorrhage prior to surgery is necessary.
Intracranial pressure monitoring, which has shown that pressure changes evolve rapidly and erratically, is used in most transplantation centers to guide the treatment of preoperative and intraoperative pressure changes. Epidural monitors are less hazardous than subdural or parenchymal devices, albeit with a lower sensitivity. A persistent cerebral perfusion pressure of less than 5.33 kPa (40 mmHg) that is refractory to treatment with mannitol should preclude transplantation.
Coagulopathy and hemorrhage are common, and decreased levels of plasma fibrinogen and clotting factors (secondary to decreased synthesis by the damaged liver) are almost universal in ALF. Decreased levels of clotting factors II, VI, VII, IX and X account for the prolonged prothrombin time and partial thromboplastin time. Low-grade fibrinolysis and intravascular coagulation may occur. Antithrombin III levels are decreased, and the level of thrombin-antithrombin complexes is increased.
Coagulation factor levels have been widely used to determine the prognosis in ALF. Pereira et al studied 22 patients with acetaminophen-induced ALF. The levels of factor V on admission were significantly higher in patients who subsequently died of ALF or required liver transplantation than in patients who recovered. A factor VIII/V ratio of greater than 30 was predictive of an adverse outcome in 10 of 11 patients on admission.
Renal failure occurs in more than 50% of patients with ALF and is a poor prognostic sign. Low urine output despite adequate cardiac output is commonly seen and may respond to low-dose dopamine loop diuretics. In 40 patients with ALF and stage III-IV encephalopathy, there were no survivors among the 22 with a creatinine level of over 200 μmol/L on admission. Six of the 18 patients without renal failure survived. Dialysis is indicated for the treatment of severe metabolic acidosis, hyperkalemia, and fluid overload.
Patients with ALF can experience marked changes in systemic and portal hemodynamics, including acute portal hypertension, systemic hyperdynamic circulation, and profound peripheral vasodilation. In one study, portal hypertension was present in 24 of 25 patients with ALF. Elevated portal pressures correlated with the degree of architectural disruption of the liver and were most evident in patients with ascites and renal failure. Patients with ALF typically had a hyperdynamic circulation with a low systemic vascular resistance and a markedly increased cardiac output. The cause is unclear, and a variety of mediators have been proposed, including bile salts, prostaglandins, glucagon, endotoxin, serotonin, nitric oxide, and nonhumoral neural substances. The circulatory changes, which can be profound, are associated with renal vasoconstriction, a decrease in cerebral perfusion pressure, and hepatopulmonary syndrome, leading to renal failure.
Hypotension may result in impaired hepatic, renal, and cerebral function due to hypoperfusion. Pulmonary problems pose a special threat to patients with ALF. Aspiration with pneumonia may occur in comatose patients, who are at a higher risk of developing low pressure pulmonary edema and adult respiratory distress syndrome, and tissue hypoxia leads to anaerobic metabolism and ultimately to lactic acidosis. Prostacyclin, which has vasodilatory effects on the microcirculation, will increase peripheral oxygen uptake. Acetylcysteine, an acetaminophen antidote, enhances oxygen delivery and consumption, possibly by opening the microcirculation through the nitric oxide-induced control of vascular tone.
Bacterial and fungal infections are common in ALF. In a study of 50 patients, 80% had culture-proven infection and 10% had a suspicion of infection. Most infections arise in the respiratory and urinary tract, and Gram-positive cocci – particularly Staphylococcus aureus – are most frequently implicated. Sepsis is common, with bacteremia documented in 20%-45% of patients. Susceptibility to infection may relate to a deficiency of complement or impaired serum opsonization and/or chemotaxis. Polymorphonuclear cell inhibition and Kupffer cell dysfunction may also increase the risk of sepsis. Patients with ALF are at risk for iatrogenic sepsis related to intravenous, arterial, or urinary catheters. Antifungal therapy should be considered prophylactically in patients with established renal failure who survived for five days post-admission. Spontaneous bacterial peritonitis is also common. Chu et al found that 32% of 82 patients developed bacterial peritonitis. These infected patients are likely to develop renal failure and gastrointestinal tract bleeding, with a significantly high mortality rate.
Hypoglycemia is common due to defective gluconeogenesis in the failing liver, as well as inadequate hepatic uptake of insulin, leading to hyperinsulinemia. Hypokalemia can occur due to central nervous system-induced respiratory alkalosis and the resultant renal excretion of potassium in exchange for hydrogen ions. Hypophosphatemia is also frequent and cardiac arrhythmias may occur.
For each ALF patient, treatment should be complex and active based on the current understanding of the cause, pathogenesis and pathophysiology – not only with modern western medicine, but also with traditional Chinese medicine. Nevertheless, basic studies are particularly necessary in ALF, since intuitive treatment approaches have thus far been of limited value.
Systemic treatments such as corticosteroids, heparin, or insulin and glucagon have shown little efficacy, but recombinant human hepatic growth factor (rhHGF), HSS and hepatitis B immune globulin may have a beneficial effect. Antiviral agents have not been used to any extent for ALF, except Chinese herbs. Interferon is beneficial to block further liver destruction and the development of ALF from acute severe viral hepatitis. Levin et al reported favorable results in the use of human interferon γ in patients with ALF secondary to hepatitis A, B, or non-A, non-B. Blood or plasma exchange, hemodialysis, or other methods to detoxify the blood may improve the coma grade. Prostaglandins initially showed some promise; however, efficacy could not be demonstrated in controlled studies.
Since there are no specific treatments with proven efficacy (except for toxin antidotes), the guiding principle of therapy in ALF is to provide good intensive care to the comatose patients. Every effort must be made to elucidate the cause, as antidotes need to be given as early as possible for acetaminophen or mushroom poisoning. Initial management includes measuring glucose (giving 560 mmol/L (10%) dextrose if necessary), acetaminophen levels, ceruloplasmin (< 50 year of age), prothrombin time, hepatitis virus markers and conducting toxicological screening. The patient's mental status, blood pressure, and urine output should be monitored carefully. H2-receptor blockers should be routinely given to prevent stress ulcers and hemorrhage. Pulmonary artery monitoring is helpful for the management of intravascular volume and optimal oxygenation. Conventional pressor treatment for shock with dobutamine or dopamine is relatively ineffective, and may further impair peripheral oxygen delivery.
The appropriateness of liver transplantation should be soon assessed, since transfer to a specialized center is best accomplished when the patient has grade I or II encephalopathy. Evidence of cerebral edema, bleeding, infection, or changes in blood pressure or oxygenation should prompt aggressive treatment. For signs of cerebral edema, 100-200 mL of 200 mL/L mannitol (0.3-0.4 g/kg) should be given by rapid intravenous infusion and may be repeated at least once after several hours. Head-up tilting of up to 45 degrees is probably deleterious, since cerebral perfusion pressure diminishes with elevations above 20 degrees.
Crystalloid or colloid solutions should be infused to maintain blood pressure, but pulmonary capillary wedge pressures of over 1.60 kPa (12 mmHg) should be avoided to minimize the risk of precipitating cerebral edema. Endotracheal intubation and mechanical ventilation will provide protection of the airway from aspiration. Patients should be monitored with continuous pulse oximetry, and hypoxia should be treated with supplemental oxygen. Regular microbial surveillance of urine, sputum, blood and, if present, ascites and open wounds should be carried out. Sites of invasive catheter insertion should be examined regularly. Early broad-spectrum antibiotic therapy is indicated at the first suggestion of sepsis. In patients with a markedly elevated white blood cell count and pyrexia unresponsive to antibiotic therapy, antifungal therapy should be initiated with amphotericin B.
Patient survival is dependent on the rapid institution of comprehensive and aggressive medical care. If the multisystem failure associated with ALF can be successfully managed, liver regeneration and patient recovery may occur. The optimal management of patients with ALF may require the services of multiple specialties including surgery, intensive care, respiratory care, gastroenterology, hematology, nephrology, and psychiatry. ALF-related problems and their management are summarized in Table 5.
| Hypoglycemia | (10%) dextrose continuous infusion | |
| Bolus (50%) dextrose solution | ||
| Encephalopathy | Lactulose per NG enemas | |
| Neomycin/metronidazole/polymycin B | ||
| GI therapy + branched chain amino acids | prostaglandins | |
| plasma exchange | ||
| Rule out sepsis, GI bleeding | hypoxia, drug effects | |
| hypoglycemia, acid-base imbalance | ||
| Cerebral edema | Restrict fluids, Avoid patient stimulation | |
| Mannitol bolus | ||
| Consider intracranial pressure | monitoring, thiopental infusion | |
| Hypotension | Consider GI bleed/hypovolemia/septic shock | |
| Optimize cardiac filling pressure | ||
| Dopamine ± norepinephrine infusion | ||
| Hypoxia | Endotracheal intubation, Mechanical ventilation | |
| Sepsis | Broad-spectrum antibiotics, Consider fungal sepsis |
Management of acetaminophen overdose has focused on preventing glutathione depletion by administration of acetylcysteine as soon as possible. However, acetylcysteine given as late as 36 h after toxic ingestion can be beneficial. The lethal dose for acetaminophen is 10-20 g. Lesser amounts of acetaminophen may cause hepatotoxicity in patients taking medications known to induce the cytochrome P450 system, such as barbiturates, and in alcoholics. Thus, in these patients the plasma acetaminophen concentration threshold for acetylcysteine treatment should be lowered by 70%.
Several centers have published their results of orthotopic liver transplantation for patients with ALF. A total of 69 patients transplanted for ALF experienced a survival rate of 65%. In 42 patients who underwent orthotopic liver transplantation for ALF, stage IV coma, bleeding, and renal failure were defined as significant risk factors for mortality. Sepsis, uncorrectable metabolic acidosis, and hemodynamic instability requiring high-dose vasopressor therapy also appeared to be significant risk factors. Overall survival was 59.4% for 1a and 45.8% for 2a, 3a, and 4a. Contraindications for transplantation include ongoing sepsis, severe chronic cardiorespiratory disease, malignancy, active substance abuse, mental instability, portomesenteric venous thrombosis, and irreversible brain damage caused by cerebral edema.
Potential advantages of heterotopic liver transplantation over orthotopic liver transplantation include avoidance of decreased cardiac output and cerebral perfusion related to the anhepatic phase, as well as caval cross-clamping, reduced surgical trauma, and decreased operative time. Potential disadvantages of heterotopic transplantation include a lack of adequate room for the graft in the abdominal cavity, technical difficulties with graft vascularization and venous drainage, and functional competition with the native liver. Heterotopic liver transplantation may have a future role as a bridging measure in patients too unstable to undergo orthotopic liver transplantation; moreover, once regeneration of the damaged native liver occurs, the patients will be spared the expense and health risks of chronic life-long immunosuppressive therapy.
In patients with ALF secondary to hepatitis B, a major concern is the recurrence of infection in the allograft. Fortunately, the risks of recurrent infection are much lower in patients with acute fulminant hepatitis B infection, perhaps due to a hyperimmune response to the virus.
Criteria are being developed to determine when transplantation is indicated. O’Grady et al received 588 patients with ALF and, using univariate and multivariate analysis, established several criteria that indicated a dismal prognosis without transplantation (Table 6).
| Cause of ALF | Criteria |
| Acetaminophen poisoning | pH < 7.3 (irrespective of grade of encephalopathy) or Prothrombin time > 100 s and serum creatinine > 300 μmol/L (3.4 mg/dL) in patients with grade III or IV encephalopathy |
| All other causes | Prothrombin time > 100 s (irrespective of grade of encephalopathy) |
| Any three of the following variables (irrespective of grade of encephalopathy): age < 10 yr or > 40 yr; liver failure caused by non-A, non-B hepatitis, halothane-induced hepatitis, or idiosyncratic drug reactions; duration of jaundice before onset of encephalopathy > 7 d; prothrombin time > 50 s; serum bilirubin > 300 mmol/L (17.5 mg/dL) |
Serial recording of sensory-evoked potentials may be very helpful to identify: (1) a subgroup of patients selected for emergency liver transplantation by King’s College criteria who may recover spontaneously without transplantation and (2) a subgroup of patients with severe, life-threatening brain dysfunction who should receive liver transplantation despite not fulfilling the King’s College criteria.
In theory, if liver function can be supported until hepatic regeneration occurs, liver transplantation or death can be avoided. Thus, an active area of investigation is the development of artificial hepatic support devices. A promising area currently being studied is the use of extracorporeal liver assist devices in which blood from patients with ALF is perfused through hollow fiber cartridges containing isolated hepatocytes. Alternate approaches, including hemodiafiltration combined with plasma exchange, have shown favorable results. Hepatocyte transplantation in ALF has been reviewed. The potential problems with this procedure include the need to transplant an adequate number of hepatocytes to reverse ALF and the need for a system that will provide prompt engraftment and functioning of the hepatocytes. The implications of hepatocyte culture systems for artificial liver support, and a summary of previous studies using these techniques, have been reviewed. Extracorporeal hybrid designs containing hepatocyte culture systems capable of treating ALF may become a reality in the future.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF
| 1. | Ross F, Ryan AM, Bennett GL, Peers D, Chamow SM, Schwall RH. Effects of recombinant human hepatocyte growth factor(rhHGF) on the liver of intact mice. Gastroenterology. 1994;106:A971. |
| 2. | Gupta S, Chowdhary JR. Hepatocyte transplantation: back to the future. Hepatology. 1992;15:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Pan BR. Therapy of hepatic encephalopathy: a progress report (in Chinese). Modern Treatment of Internal Medicine. Tianjin: Tianjin Sci & Tech Translation Pub Co 1987; 109-115. |
| 4. | Ma ZH. Diagnosis and management of acute liver failure (progress). Yang RK, et al. Progress in diagnosis and treatment of digestive system diseases (in Chinese). Haerbin: Heilongjiang Sci & Tech Pub Co 1991; 109-117. |
| 5. | Mao XH, Pan BR. Hepatic encephalopathy and plasma amino acids (in Chinese). Linchuang Gandanbing Zazhi. 1986;2:1-2. |
| 6. | Pan BR, Wu JP. Management of potassium imbalance in liver diseases (in Chinese). Clin Focus Complex Med J. 1987;2:385-387. |
| 7. | Pan BR. Blood amoniuma2reducing therapy (in Chinese). Linchuang Gandanbing Zazhi. 1988;4:26-27. |
| 8. | Pan BR. Hepatic coma: clinical research 1 (in Chinese). Jixu Yixue Jiaoyu. 1989;3:85-88. |
| 9. | Pan BR. Hepatic coma: clinical reseach 2 (in Chinese). Jixu Yixue Jiaoyu. 1989;3:146-148. |
| 10. | Pan BR, Zhou SZ. Progress in pathogenesis and treatment of hepatic coma. Huabei Yixue. 1980;6:18-24. |
| 11. | Hoofnagle JH, Carithers RL, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 441] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Fingerote RJ, Bain VG. Fulminant hepatic failure. Am J Gastroenterol. 1993;88:1000-1010. [PubMed] |
| 14. | O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 412] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Fukui H, Tsujii T. Pathogenesis of fulminant hepatitis(in Janpanese). Igaku no Ayumi. 1994;171:1027-1030. |
| 16. | Watanabe S, Nishioka M. Treatment of fulminant hepatitis(in Japanese). Igaku no Ayumi. 1994;171:1031-1036. |
| 17. | Yoshiba M, Sekiyama K, Inoue K, Fujita R. Early prediction of fulminant hepatic failure and its prevention by interferon in acute severe viral hepatitis. Hepatology. 1994;20:416A. |
| 18. | Grimm G, Madl CH, Kramer L, Gerenci P, Lenz K. Serial recording of sensory evoked potentials: a nona2invasive prognostic indicator in fulminant hepatic failure. Hepatology. 1995;30:140A. |
| 19. | Shiodt FV, Bondesen S, Tygstrup N. Gc globulin in fulminant hepatic failure: relation to survival. Hepatology. 1994;20:369A. |
| 20. | Larsen FS, Eilersen E, Therkelsen K, Mogensen T. Endothelina21 and systemic circulation in patients with fulminant hepatic failure. Hepatology. 1994;20:368A. |
| 21. | Akivama K, Yoneda M, Nakamura K, Kimura A, Kato T, Tamori K, et al. Plasma thrombomodulin concentrations and hepatic immunohistochemical staining of TM in patients with liver diseases. Gastroenterology. 1995;108:A1024. [DOI] [Full Text] |
| 22. | Ortiz D, Westerberg S, Munoz SJ. Effect of total plasma exchange on cerebral edema of fulminant hepatitis. Gastroenterology. 1995;108:A1139. |
| 23. | Ruan E, Curo KA, Komorowski RA, Varma RR. Role of hepatitis B immune globulin in the treatment of severe hepatitis B after liver transplantation. Gastroenterology. 1994;106:A971. |