Copyright
©The Author(s) 2025.
World J Gastroenterol. Jun 7, 2025; 31(21): 107395
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.107395
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.107395
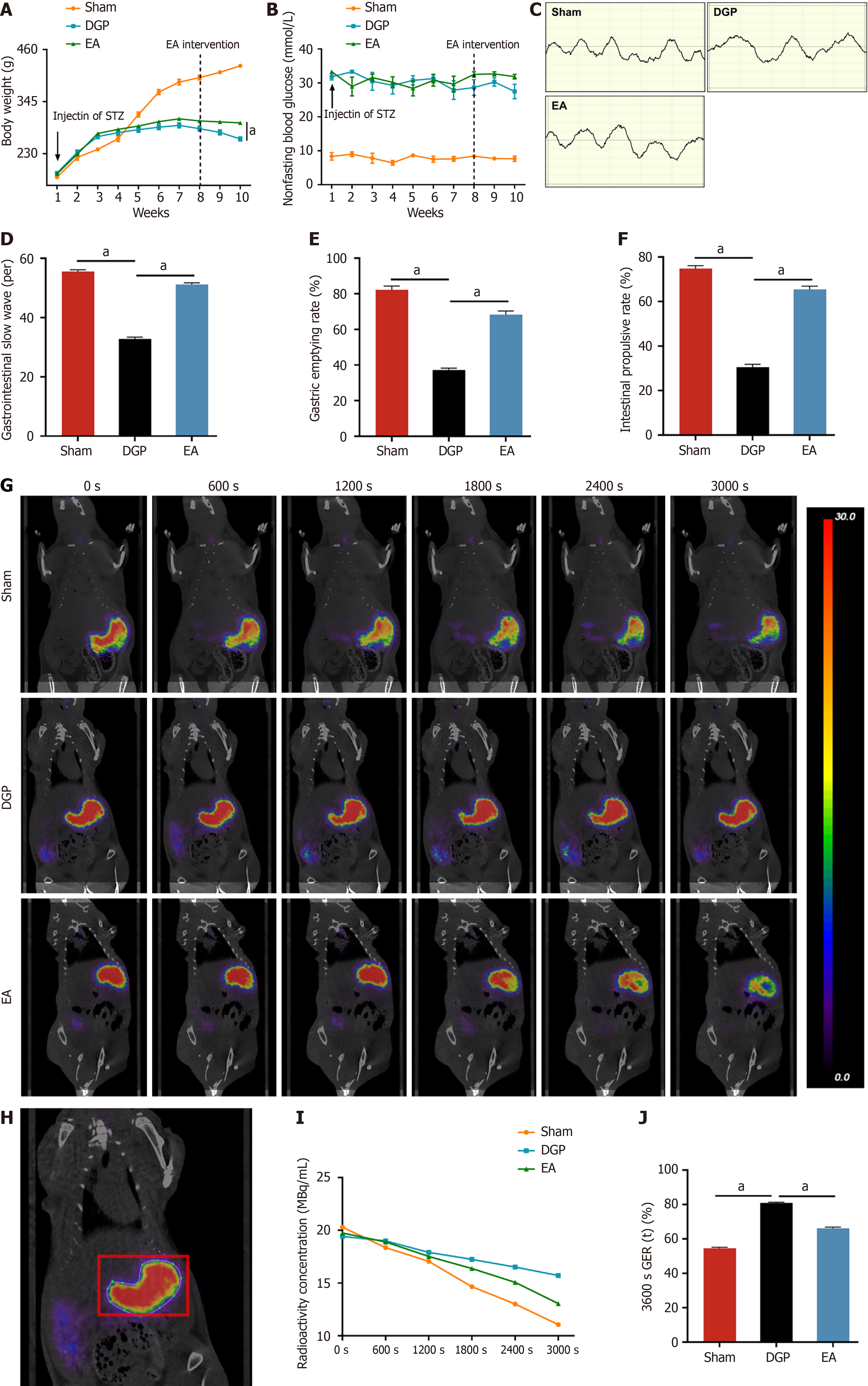
Figure 1 Electroacupuncture has been demonstrated to promote gastric emptying in diabetic gastroparesis rats.
A: Body weight (n = 10); B: Non-fasting blood glucose level (n = 10); C: Electrographic recording of gastric motor activity; D: Number of slow wave discharges in the gastric antrum within 5 minutes (n = 5); E: Gastric emptying rate (n = 5); F: Small intestine propulsion rate (n = 5); G: Representative positron emission tomography images; H: Region of interest in the stomach; I: Radioactive concentration in the region of interest in the stomach (n = 5); J: Gastric emptying rate at 3600 seconds (n = 5). Data are expressed as the mean ± SD. aP < 0.05. STZ: Streptozotocin; DGP: Diabetic gastroparesis; EA: Electroacupuncture.
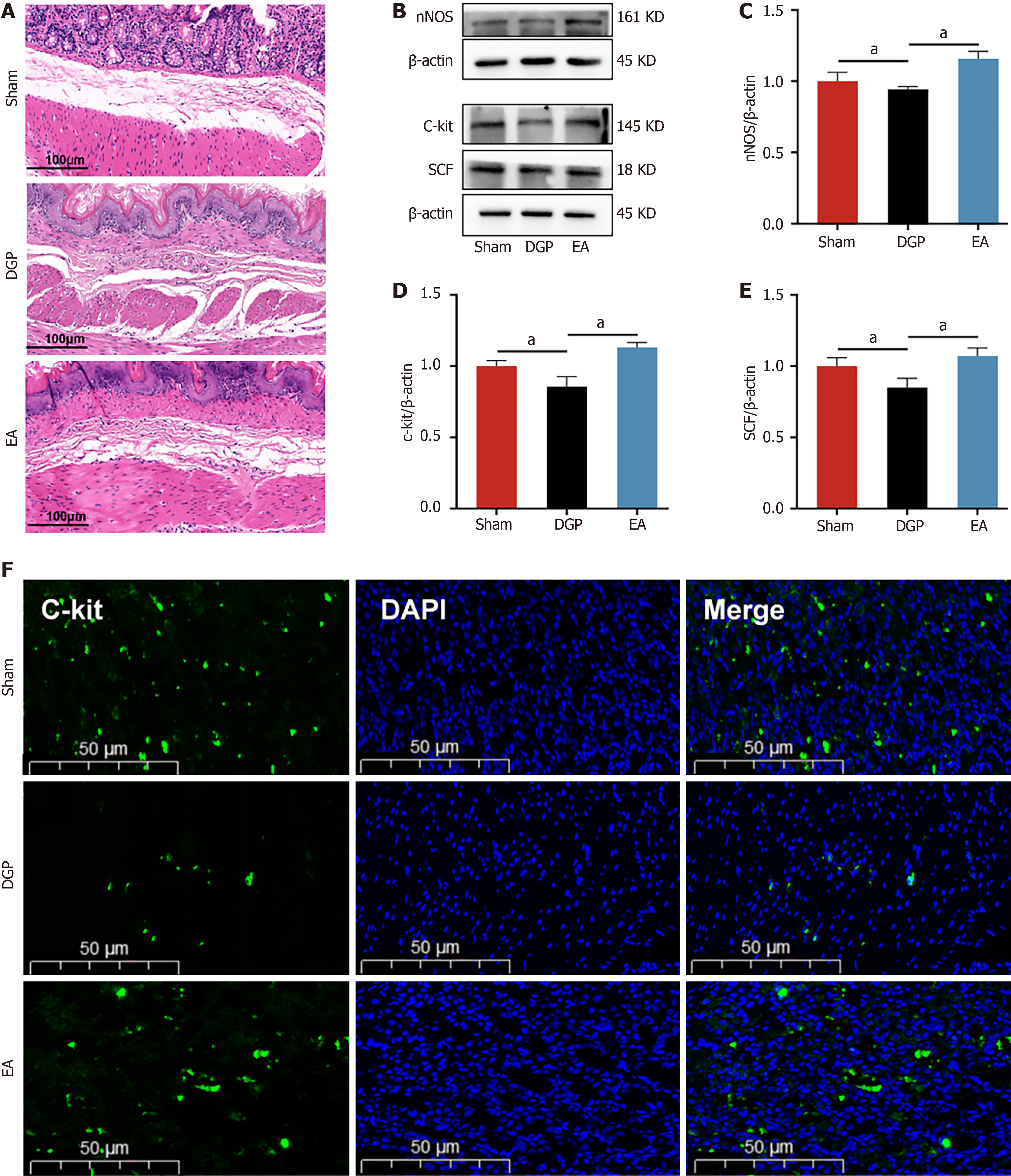
Figure 2 Electroacupuncture improves the dysfunction of gastric smooth muscle in diabetic gastroparesis rats.
A: Representative images of hematoxylin-eosin in the stomach. Scale bar: 100 μm; B-E: Western blotting analysis and quantification of neuronal nitric oxide synthase, cluster of differentiation 117 (C-kit) and stem cell factor protein levels in stomach tissue (n = 5); F: Representative immunofluorescence images of C-kit. Scale bar: 50 μm. Data are expressed as the mean ± SD. aP < 0.05. DGP: Diabetic gastroparesis; EA: Electroacupuncture; nNOS: Neuronal nitric oxide synthase; C-kit: Cluster of differentiation 117; SCF: Stem cell factor; DAPI: 4’,6-diamidino-2-phenylindole.
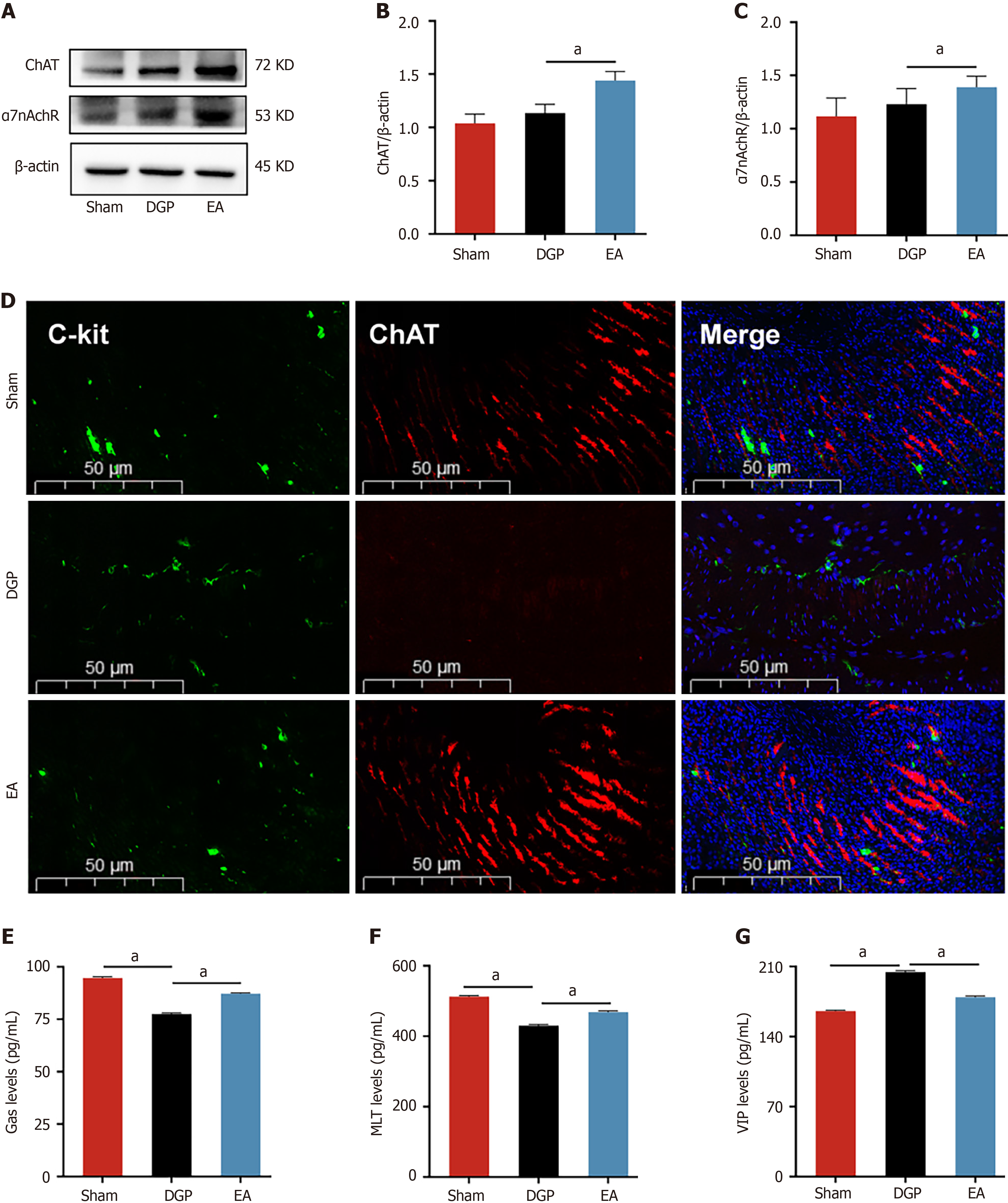
Figure 3 Electroacupuncture improves gastric motility and is associated with vagus nerve targets.
A-C: Western blotting analysis and quantification of choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor protein levels in stomach tissue (n = 5); D: Expression of cluster of differentiation 117 +/ChAT + in the stomach in each group. Scale bar: 50 μm; E-G: Enzyme-linked immunosorbent assay to detect the concentration of gastrin, motilin and vasoactive intestinal peptide in supernatant (n = 5). Data are expressed as the mean ± SD. aP < 0.05. C-kit: Cluster of differentiation 117; DGP: Diabetic gastroparesis; EA: Electroacupuncture; ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; Gas: Gastrin; MLT: Motilin; VIP: Vasoactive intestinal peptide.
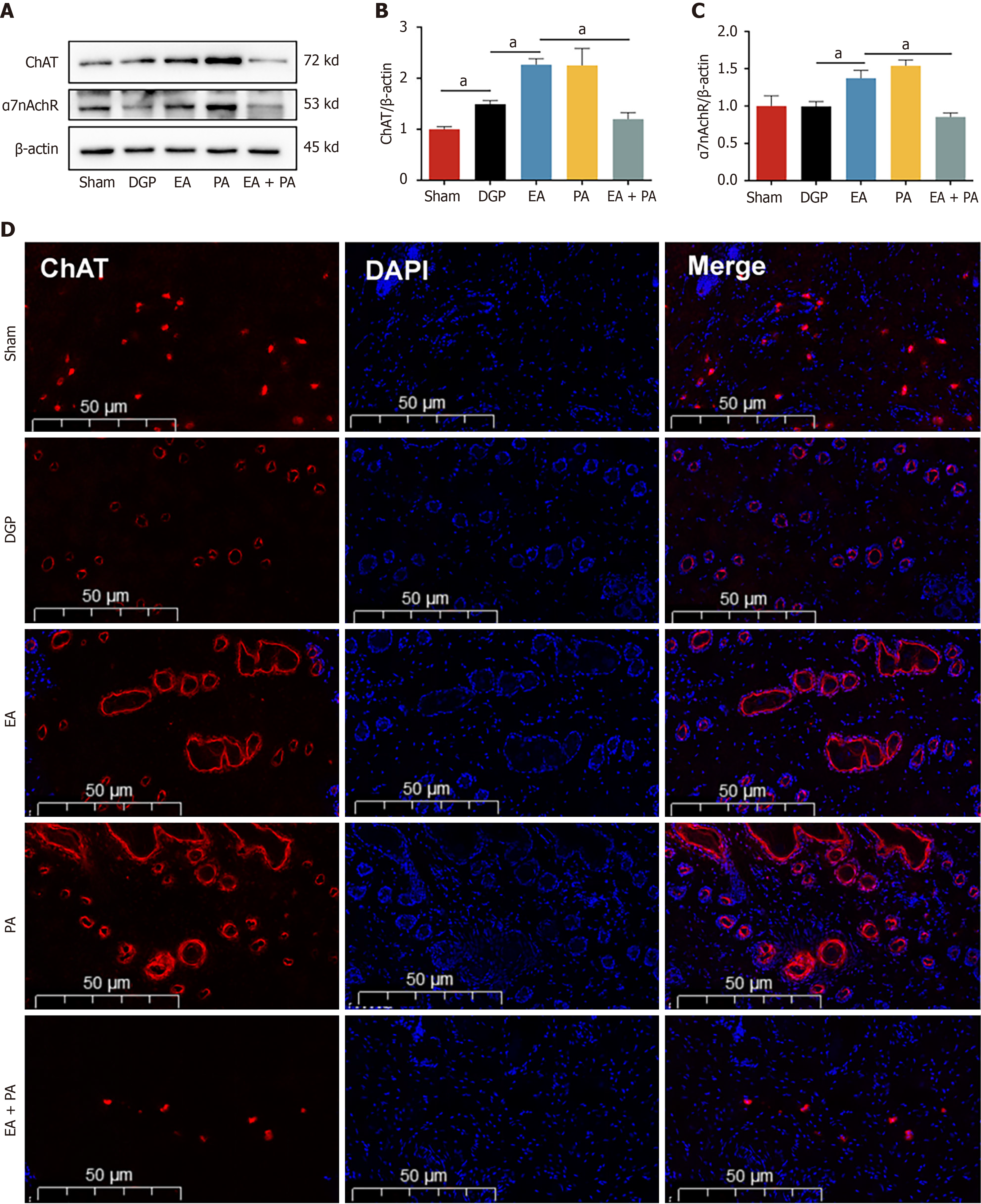
Figure 4 Electroacupuncture activates the vagus nerve target in the ST36 acupoint area.
A-C: Western blotting analysis and quantification of choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor protein levels in acupoint skin (n = 5); D: Representative immunofluorescence images of ChAT. Scale bar: 50 μm. Data are expressed as the mean ± SD. aP < 0.05. DGP: Diabetic gastroparesis; EA: Electroacupuncture; ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; PA: Agonist polygalacic acid; AP: Antagonist alpha-NETA; DAPI: 4’,6-diamidino-2-phenylindole.
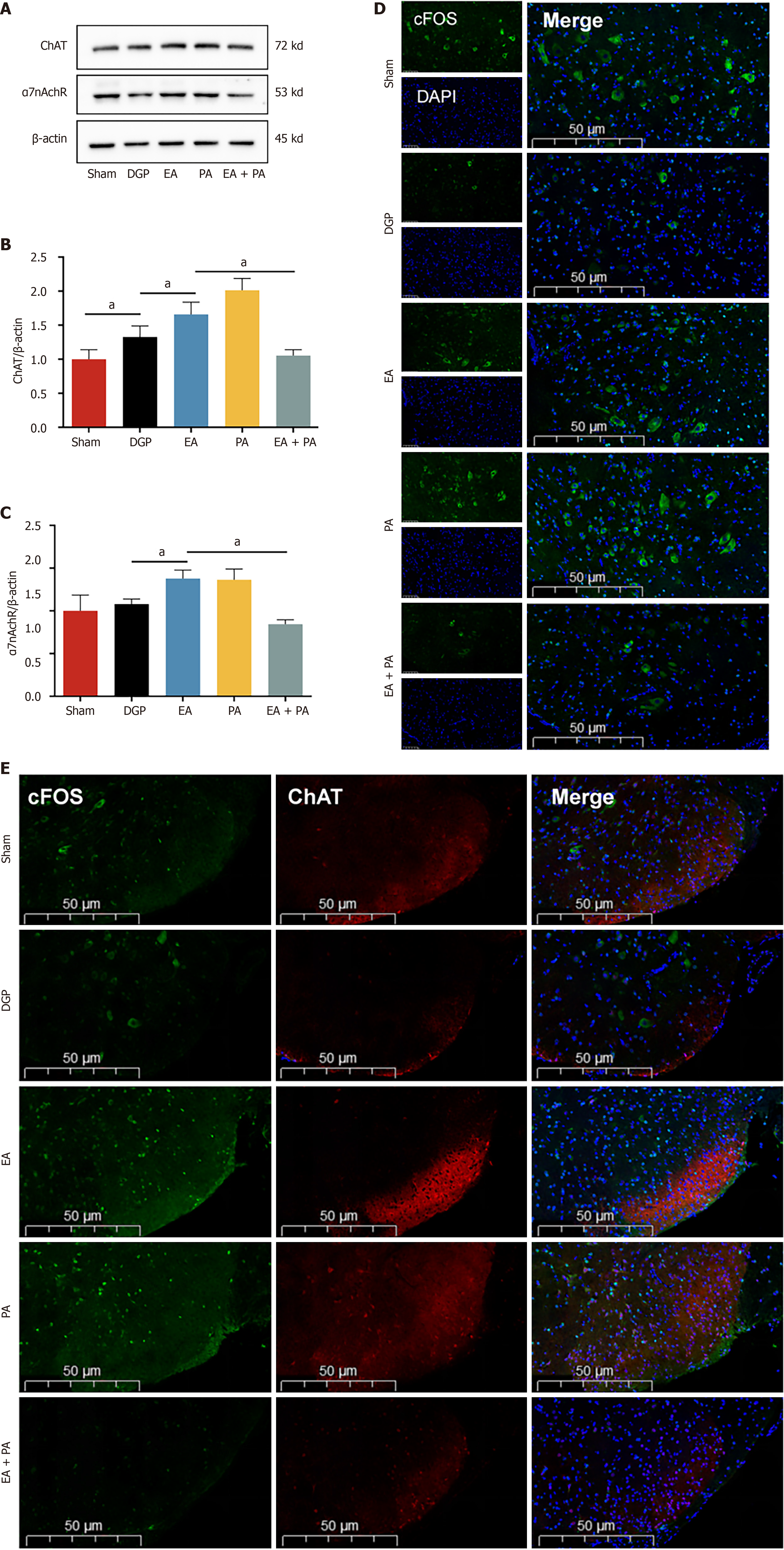
Figure 5 Electroacupuncture activates the vagus nerve target at the L4-L6 segment.
A-C: Western blotting analysis and quantification of choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor protein levels in L4-L6 (n = 5); D: Representative immunofluorescence images of c-FOS. Scale bar: 50 μm; E: Expression of c-FOS +/ChAT + in the stomach in each group. Scale bar: 50 μm. Data are expressed as the mean ± SD. aP < 0.05. DGP: Diabetic gastroparesis; EA: Electroacupuncture; ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; PA: Agonist polygalacic acid; AP: Antagonist alpha-NETA; DAPI: 4’,6-diamidino-2-phenylindole.
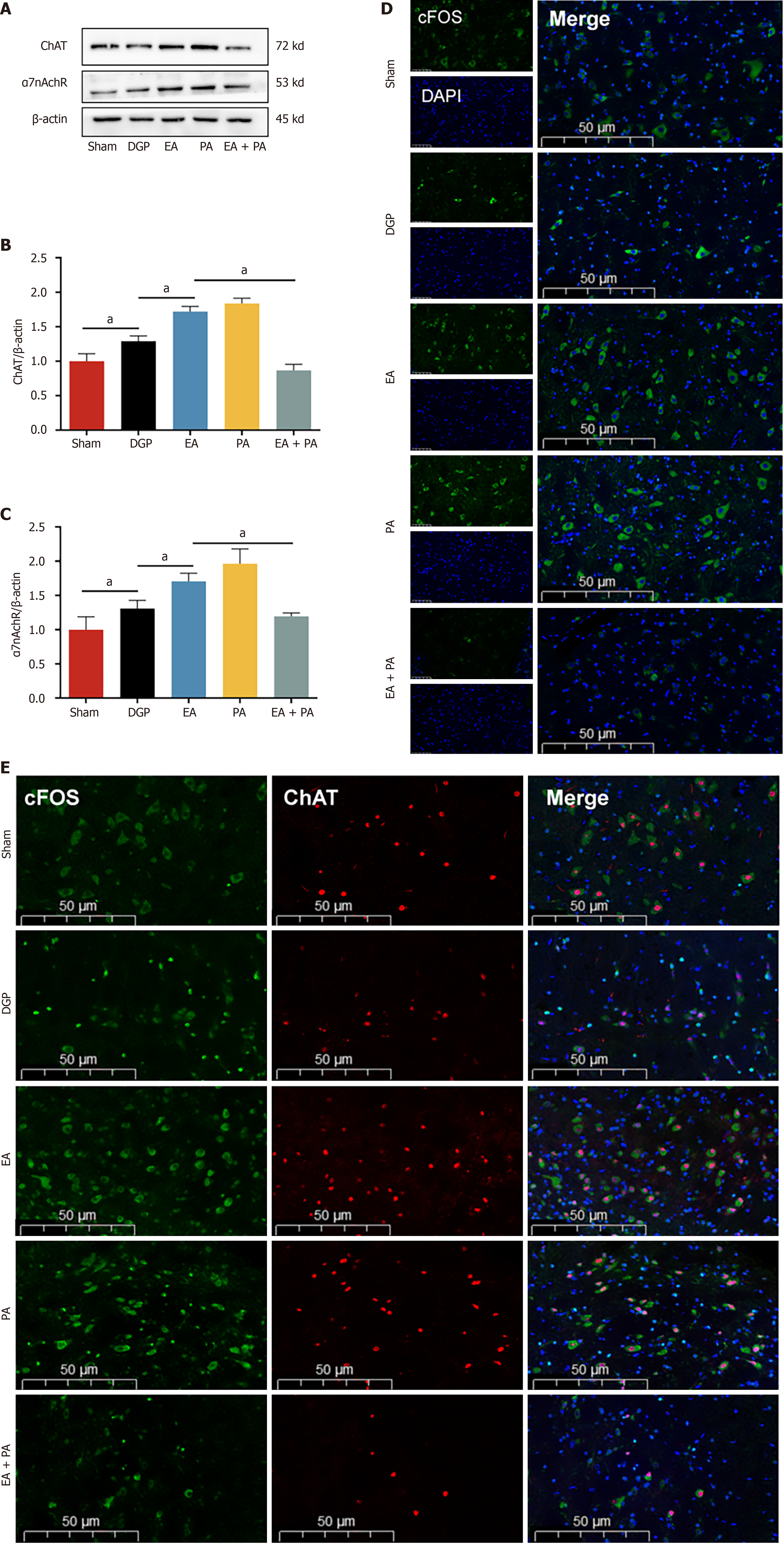
Figure 6 Electroacupuncture activates the vagus nerve target of nucleus tractus solitarius.
A-C: Western blotting analysis and quantification of choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor protein levels in nucleus tractus solitarius (n = 5); D: Representative immunofluorescence images of c-FOS. Scale bar: 50 μm; E: Expression of c-FOS +/ChAT + in the stomach in each group. Scale bar: 50 μm. Data are expressed as the mean ± SD. aP < 0.05. DGP: Diabetic gastroparesis; EA: Electroacupuncture; ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; PA: Agonist polygalacic acid; AP: Antagonist alpha-NETA; DAPI: 4’,6-diamidino-2-phenylindole.
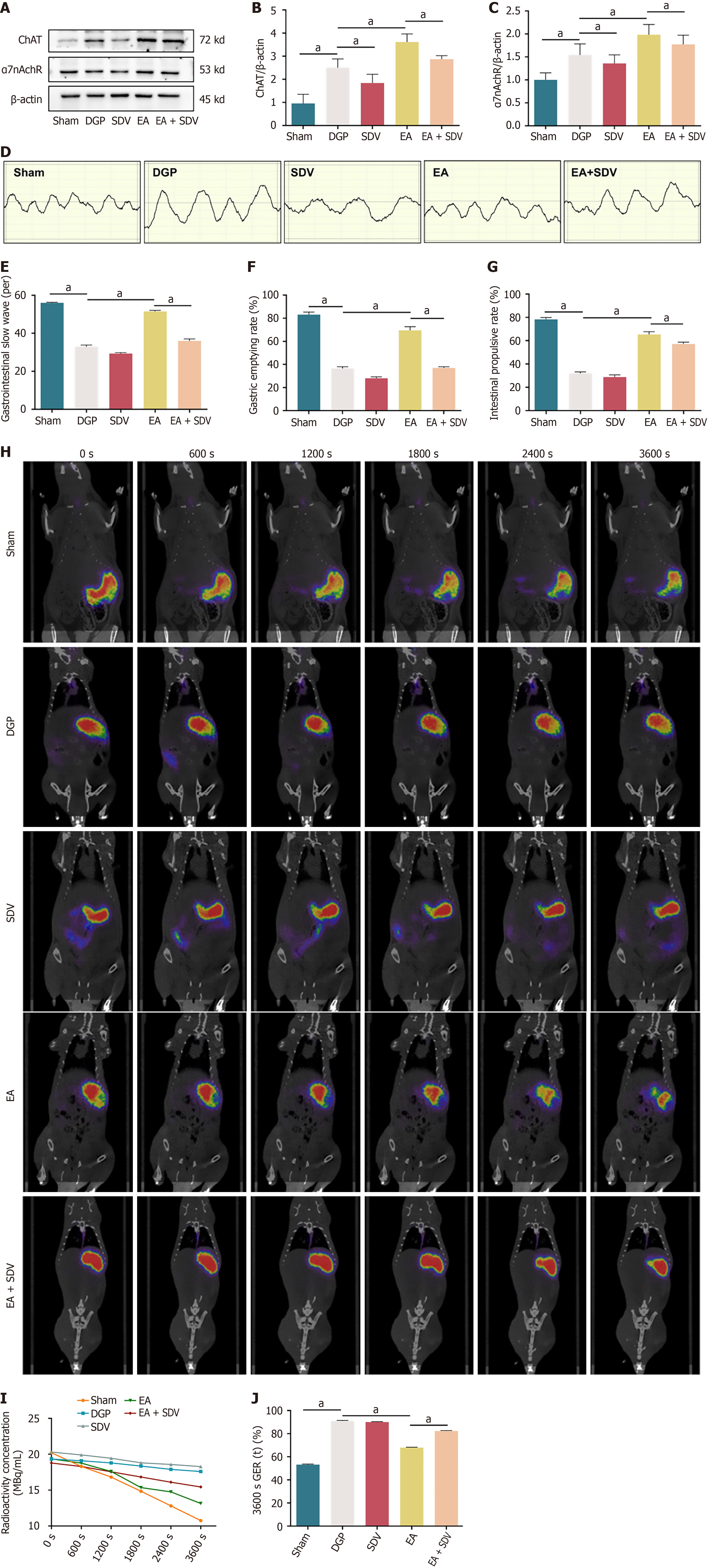
Figure 7 The regulatory effect of electroacupuncture stimulation on gastric emptying after subdiaphragmatic vagotomy.
A-C: Western blotting analysis and quantification of choline acetyltransferase (ChAT) and α7 nicotinic acetylcholine receptor protein levels in stomach tissue (n = 5); D: Electromyogram of the rat stomach; E: Number of slow wave discharges in the antrum of the stomach within 5 minutes (n = 5); F: Gastric emptying rate (n = 5); G: Small intestine propulsion rate (n = 5); H: Representative positron emission tomography images; I: Radioactive concentration in the gastric region of interest (n = 5); J: Gastric emptying rate at 3600 seconds (n = 5). Data are expressed as the mean ± SD. aP < 0.05. ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; DGP: Diabetic gastroparesis; EA: Electroacupuncture; GER: Gastric emptying rate; SDV: Subdiaphragmatic vagotomy.
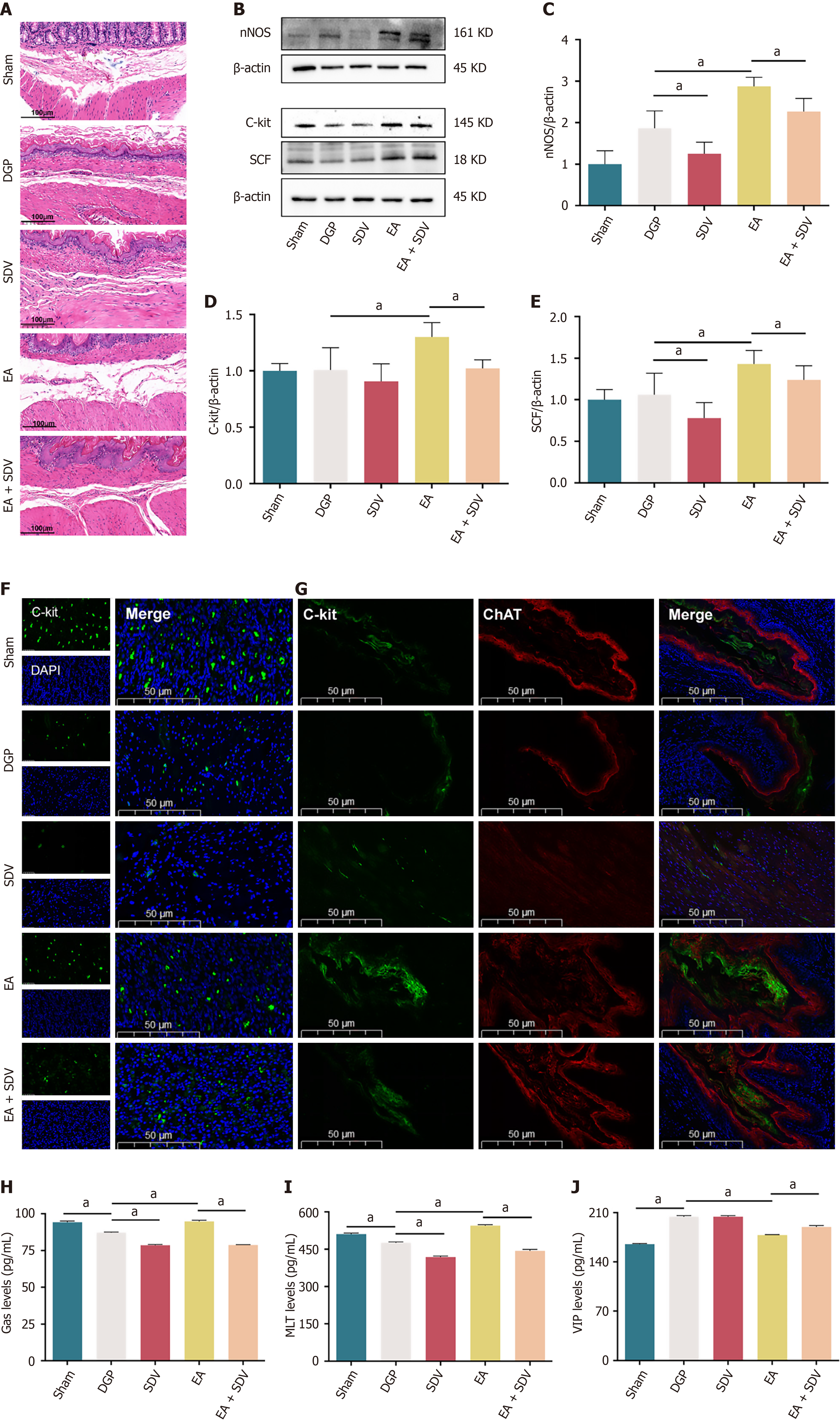
Figure 8 The effect of electroacupuncture on the function of smooth muscle after subdiaphragmatic vagotomy.
A: Representative images of hematoxylin-eosin in the stomach. Scale bar: 100 μm; B-E: Western blotting analysis and quantification of neuronal nitric oxide synthase, cluster of differentiation 117 (C-kit) and stem cell factor protein levels in stomach tissue (n = 5); F: Representative immunofluorescence images of C-kit. Scale bar: 50 μm; G: Expression of C-kit +/choline acetyltransferase + in the stomach in each group. Scale bar: 50 μm; H-J: Enzyme-linked immunosorbent assay to detect the concentration of gastrin, motilin and vasoactive intestinal peptide in supernatant (n = 5). Data are expressed as the mean ± SD. aP < 0.05. DGP: Diabetic gastroparesis; EA: Electroacupuncture; SDV: Subdiaphragmatic vagotomy; nNOS: Neuronal nitric oxide synthase; C-kit: Cluster of differentiation 117; SCF: Stem cell factor; ChAT: Choline acetyltransferase; DAPI: 4’,6-diamidino-2-phenylindole; Gas: Gastrin; MLT: Motilin; VIP: Vasoactive intestinal peptide.
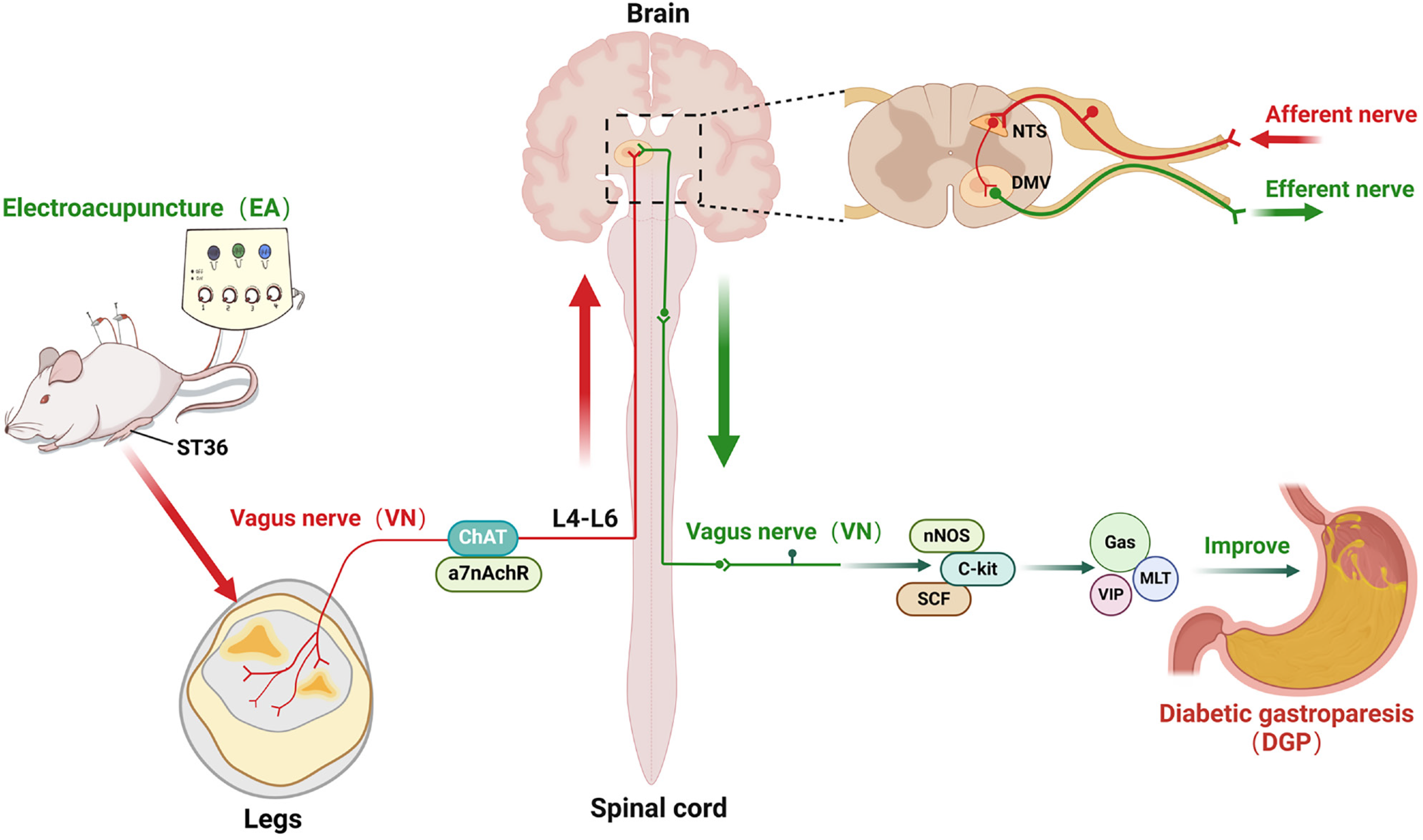
Figure 9 This schematic diagram illustrates the vagus nerve pathway through which electroacupuncture at ST36 enhances diabetic gastroparesis.
Electroacupuncture activates the choline acetyltransferase target of the vagus nerve by intervening in the ST36 acupoint area, which is transmitted up the spinal cord from L4-L6 to the intracranial nucleus tractus solitarius. This, in turn, regulates the gastric smooth muscle-related factors neuronal nitric oxide synthase, cluster of differentiation 117 and stem cell factor, as well as gastrointestinal peptides, through the subdiaphragmatic vagus nerve. This, in turn, improves the gastric motility disorder of diabetic gastroparesis. ChAT: Choline acetyltransferase; α7nAchR: α7 nicotinic acetylcholine receptor; nNOS: Neuronal nitric oxide synthase; C-kit: Cluster of differentiation 117; SCF: Stem cell factor; Gas: Gastrin; MLT: Motilin; VIP: Vasoactive intestinal peptide; NTS: Nucleus tractus solitarius; DMV: Dorsal motor nucleus of the vagus.
- Citation: Zhang Y, Tang YW, Zhou J, Wei YR, Peng YT, Yan Z, Yue ZH. Electroacupuncture at ST36 ameliorates gastric dysmotility in rats with diabetic gastroparesis via the nucleus tractus solitarius-vagal axis. World J Gastroenterol 2025; 31(21): 107395
- URL: https://www.wjgnet.com/1007-9327/full/v31/i21/107395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i21.107395