Copyright
©The Author(s) 2021.
World J Gastroenterol. Jan 14, 2021; 27(2): 189-207
Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.189
Published online Jan 14, 2021. doi: 10.3748/wjg.v27.i2.189
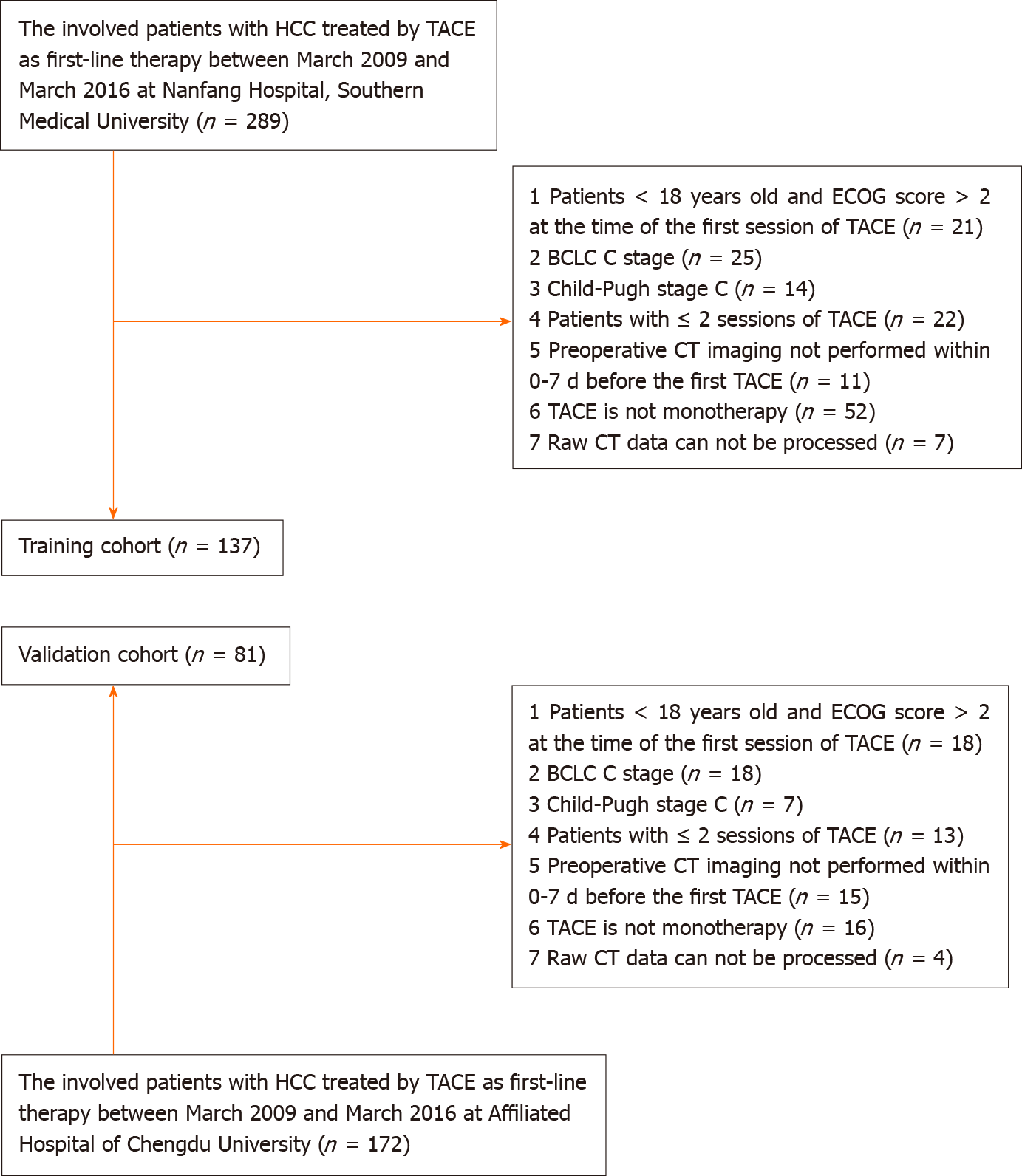
Figure 1 Flowchart for inclusion and exclusion of patients within the training and validation cohort.
TACE: Transarterial chemoembolization; ECOG: Eastern Cooperative Oncology Group; BCLC: Barcelona Clinic Liver Cancer; CT: Computed tomography; HCC: Hepatocellular carcinoma.
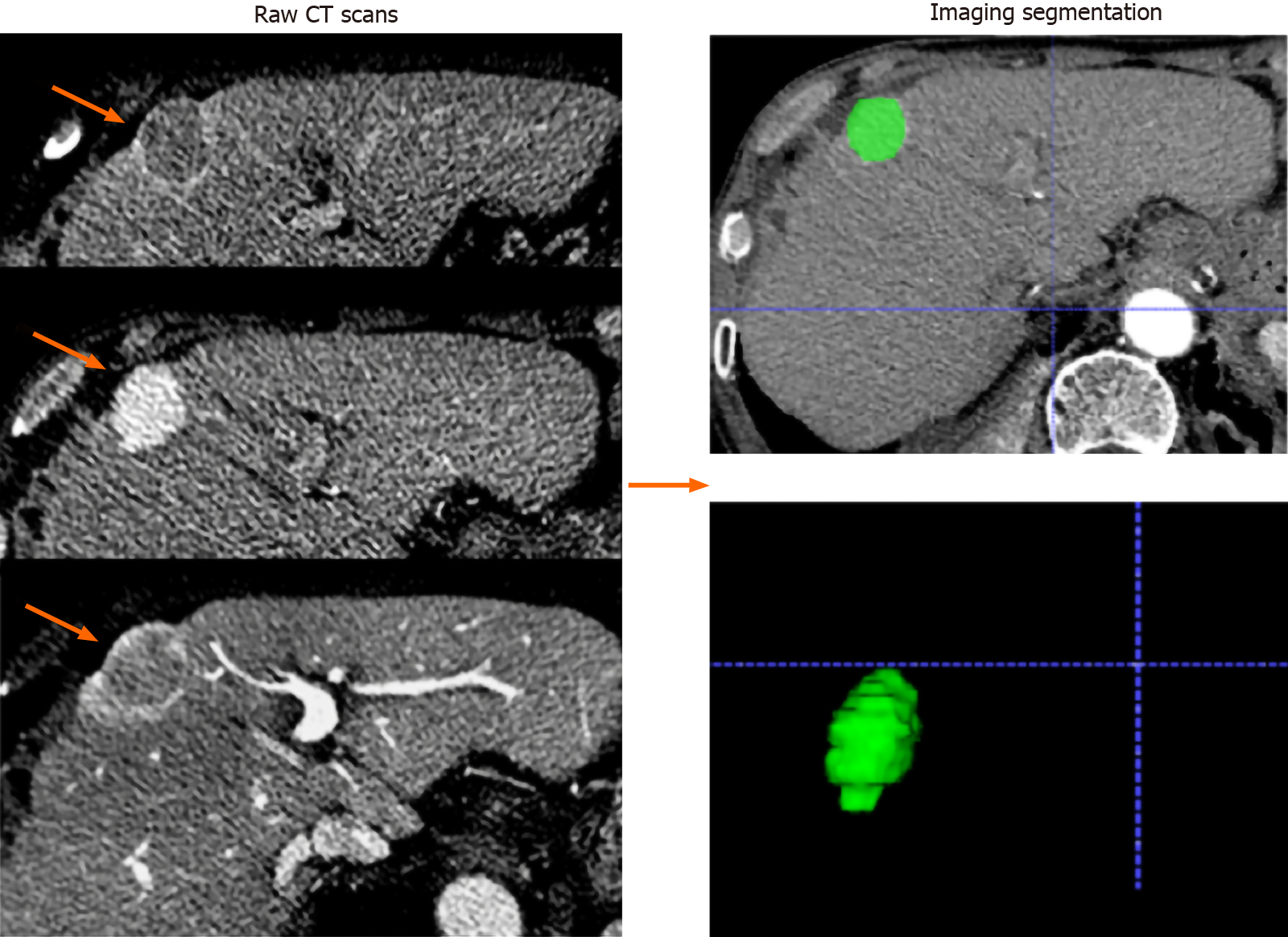
Figure 2 Imaging segmentation.
The images were acquired form a 65-year-old man with hepatocellular carcinoma (orange arrows). Serial raw computed tomography scans obtained before transarterial chemoembolization treatment; regions of interest were manually depicted along with the tumor outline on each axial slice and automatically merged into a volume of interest (green area).
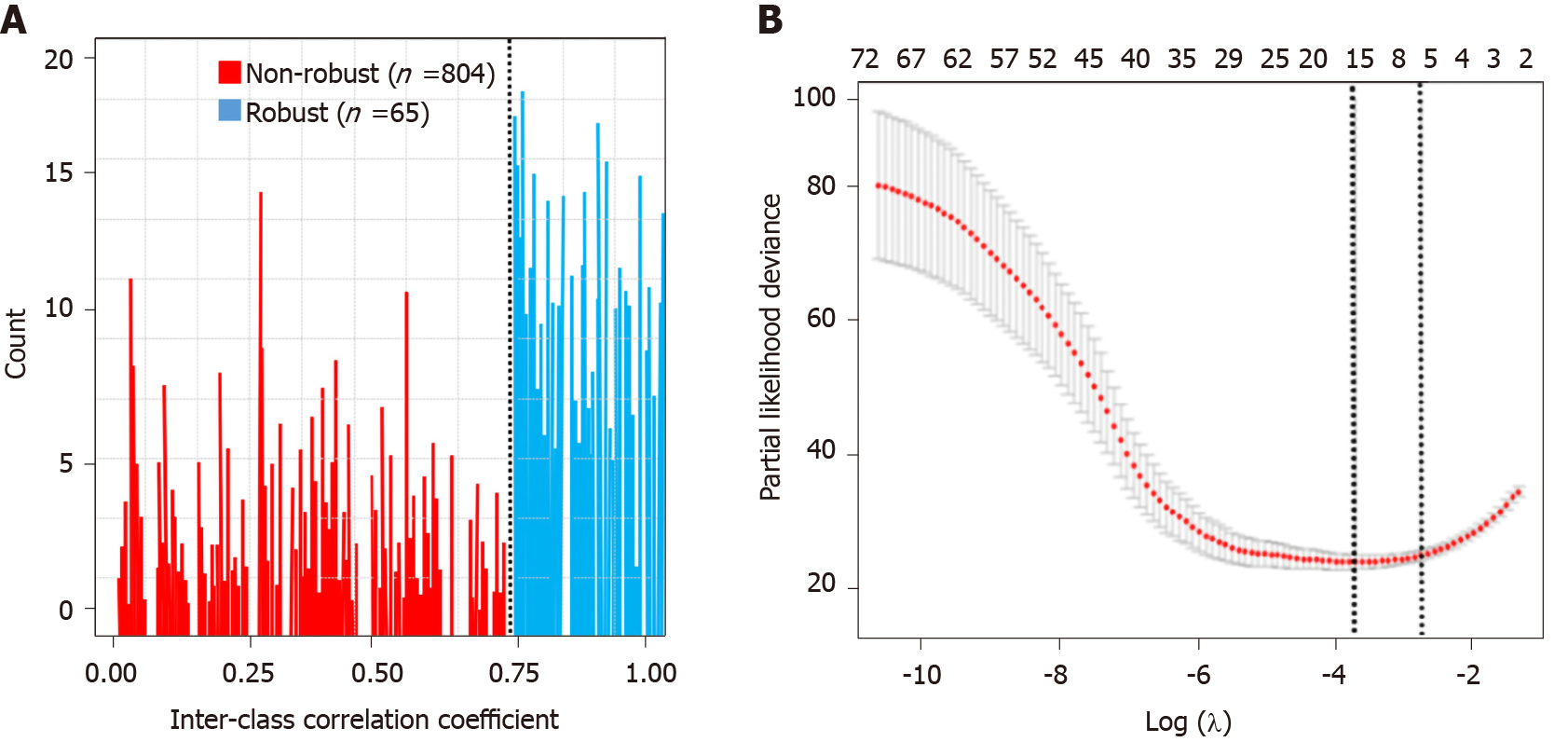
Figure 3 Feature selection.
A: Histogram of the inter-class correlation coefficient. The intraclass correlation coefficient (ICC) was used to determine the stability of the features. Features with an ICC < 0.8 were excluded from the analysis. After robustness test, 65 of the initial 869 computed tomography image features in the arterial phase were retained; B: Partial likelihood deviance was drawn vs log (λ) in the arterial phase.
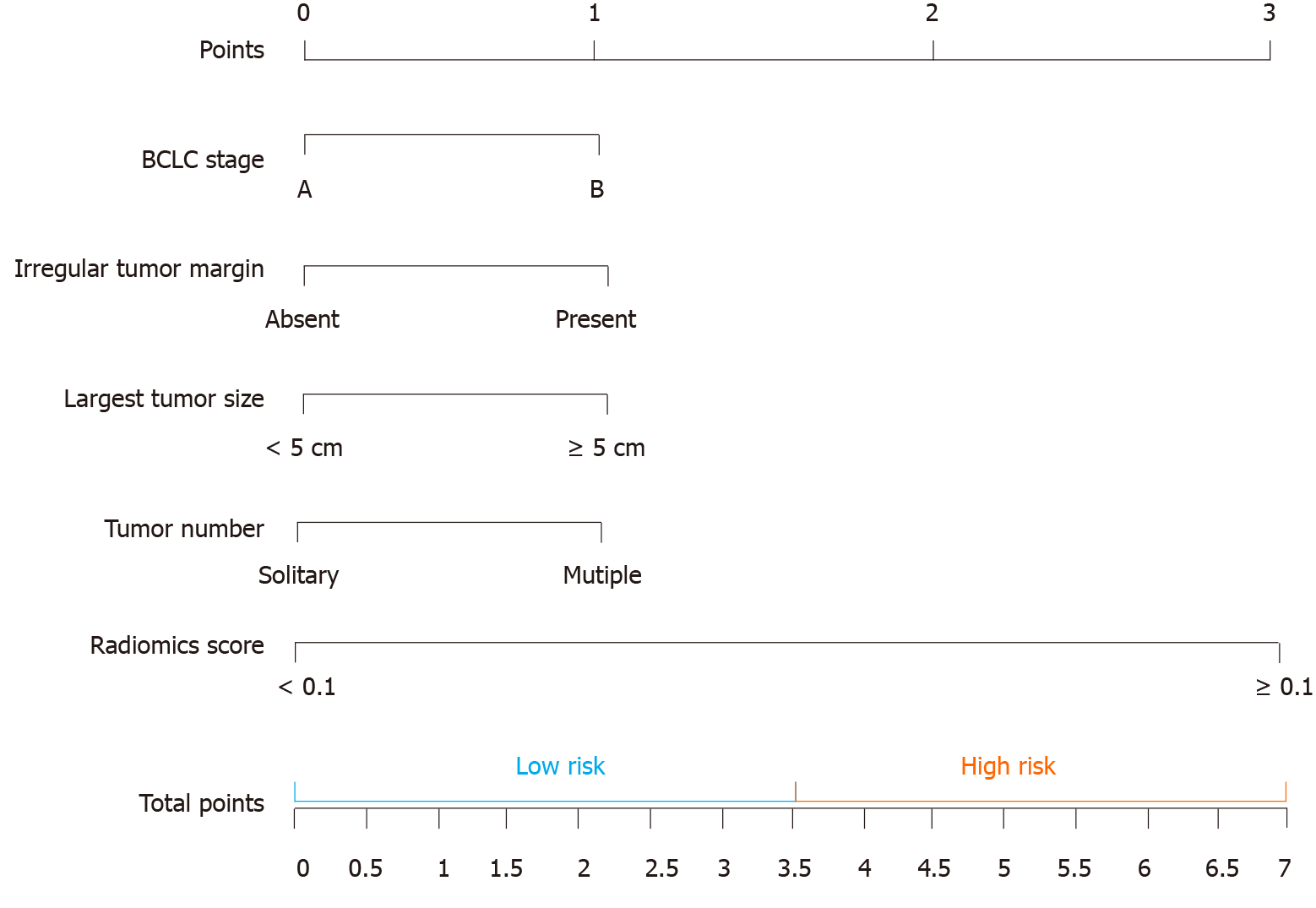
Figure 4 The computed tomography-based nomogram was built.
The computed tomography (CT)-based nomogram was obtained by combining the effective clinical factors and radiomics signature of artery phase contrast-enhanced CT images. We choose the median value (3.5) to classify our patients into high and low-risk groups. BCLC: Barcelona Clinic Liver Cancer.
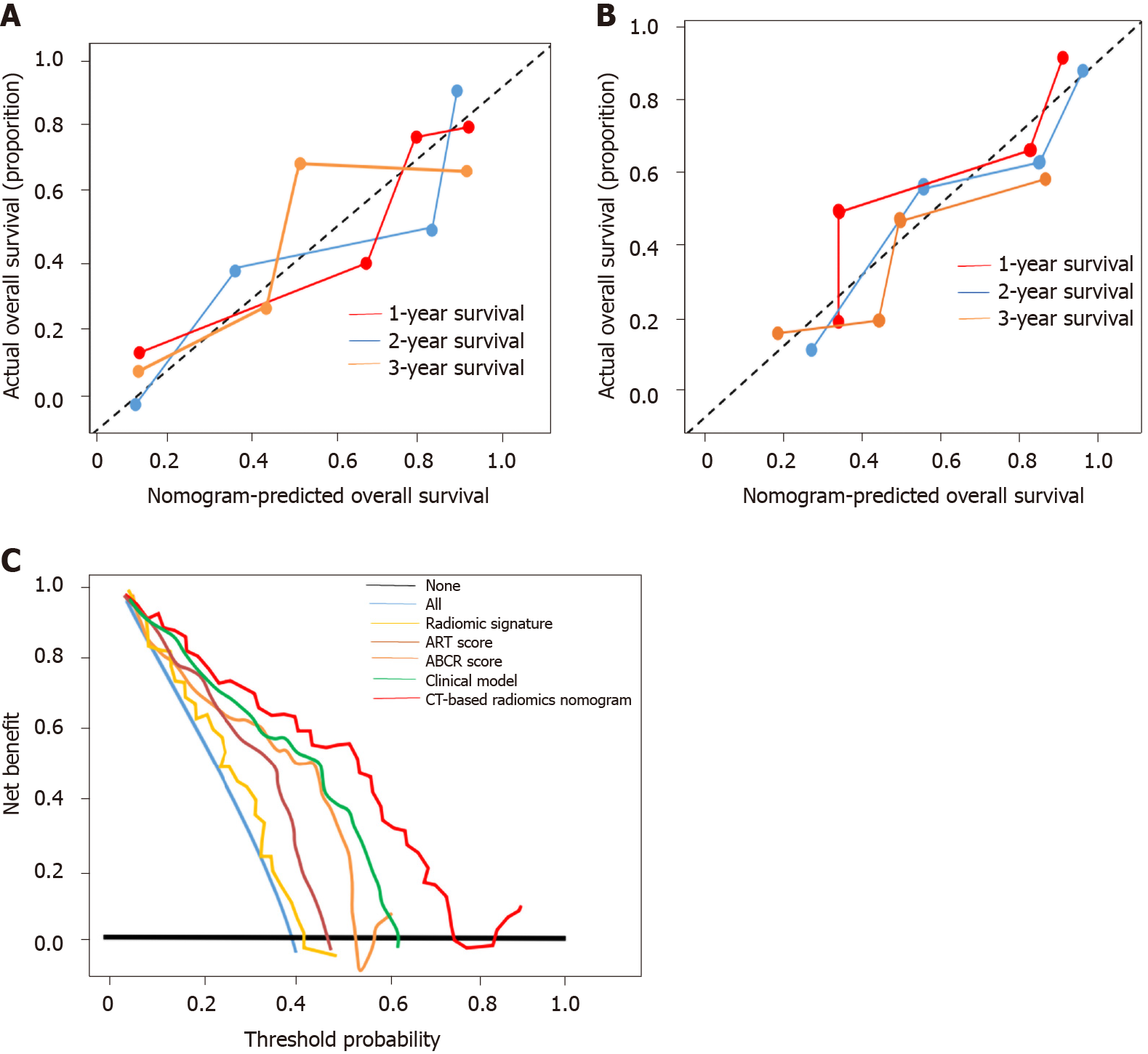
Figure 5 Calibration curve and clinical utility analyses.
A: Calibration curves of the nomogram on the training dataset. The Hosmer-Lemeshow test yielded a P value of 0.137 in the training dataset; B: Calibration curves of the nomogram on the validation dataset. The Hosmer-Lemeshow test yielded a P value of 0.165 in the validation dataset; C: Decision curve analysis for each model in the validation dataset. The Y-axis measures the net benefit, which is calculated by summing the benefits (true-positive findings) and subtracting the harms (false-positive findings). The decision curve showed that if the threshold probability is over 10%, the application of computed tomography-based radiomics nomogram to predict transarterial chemoembolization-refractoriness adds more benefit compared with the other scores/models. ART: Assessment for Retreatment with Transarterial Chemoembolization; ABCR: α-Fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response; CT: Computed tomography.
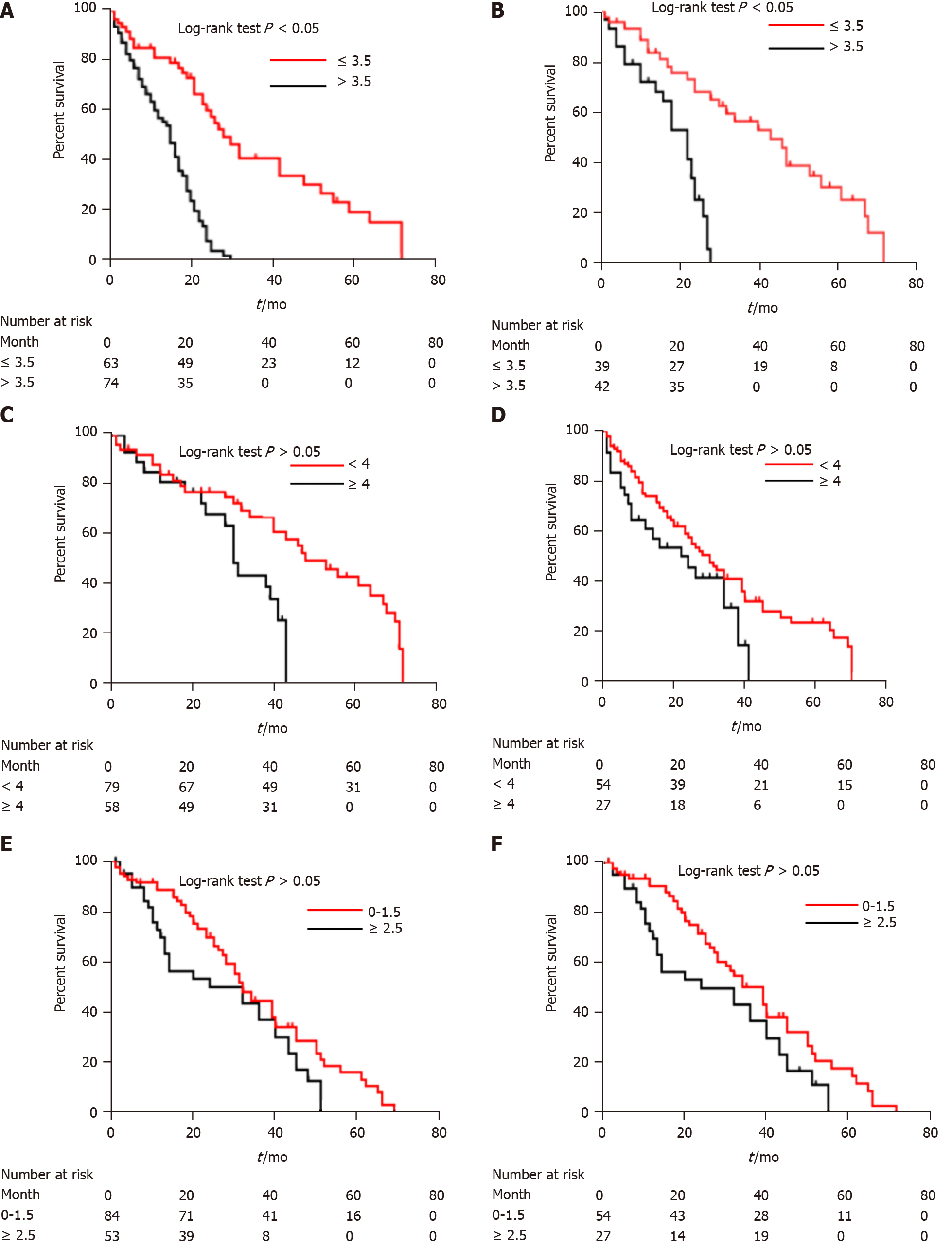
Figure 6 Kaplan-Meier curves of survival outcomes in different patient groups.
A: Kaplan-Meier analysis of overall survival of high-risk (> 3.5) and low-risk (≤ 3.5) patients according to computed tomography (CT)-based radiomics nomogram in the training cohort; B: Kaplan-Meier analysis of overall survival of high-risk (> 3.5) and low-risk (≤ 3.5) patients according to CT-based radiomics nomogram in the validation cohort; C: Kaplan-Meier analysis of overall survival of high-risk (≥ 4) and low-risk (< 4) patients according to α-fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response (ABCR) score in the training cohort; D: Kaplan-Meier analysis of overall survival of high-risk (≥ 4) and low-risk (< 4) patients according to ABCR score in the validation cohort; E: Kaplan-Meier analysis of overall survival of high-risk (≥ 2.5) and low-risk (0-1.5) patients according to Assessment for Retreatment with Transarterial Chemoembolization (ART) score in the training cohort; F: Kaplan-Meier analysis of overall survival of high-risk (≥ 2.5) and low-risk (0-1.5) patients according to ART score in the validation cohort.
- Citation: Niu XK, He XF. Development of a computed tomography-based radiomics nomogram for prediction of transarterial chemoembolization refractoriness in hepatocellular carcinoma. World J Gastroenterol 2021; 27(2): 189-207
- URL: https://www.wjgnet.com/1007-9327/full/v27/i2/189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i2.189